Genital tuberculosis: current status of diagnosis and management
Introduction
Even more than a century after its isolation and description by Robert Koch, Tuberculosis (Tb) still remains a global health problem. With nearly one third of the world’s population infected, it is responsible for maximum number of deaths in adults resulting from a single infectious cause (1). Of its world health burden, more than 90% of the infected individuals belong to the developing countries where the incidence ranges from 192 to 232 per 100,000 population with 19 to 30 deaths per 100,000 population per year (2). Seventy five percent of these infected individuals belong to the economically productive age group (3). Despite the advent of effective chemotherapy and increased awareness, which caused the initial decline, recent decades have noted an increase in the incidence of Tb (3). This re-emergence has been attributed to the AIDS pandemic, emergence of multi drug resistant bacilli (MDR) and immigration (4). Twenty five to fifty percent of the HIV infected population have active Tb, which occurs at a younger age and is often extra-pulmonary (5). MDR Tb is often the result of poor compliance to pharmacotherapy and with its increasing incidence, is another cause for concern. Extra-pulmonary Tb (EPTB), which accounts for 4.5–47.9% (about 10%) of the overall Tb burden, most commonly affects the lymph nodes, urogenital organs, pleura and the skeletal system (3). Thus, although the global prevalence and death rate of Tb is declining, the number of individuals infected is actually increasing (3).
About half a century after its first description, the term genitourinary Tb (GUTB) was first suggested by Wildbolz in 1937. In more than half of the cases, both urinary and genital organs are involved (6). Since renal affliction is always primary and more common, some authors prefer the term urogenital Tb instead (6). GUTB, which accounts for 20–40% of EPTB cases, is the second most common site in developing and third most common site in developed nations (3,6,7). Despite the fact that involvement of genitourinary organs is almost always secondary to Tb elsewhere in the body and is rarely contagious, GUTB can result in a significant morbidity and even mortality due to renal failure. Isolated involvement of genital organs is reported in about 5–30% of the cases of GUTB, with higher rates in epidemic situations (6,8,9). The epididymis or the prostate can be involved by hematogenous spread, with the rest of the genital organs being involved by canalicular, urinary or contiguous spread (3,4,9). Besides significant morbidity, involvement of reproductive organs can result in infertility, which can even be the presenting complaint (10).
This review focuses on genital Tb, its etiopathogenesis, clinical features, current diagnostic modalities and the management strategies, both medical and surgical. A separate section will discuss Tb related infertility, its diagnostic evaluation and management.
Etiopathogenesis
Tubercular involvement of the genitourinary organs is almost always secondary to pulmonary infection. Aerosolized Mycobacterium tuberculosis bacilli infect and invade the pulmonary alveoli and cause primary pulmonary tuberculosis. This subclinical pulmonary infection leads to bacillemia and hematogenous implantation of the Tb bacilli in the kidneys, epididymis and the prostate amongst various other organs of the body. Initially, the implantation occurs in the more vascular parts such as cortex of the kidneys and globus minor of the epididymis, and is bilateral. With the development of immunity, these lesions cicatrize in about 6 months or so, and a latent phase then ensues.
Reactivation of the latent foci results from a decrease in the host immunity, usually after a long latent period of about 30 years, ranging from 1–46 years (11). The reactivation is usually of a single focus which accounts for the greater frequency of a unilateral clinical disease, but can be multifocal, either concurrent or sequential. Amongst the genitourinary organs, renal involvement is the commonest, in about 80%, and the epididymis are the most commonly affected genital organs in about 22–55% of patients (11,12). Genital Tb can result from primary reactivation of the latent bacilli either in the epididymis or the prostate or by secondary spread from the already infected genitourinary organs via the urinary system, by canalicular spread both antegrade and retrograde or by lymphatic spread which probably plays a minor role (13). The epididymis are more frequently involved hematogenously but retrograde canalicular spread from infected prostate and urinary system has also been described. The disease usually starts in the globus minor, which is commonest site for hematogenous spread as well as the first site in retrograde canalicular spread, and then spreads to the other parts. Because of blood testes barrier, testes can only be involved by direct extension from the epididymis and isolated testicular involvement with normal epididymis should raise concerns for a malignant testicular lesion. In tubercular prostatitis, the lateral and peripheral lobes are frequently involved with mucosal or submucosal lesion being seen only in advanced cases, suggesting hematogenous spread as the primary mechanism of involvement rather than direct extension from the urinary tract (14). Seminal vesicles are involved by the canalicular route and this involvement is seldom isolated. Despite its constant exposure to infected urine, urethral Tb is rare and is never isolated.
Very rarely, Tb can infect genital organs primarily, without prior pulmonary involvement. Penile Tb has been described after sexual contact with an infected partner, after contact with infected clothing and in infants after ritual circumcision, by the act of sucking at the penis by operators who had active pulmonary Tb (15-17). Sexual transmission is thought to be possible as viable bacilli have been demonstrated in semen of the patients with pulmonary as well as prostatic Tb and such transmission was confirmed by molecular typing which showed identical organisms isolated from penile ulcer and endometrial biopsy of a couple (7,15,16). The normal mucosa is highly resistant to Tb bacilli, thus it has been postulated that the mucosal abrasions caused by vigorous sexual activity permits their inoculation (18). Besides these, reinoculation of the male partner through his own infected ejaculate and secondary involvement via urethra may also lead to the development of penile lesions in a patient of genital Tb (16).
