Understanding the cellular basis and pathophysiology of Peyronie’s disease to optimize treatment for erectile dysfunction
Introduction
Erectile dysfunction (ED) is the most prevalent and thoroughly researched male sexual health problem in the world. It is defined as the persistent inability to achieve and maintain a penile erection of sufficient rigidity to permit satisfactory sexual activity for at least 3 months (1). ED is estimated to carry an overall adult male prevalence rate of almost 20% worldwide. An age correlation exists for the prevalence of ED, with worldwide rates of 1% to 10% for men younger than 40, as high as 30% for men age 50 to 59 years, and 50% to 100% for men in their 70s and 80s (2). ED is associated with obesity, hypertension, benign prostatic hyperplasia, cardiovascular disease, central neurologic conditions, smoking, depression, diabetes mellitus, and other endocrine disorders, such as hypogonadism and hyperprolactinemia (3-5). Due to the complex nature of the pathophysiology and multiple contributing