Epidemiology
GUTB is classified as a severe form of EPTB with a high bacillary load (19). Because of its non-specific symptoms, it is recommended that the diagnosis of GUTB should always be considered in a patient presenting with long standing vague urinary symptoms with no obvious cause (19-21). It is estimated that upto 20% of the patients with clinical pulmonary Tb have genitourinary organ involvement; 2–10% in developed countries and 15–20% in developing countries (3,4,22). Evidence of previous or active pulmonary involvement can be found in 56–87% of the patients with GUTB (9,23). Genitourinary organs are commonly involved together rather than in isolation and it is the duration of disease prior to diagnosis that dictates the number of organs involved. Involvement of urinary organs is commoner and in 53% of the cases of renal Tb, an associated genital lesion can be identified (11,12,20). Similarly, 49–88% cases of genital Tb have an associated lesion in the urinary tract (8,23). Isolated involvement of genital organs is rare, 5–30% of all the cases, and it depends on the burden of Tb in the community, ethnic status and thoroughness of evaluation for Tb at other sites (6,8,9). Also, there is some evidence that genital involvement from Tb might be commoner in India as compared to other countries (24). Because of all these factors and its asymptomatic nature, the true incidence of genital Tb is largely unknown.
Clinical manifestations
Genital Tb can present at any age, although it commonly affects men at 30–50 yrs and is uncommon in children due to long latency periods (3,4,8,20,23). Clinical presentation may range from a painless scrotal mass, which is the commonest, to irritative lower urinary tract symptoms, hematuria, dysuria, hemospermia, infertility, ulcerative penile lesion to being completely asymptomatic and incidentally diagnosed on histopathology (21,25). Patients may have symptoms of associated upper tract disease but constitutional symptoms per se are less common and may suggest an extra-genital disease (11,21). History of prior Tb or contact with a patient of Tb, immune-compromised status, immigration or travel to endemic region must be elicited although in a significant majority, such a history may not be present. Commonly multiple genital organs are affected, even in isolated cases of genital Tb and thus clinical picture may overlap.
Affected organs
Epididymis and testes
The epididymis are the commonest site of involvement in male genital Tb and is involved in 48.9% of patients with GUTB, which can be the first or the only presenting feature (4,19). Although termed epididymo-orchitis, 45% have involvement of epididymis alone, with testicular involvement developing later as the disease progresses (26). Eighty percent of patients with tubercular epididymo-orchitis present with a scrotal mass, which can be painful in 40–44% (26,27). Bilateral involvement is seen in 34% cases, 4–50% may present late with an abscess or a fistula and 5–10% may have an associated hydrocoele (4,26,27). Presentation as rapidly enlarging painful hydrocoele due to isolated involvement of tunica albugniea and vaginalis has also been described (28). Clinical finding of non tender or tender nodules with or without induration in the vas and epididymis with or without involvement of testes is suggestive. Presence of multiple pus discharging sinuses on posterior surface of scrotum, although rare, is characteristic. Isolated testicular involvement is rare and should raise the suspicion of a primary testicular malignancy, as should a lesion which fails to respond after 3 weeks of antitubercular chemotherapy (19).
Prostate and seminal vesicle
Prostate is the second most commonly involved genital organ in GUTB, being clinically involved in 22% to 49% of the cases (4). However, because of the asymptomatic nature of its involvement, many cases of tubercular prostatitis may remain undetected as reflected in autopsy series which depict a 77% involvement in patients dying of Tb (7). Similar higher rates of involvement may also be seen in series specifically assessing for microbiologic evidence of Tb on prostate specific tests such as expressed prostatic secretion, semen and prostate biopsy specimens (7). Prostatic involvement most commonly presents with storage lower urinary tract symptoms, that is urinary frequency, nocturia usually without urgency (25). There may be associated dysuria, hematuria or hemospermia. Isolated hemospermia is considered benign, but persistent, frequently recurring or high volume hemospermia may be associated with genital Tb in 15% of cases (29). Neglected cases or immunocompromised patients may present with tubercular prostatic abscess or discharging perineal sinuses (30). Rectal examination may reveal non tender nodular prostate mimicking malignancy, but soft areas of caseous necrosis may also be appreciated. Tubercular prostatitis can also be clinically silent and detected incidentally on histopathologic specimens of transurethral prostatic resection (TURP) (31,32). Direct contiguous involvement or canalicular spread to seminal vesicles and ejaculatory ducts may cause a progressive decline in volume of ejaculate with oligo or azoospermia or even aspermia and the resultant infertility (33).
Penis and urethra
Tb of penis is very rare and accounts for less than 1% of cases of GUTB and can involve both the skin and the corpora (3). Cutaneous penile Tb results from veneral transmission and usually manifests as a single or multiple superficial necrotic ulcers over glans or corona with or without tender inguinal lymphadenopathy (16). Presentation as a nodule or a fungating mass with induration although uncommon, can mimic penile carcinoma (16). Cavernosal involvement is even rarer and presents with destruction of glans penis or erectile dysfunction (16). Penis may also be a rare site for cutaneous eruption in response to internal focus of Tb (34). These tuberculids present as asymptomatic, symmetric, dusky red papules and pustules over glans penis which occurs in crops and heal with scarring. Such cutaneous eruptions are associated with a deep focus of Tb in about a third of cases and result from acute leukocytoclastic vasculitis and thrombosis of dermal vessels (34).
Urethral Tb affects 1.9–4.5% cases of GUTB and is never isolated (4,35). Acute cases present with urethral discharge and endoscopy may reveal beefy red urethral mucosa with ulceration (36,37). Chronic urethral infections presents with stricture and Tb accounts for 1–2% of all the cases of strictures and 3.5% of the unusually difficult or the intractable ones (35). If the stricture forms during the diagnosis or treatment of Tb, because of the rarity of this condition, it becomes difficult to ascertain whether the stricture is a result of instrumentation or Tb itself. Any segment of the urethra can be involved and long strictures involving the entire anterior and prostatic urethra along with prostatic abscess have been described (35). Delayed cases are characterized by multiple discharging penile sinus or fistulas, recto-urethral fistulas or extensive involvement in the form of a watering can perineum (35).
Diagnosis
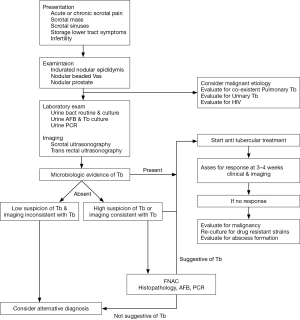
Tuberculosis is characterized by extensive destruction and fibrosis, thus early diagnosis may prevent function and organ loss. In developed countries, due to diagnosis at an early stage, the disease tends to be less serious with fewer instances of non functioning renal units and renal failure (9). Similarly, treatment of Tb epididymitis at an early stage may preserve fertility (38). However, non specific presentation and lack or inaccessibility to a medical facility may result in delayed diagnosis and worsens morbidity. Because of common co-existence, it is recommended that all patients of suspected or confirmed genital Tb should be evaluated for pulmonary and urinary Tb as well as HIV. Figure 1 summarizes a proposed algorithm for the management of genital Tb.
Laboratory and microbiologic findings
The gold standard for the diagnosis of tuberculosis at any site is isolation and culture of Mycobacterium tuberculosis bacilli and, in the cases of suspected genitourinary Tb, it is commonly looked for in the urinary samples. Traditionally, presence of ‘sterile pyuria’ on microscopic examination of urine was considered a classic finding of genitourinary involvement. However, superadded infection with usual coliform bacilli is present in about 30% of the patients (20,39). Other findings such as leukocyturia, microscopic (50%) or macroscopic (10%) hematuria and acidic urine are non specific and may be absent in 20% of cases (19,25). Staining with Ziel-Neelsen or auramine stain can identify acid fast tuberculosis bacilli with a sensitivity of 37.1% to 52.1% (20,40,41). Sporadic shedding of bacilli in low concentrations explains the poor sensitivity and at least 3 or preferably 5 or even 9 early morning samples should be analyzed (6,25,41). Contamination with Mycobacterium smegmatis or fragments of sperm can give a false positive smear result (19). Culture, although is gold standard, has a widely variable sensitivity of 10.7 to 80% and can take about 6 to 8 weeks, but has the advantage of providing antibiotic sensitivity testing (20,40,41). Nucleic acid amplification (NAA) allows for rapid diagnosis with significantly higher yield even in low bacillary concentrations and can detect Mycobacterial DNA in 80.9% patients with suspected GUTB (41). The sensitivity and specificity of polymerase chain reaction (PCR) on urinary samples range from 94.3% to 95.6% and 85.7% to 94.3% respectively (40,41). The disadvantage of PCR is its inability to differentiate between active and latent infection and thus the need of therapy in equivocal cases. The more recent self-contained cassette based analysis (GeneXpert®) avoids contamination and provide results within 2 hours with the advantage of identification of rifampicin resistance. But, the detection rates of genital TB on urinary analysis may be lower than that for urinary Tb as the infected sites may not be in direct contact with urinary system or the concentration of bacilli may be too low. Thus, it becomes necessary to analyze for evidence of disease on semen and prostatic secretions also (7).
For suspected cases of isolated genital Tb, analysis of expressed prostatic secretions (EPS), post massage urine and ejaculate for mycobacterium by microscopy, culture and PCR may be helpful (6). EPS can be collected using with either the standard four glass test or the modified 3 glass test that is proposed to have lesser contamination of urine samples with prostatic secretions (7). At least 3 but 5 or more samples should be collected and plated within 40 min of collection (6). But the search of Mycobacterium in semen or EPS samples by these methods is often futile even in diagnosed cases of genital Tb or may be incidentally positive in clinically asymptomatic men undergoing infertility analysis (42). All other obtainable body fluid specimens from possible sites of infection, such as pus from epididymal or prostatic abscess and discharge from penile lesion or perineal or scrotal sinuses, must be subjected to smear, culture and possibly PCR for detection of bacilli.
About a third of patients with tubercular prostatitis may have raised in serum prostate specific antigen (PSA) that resolved in half after anti-tubercular chemotherapy, but persistent rise was not predictive of relapse (31). Raised ESR or a positive Mantoux test are neither diagnostic nor localizing but can be used as supportive evidence in absence of microbiologic evidence (20,21). A provocation test, although seldom used clinically, has been described to increase the detection rates especially in obscure or latent forms of Tb (6,7). The test is performed by injecting 20, 50 or 100 units of tuberculin subcutaneously and leukocytosis, leukocyturia, lymphopenia and increased body temperature after 24 and 48 hrs is considered as positive. After provocation, a 16% increase in detection of mycobacterium bacilli by culture or PCR has also been reported (6,7).
Radiologic findings
In cases lacking microbiologic evidence, imaging findings often provide valuable supportive evidence and can even form the basis for anti-tubercular therapy (43). Plain X-ray of the chest should be obtained is all suspected cases (19). High resolution ultrasonography is the current imaging modality of choice for evaluating a patient presenting with scrotal mass or pain (44). The most important differential in such a clinical scenario is a testicular tumor. A mass primarily arising from the testes and involving the epididymis in the later stages suggest a testicular pathology likely malignancy. Whereas in tuberculosis, the infection starts in the epididymis and involves the testis at a later stage. A heterogenous hypoechoic diffuse or nodular enlargement of the epididymis is suggestive of tubercular involvement. An associated abscess cavity that is larger in size and has lesser peripheral blood flow is more likely to be tubercular than pyogenic (45). Other findings such as presence of hydrocele, extra-testicular calcification and sinus formation also favor tubercular etiology. Thus, a testicular lesion associated with epididymal involvement and skin thickening favors Tb rather than tumor.
Transrectal sonography may be utilized in patients suspected to have prostatic and seminal vesicle involvement. Tubercular prostatitis presents as multiple hypoechoic areas in the peripheral zones of prostate with areas of calcification or abscess formation (46). In absence of calcification, these lesions may be indistinguishable from malignancy and magnetic resonance imaging (MRI) may be of help. The presence of peripheral enhancement or the watermelon skin sign on MRI suggests Tb (47).
Histopathology
Genital Tb may present as an isolated tubercular epididymo-orchitis and in the absence of microbiologic evidence of Tb together with high suspicion of malignancy, such cases are often diagnosed on inguinal exploration (48). In cases where clinical and radiologic suspicion of testicular malignancy is low, fine needle aspiration cytology (FNAC) can provide a histologic diagnosis avoiding open surgical biopsy in 90% of the patients with epididymal nodules (49). When compared with open epididymal biopsy in cases of tubercular epididymitis, FNAC showed a sensitivity and specificity of 87% and 93% respectively (50). The presence of epithelioid cell granuloma with multinucleate giant cell and caseous necrosis can be considered diagnostic for Tb, but even on histopathology, a definite evidence can only established by demonstrating mycobacteria on smear or culture. Out of 40 cases of tubercular epididymo-orchitis, AFB could be demonstrated in 60% of the FNAC specimens and the diagnosis of Tb could be established in 67.5% (26). Aspiration of sperms can give false positive acid fast positivity and other granulomatous conditions such as idiopathic granulomatous orchitis, leprosy, sarcoidosis, fungal infections such as Blastomyces and Cryptococcus and Mycobacterium other than tuberculosis must be considered as the differential diagnosis and ruled out (25,26). In inconclusive cases of granulomatous inflammations on histopathology, tissue PCR for Tb can be used to augment the diagnostic accuracy and has been shown to have a sensitivity and specificity of 87.5% and 86.7% respectively (51). The treatment of tubercular epididymo-orchitis is mostly medical and FNAC has replaced surgical explorations like epididymectomy or orchiectomy as the current primary diagnostic measure.
Tb can be incidentally diagnosed in TURP or needle biopsy specimens performed for obstructive symptoms or suspicion of carcinoma (36). However, sometimes, trans rectal ultrasonography (TRUS) guided biopsy may be specifically performed to diagnose or monitor efficacy of therapy for tubercular prostatitis (7,31). Such an approach much be followed cautiously as miliary tuberculosis has been reported after TRUS guided biopsy performed for raised PSA in case of tubercular prostatitis and there is always an inherent risk of sepsis and hemorrhage associated with such a procedure (52).
Management
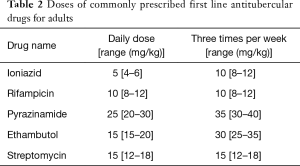
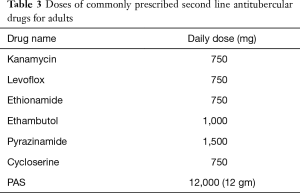
GUTB commonly presents as vague long standing urinary symptoms with or without a positive urine culture and most of these patients are treated with broad spectrum antibiotics, mainly due to the lack of clinical suspicion (19). It is recommended to consider Tb as a differential in all the cases of urinary tract infection and quinolones should be excluded from the first line of therapy (7). Like for the most forms of Tb, anti-tubercular chemotherapy has become the first line of management for all forms of genital Tb (19). An optimal schedule and treatment duration specific for GUTB remains to be defined, but there is a gradual change from the longer 18–24 months treatment to a shorter 6 months course, and the latter has become the current standard of care (19). Most patients are started with four drugs in the first two months ‘the intensive phase’ of the regimen (rifampicin, isoniazid, pyrazinamide and ethambutol) and are then shifted to two drugs in the ‘continuation phase’ (rifampicin and isoniazid) (Tables 1-3 summarizes schedules and doses of commonly prescribed medications). Longer duration therapy (9 to 12 months) may be required in immunocompromised or HIV/AIDS co-infection. There are some reports that addition of corticosteroids may be beneficial in tubercular epididymo-orchitis, but there is insufficient evidence to support its use in genital tuberculosis (39). Pharmacologic treatment has been shown to induce fibrosis and to aggravate ureteric obstruction and vesicle contraction. Although such fibrosis is not reported in genital system, it can be one of the causes of persistent azoospermia after chemotherapy for tubercular epididymo-orchitis (33).

Full table
Full table
Full table
Surgical management
Effective chemotherapy has limited the requirement of surgical intervention to management of either the complications or the sequale of Tb (19). Even extensive disease and abscesses can be managed by anti-tubercular chemotherapy alone or with addition of image guided percutaneous drainage. Most patients with tubercular epididymo-orchitis respond to antitubercular therapy but a caseating abscess not responding to therapy may require open or percutaneous drainage (19). A scrotal mass that does not respond or increases in size despite 3 weeks of antitubercular therapy must be explored by an inguinal incision to rule out testicular malignancy (19). An incidentally diagnosed tubercular prostatitis, on biopsy or TURP, should be treated with a full six months course (36). Prostatic abscesses not resolving with drug therapy may be subjected to transrectal ultrasonography guided aspiration (36). Most of the cases of penile tuberculosis resolve with anti-tubercular therapy and seldom require surgical intervention (16). Rare cases of tubercular urethral strictures are managed on similar lines as non-tubercular strictures but surgical intervention should be deferred till at least 4–6 weeks after initiation of chemotherapy (19,36).
Response to therapy must be assessed clinically, with the need of imaging being reserved in non-responsive cases. If the lesion fails to resolve and adherence to therapy has been confirmed, fresh samples must be collected and subject to antibiotic sensitivity to exclude MDR strains (16). Other differentials, especially malignancy must also be considered. With the current multidrug regimes, relapse rates are low, still some authors recommend an annual follow up for 3-5 years or even 10 years following treatment (6).
Genital tuberculosis and male infertility
Genital Tb most commonly affects young males in their reproductive years, with the mean age at diagnosis being 29.6–32 years and majority of the patients being in their twenties (26,50). Because of its destructive nature and the resultant fibrosis and scarring, infertility resulting from tubercular affliction of genitalia is multifactorial in origin and may persist even after successful chemotherapy (50,53). About 10% of the patients with genital Tb may present with infertility and around 4% to 9.1% of men with infertility with a clinical diagnosis of obstructive azoospermia have Tb as the cause (50,53,54). A previous history of epididymo-orchitis or pulmonary Tb may not be elicited and infertility may be the first presentation (10). Tubercular involvement of the epididymis and vas results in luminal obstruction, either by the granulomatous masses in the acute phase or by fibrosis and scarring as the disease progresses or post therapy (33). Findings of a nodular indurated enlarged epididymis and vas deferens, which may be frequently bilateral, with or without sinus formation on local examination suggests tuberculosis. In cases with isolated epididymal or vassal involvement, semen parameters is suggestive of obstructive azoospermia and reveal azoospermia or severe oligospermia with normal volume fructose positive ejaculate. Further evaluation usually reveals a normal hormonal profile and spermatogenesis. In patients with clinically palpable epididymal nodules, a thorough search for other sites of involvement is recommended as discussed, and if there is lack of microbiologic evidence, FNAC should be performed from the nodule, if required under ultrasonographic guidance. Some authors recommend against biopsy and to treat empirically with anti-tubercular therapy and steroids and have noted reappearance of sperms in the ejaculate in a third of cases (38). Traditionally, the diagnosis could only be made on excisional biopsy, but currently, FNAC has replaced biopsy as the first line of investigation and the ill effects of this minimally invasive procedure in already inflamed epididymal tissue are debatable. Genital Tb may also be suspected as an intra-operative finding in patients with a provisional diagnosis of idiopathic obstructive azoospermia who on scrotal exploration are found to have inflamed epididymis with non distended system not amenable to reconstruction. Rarely, Tb may also present as a discrete obstruction within the vas or at vaso-epididymal junction. Such cases are usually explored, the obstructive lesion excised and subjected to histopathology and if the fluid from the proximal end has motile sperms and a vasogram or saline injection confirms distal patency, a vaso-vasal or vaso-epididymal anastomosis may be performed (33).
Concomitant or isolated involvement of the prostate, seminal vesicle and ejaculatory ducts by tubercular inflammation or scarring clinically presents as low volume fructose negative acidic ejaculate, features similar to ejaculatory duct obstruction (EDO). A gradually progressing decline in the volume of ejaculate associated with azoospermia and progressing to aspermia is thought to be characteristic of tubercular affliction (55). A thorough clinical examination rules out epididymal and vassal lesions and documents the presence of vas. Once congenital absence of vas and retrograde ejaculation have been excluded, a trans-rectal ultrasonogram may be performed to assess seminal vesicles, which are typically fibrotic and atrophic in tuberculosis (33,56). Features suggestive of EDO with non distended atrophic seminal vesicles has been considered diagnostic of Tb and most of these patients have multifocal obstruction preventing surgical reconstruction and benefit by assisted reproduction (54,56). However, few patients have discrete obstruction at the level of ejaculatory ducts with dilated seminal vesicles (33). These selected handfuls of patients should undergo transurethral resection of ejaculatory duct (TURED) or its modification, the trans-urethral incision-resection of ejaculatory ducts (TUIRED), which does not require instillation of blue dye into seminal vesicles under ultrasonographic guidance immediately prior to surgery (33).
All patients presenting for infertility evaluation must be thoroughly examined, but if there are no features suggestive of genital Tb, extensive laboratory workup in form of semen and EPS smear, culture and PCR is probably not required (53). Also, although venereal transmission is documented, male partners of females with genital TB with a normal examination and semen analysis should not be evaluated further for genital Tb (42). Such an extensive search is probably not beneficial, as in patients with azoospermia, no further improvement is expected in semen parameters post anti-tubercular therapy and in patients with normal semen parameters or in those previously treated for Tb, a positive PCR may be indicative of a latent infection or residual DNA of killed bacteria.
Multiple organ involvement with obstruction at several sites is characteristic of genital Tb and most of these cases are not amenable to surgical reconstruction (54,56). Thus, most patients with tuberculous infertility will need assisted reproduction, and in-vitro fertilization or intra-cytoplasmic sperm injection is usually feasible (55,57). The epididymis are usually involved by the inflammatory process and because of relative testicular sparing, testicular sperm extraction remains an option for sperm retrieval if epididymal extraction fails. The outcomes of sperm retrieval and pregnancy are similar between tubercular and non-tubercular causes of obstructive azoospermia, but with a higher preponderance of using testicular sperms in tubercular patients (57).
Intravesicle BCG therapy and genital tuberculosis
Intravesical Bacille Calmette-Guerin is widely used to reduce the chances of recurrence and delay the progression of transitional cell cancer of bladder. Post instillation, systemic BCG infection occurs in 4.3% of the patients treated and 12.9% of the infections are limited to the genital organs, penis being the commonest site along with the epididymis and the prostate (58). Although few studies report better side effect profile of one strain over the other but these results may be biased by the differences in dose and schedule used and the choice of strain for instillation mostly depends on its availability rather than possibility of local complications (58,59). After instillation into the bladder, reflux of the contaminated urine into the epididymis and prostate results in this granulomatous inflammation, whereas the penile lesions usually develop after an incidence of local trauma along with spillage (60,61). Clinical presentation is similar to that of infections caused by Mycobacterial tuberculosis and can only be differentiated on tubercular culture and should be suspected if there is a recent history of vaccine instillation (58). Antitubercular therapy in form of isoniazid and rifampicin for a period of 3 months is recommended along with suspension of any further intravesical therapy (60).
Summary
Genital Tb is an uncommon extra-pulmonary manifestation of tuberculosis with a potential to cause significant morbidity. Because of the high burden of Tb, especially in the developing countries, even with its low overall incidence, genital Tb causes a significant burden on the health care system. Its non specific vague symptoms and low sensitivity of the available microbiologic tests, delays the diagnosis, increasing the chances of irreversible organ damage and later need of ablative therapy. Coexistent pulmonary and urinary tract involvement is common and should be thoroughly evaluated for. Malignancy must be ruled out before embarking upon medical management and in equivocal cases urgent surgical intervention is preferred. A short course of multidrug therapy lasting for a period of 6 months, 4 drugs for first two months of intensive phase and 2 drugs for another 4 months as continuation phase, is considered adequate. Response must be assessed at 3–4 weeks clinically, with addition of imaging if indicated and those not responding must be re-assessed for co-existent or missed malignant pathology or infection with drug resistant strains. Infertility resulting from genital Tb is multifactorial and multifocal involvement with fibrosis and anatomic distortion prevents surgical reconstruction in the most. The result of assisted reproduction techniques with testicular sperms is not affected by genital Tb and reports good overall success. Rare instances of genital Tb with BCG, post intra-vesical therapy, have been reported and should be treated with a 2 drug regimen for a period of 3 months. Post treatment, regular annual follow up is recommended even though, with the current multi drug therapy, the chances of relapse are low.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Raviglione MC, Snider DE Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 1995;273:220-6. [Crossref] [PubMed]
- WHO. Global Tuberculosis report 2015. Available online: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1
- Abbara A, Davidson RN. Etiology and management of genitourinary tuberculosis. Nat Rev Urol 2011;8:678-88. [Crossref] [PubMed]
- Figueiredo AA, Lucon AM. Urogenital Tuberculosis: Update and review of 8961 cases from world literature. Rev Urol 2008;10:207-17. [PubMed]
- Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med 1999;340:367-73. [Crossref] [PubMed]
- Kulchavenya E. Best practice in the diagnosis and management of urogenital tuberculosis. Ther Adv Urol 2013;5:143-51. [Crossref] [PubMed]
- Kulchavenya E, Kim C, Bulanova O, et al. Male genital tuberculosis: epidemiology and diagnostic. World J Urol 2012;30:15-21. [Crossref] [PubMed]
- Kulchavenya E, Khomyakov V. Male genital tuberculosis in Siberians. World J Urol 2006;24:74-8. [Crossref] [PubMed]
- Figueiredo AA, Lucon AM, Gomes CM, et al. Urogenital tuberculosis: patient classification in seven different groups according to clinical and radiologic presentation. Int Braz J Urol 2008;34:422-32. [Crossref] [PubMed]
- Kumar R, Hemal AK. Bilateral epididymal masses with infertility. ANZ J Surg 2004;74:391. [Crossref] [PubMed]
- Christensen WI. Genitourinary tuberculosis. Review of 102 cases. Medicine (Baltimore) 1974;53:377-90. [Crossref] [PubMed]
- Mochalova TP, Starikov IY. Reconstructive surgery for treatment of urogenital tuberculosis: 30 years of observation. World J Surg 1997;21:511-5. [Crossref] [PubMed]
- Duchek M, Winblad B. Experimental male genital tuberculosis—the possibility of lymphatic spread. Urol Res 1973;1:170-6. [Crossref] [PubMed]
- Sporer A, Auerbach O. Tuberculosis of prostate. Urology 1978;11:362-65. [Crossref] [PubMed]
- Angus BJ, Yates M, Conlon C, et al. Cutaneous tuberculosis of the penis and sexual transmission of tuberculosis confirmed by molecular typing. Clin Infect Dis 2001;33:E132-4. [Crossref] [PubMed]
- Venyo AK. Tuberculosis of the Penis: A Review of the Literature. Scientifica 2015. Available online: https://www.hindawi.com/journals/scientifica/2015/601624/
- Lewis EL. Tuberculosis of the penis; a report of 5 new cases, and a complete review of the literature. J Urol 1946;56:737-45. [PubMed]
- Sekhon GS, Lal MM, Dhall JC. Tuberculosis of penis. J Indian Med Assoc 1971;56:316-18. [PubMed]
- Cek M, Lenk S, Naber KG, et al. EAU Guidelines for the Management of Genitourinary Tuberculosis. Eur Urol 2005;48:353-62. [Crossref] [PubMed]
- Gupta NP, Kumar R, Mundada OP, et al. Reconstructive surgery for the management of genitourinary tuberculosis: a single center experience. J Urol 2006;175:2150-4. [Crossref] [PubMed]
- Hemal AK, Aron M. Orthotopic neobladder in management of tubercular thimble bladders: initial experience and long-term results. Urology 1999;53:298-301. [Crossref] [PubMed]
- Psihramis KE, Donahoe PK. Primary genitourinary tuberculosis: rapid progression and tissue destruction during treatment. J Urol 1986;135:1033-36. [PubMed]
- Gorse GJ, Belshe RB. Male genital tuberculosis: a review of the literature with instructive case reports. Rev Infect Dis 1985;7:511-24. [Crossref] [PubMed]
- Ormerod LP. Why does genitourinary tuberculosis occur less often than might be expected in the ethnic Indian subcontinent population living in the United Kingdom? J Infect 1993;27:27-32. [Crossref] [PubMed]
- Jacob JT, Nguyen TM, Ray SM. Male genital tuberculosis. Lancet Infect Dis 2008;8:335-42. [Crossref] [PubMed]
- Sah SP, Bhadani PP, Regmi R, et al. Fine needle aspiration cytology of tubercular epididymits and epididymo-orchitis. Acta Cytol 2006;50:243-49. [Crossref] [PubMed]
- Suankwan U, Larbcharoensub N, Viseshsindh W, et al. A clinicopathologic study of tuberculous epididymo-orchitis in Thailand. Southeast Asian J Trop Med Public Health 2012;43:951-58. [PubMed]
- Khan S, Haroon N, Azami R, et al. Isolated tuberculosis of tunica albuginea and tunica vaginalis presenting as acute hydrocoele: a diagnostic dilemma. BMJ Case Rep 2015;2015. pii: bcr2014207744.
- Zargooshi J, Nourizad S, Vaziri S, et al. Hemospermia: long term outcomes in 165 patients. Int J Impot Res 2014;26:83-6. [Crossref] [PubMed]
- Trauzzi SJ, Kay CJ, Kaufman DG, et al. Management of prostatic abscess in patients with human immunodeficiency syndrome. Urology 1994;43:629-33. [Crossref] [PubMed]
- Lee Y, Huang W, Huang J, et al. Efficacy of chemotherapy for prostatic tuberculosis-a clinical and histologic follow-up study. Urology 2001;57:872-7. [Crossref] [PubMed]
- Hemal AK, Aron M, Nair M, et al. 'Autoprostatectomy': an unusual manifestation in genitourinary tuberculosis. Br J Urol 1998;82:140-1. [Crossref] [PubMed]
- Kumar R. Reproductive tract tuberculosis and male infertility. Indian J Urol 2008;24:392-95. [Crossref] [PubMed]
- Murthy SC, Udagani MM, Kajagar BM. Tuberculous epididymo-orchitis and papulonecrotic tuberculids of the glans penis. Indian J Dermatol Venereol Leprol 2003;69:408-10. [PubMed]
- Symes JM, Blandy JP. Tuberculosis of the male urethra. Br J Urol 1973;45:432-6. [Crossref] [PubMed]
- Gupta N, Mandal AK, Singh SK. Tuberculosis of the prostate and urethra: A review. Indian J Urol 2008;24:388-91. [Crossref] [PubMed]
- Singh I, Hemal AK. Primary urethral tuberculosis masquerading as a urethral caruncle: a diagnostic curiosity! Int Urol Nephrol 2002;34:101-3. [Crossref] [PubMed]
- Shah RS. Obstructive azoospermia following genital tuberculosis may be reversible with medical therapy. AUA 2004:Abstract 1600.
- Gokce G, Kilicarslan H, Ayan S, et al. Genitourinary tuberculosis: a review of 174 cases. Scand J Infect Dis 2002;34:338-40. [Crossref] [PubMed]
- Moussa OM, Eraky I, El-Far MA, et al. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol 2000;164:584-8. [Crossref] [PubMed]
- Hemal AK, Gupta NP, Rajeev TP, et al. Polymerase chain reaction in clinically suspected genitourinary tuberculosis: comparison with intravenous urography, bladder biopsy, and urine acid fast bacilli culture. Urology 2000;56:570-4. [Crossref] [PubMed]
- Regmi SK, Singh UB, Sharma JB, et al. Relevance of semen polymerase chain reaction positive for tuberculosis in asymptomatic men undergoing infertility evaluation. J Hum Reprod Sci 2015;8:165-9. [Crossref] [PubMed]
- Kapoor R, Ansari MS, Mandhani A, et al. Clinical presentation and diagnostic approach in cases of genitourinary tuberculosis. Indian J Urol 2008;24:401-5. [Crossref] [PubMed]
- Muttarak M, Peh WC, Lojanapiwat B, et al. Tuberculous epididymitis and epididymo-orchitis: sonographic appearances. AJR Am J Roentgenol 2001;176:1459-66. [Crossref] [PubMed]
- Yang DM, Yoon MH, Kim HS, et al. Comparison of tuberculous and pyogenic epididymal abscesses: clinical, gray-scale sonographic, and color Doppler sonographic features. AJR Am J Roentgenol 2001;177:1131-5. [Crossref] [PubMed]
- Engin G, Acunas B, Acunas G, et al. Imaging of extrapulmonary tuberculosis. Radiographics 2000;20:471-88. [Crossref] [PubMed]
- Wang JH, Sheu MH, Lee RC. Tuberculosis of the prostate: MR appearance. J Comput Assist Tomogr 1997;21:639-40. [Crossref] [PubMed]
- Kho VK, Chan PH. Isolated tuberculous epididymitis presenting as painless scrotal tumor. J Chin Med Assoc 2012;75:292-5. [Crossref] [PubMed]
- Gupta N, Rajwanshi A, Srinivasan R, et al. Fine needle aspiration of epididymal nodules in Chandigarh, north India: an audit of 228 cases. Cytopathology 2006;17:195-8. [Crossref] [PubMed]
- Viswaroop BS, Kekre N, Gopalakrishnan G. Isolated tuberculous epididymitis: A review of forty cases. J Postgrad Med 2005;51:109-11. [PubMed]
- Chawla A, Chawla K, Reddy S, et al. Can tissue PCR augment the diagnostic accuracy in genitourinary tract tuberculosis? Urol Int 2012;88:34-8. [Crossref] [PubMed]
- Kim CJ, Sano T, Takimoto K. Miliary tuberculosis following transrectal ultrasonography (TRUS)-guided prostate biopsy. Korean J Urol 2011;52:425-7. [Crossref] [PubMed]
- Gupta R, Singh P, Kumar R. Should men with idiopathic obstructive azoospermia be screened for genitourinary tuberculosis. J Hum Reprod Sci 2015;8:43-7. [Crossref] [PubMed]
- Pryor JP, Hendry WF. Ejaculatory duct obstruction in subfertile males: Analysis of 87 patients. Fertil Steril 1991;56:725-30. [Crossref] [PubMed]
- Fraietta R, Mori MM, De Oliveira JM, et al. Tuberculosis of seminal vesicles as a cause of aspermia. J Urol 2003;169:1472. [Crossref] [PubMed]
- Paick J, Kim SH, Kim SW. Ejaculatory duct obstruction in infertile men. BJU Int 2000;85:720-4. [Crossref] [PubMed]
- Moon SY, Kim SH, Jee BC, et al. The outcome of sperm retrieval and intracytoplasmic sperm injection in patients with obstructive azoospermia: Impact of previous tuberculous epididymitis. J Assist Reprod Genet 1999;16:431-5. [Crossref] [PubMed]
- Pérez-Jacoiste Asín MA, Fernández-Ruiz M, López-Medrano F, et al. Bacillus Calmette-Guérin (BCG) infection following intravesical BCG administration as adjunctive therapy for bladder cancer: incidence, risk factors, and outcome in a single-institution series and review of the literature. Medicine (Baltimore) 2014;93:236-54. [Crossref] [PubMed]
- Decaestecker K, Oosterlinck W. Managing the adverse events of intravesical bacillus Calmette–Guérin therapy. Res Rep Urol 2015;7:157-63. [PubMed]
- Witjes JA, Palou J, Soloway M, et al. Clinical practice recommendations for the prevention and management of intravesical therapy-associated adverse events. Eur Urol Suppl 2008;7:667-74. [Crossref]
- Linden-Castro E, Pelayo-Nieto M, Alias-Melgar A. Penile tuberculosis after intravesical bacille Calmette-Guérin immunotherapy. Urology 2014;84:e3. [Crossref] [PubMed]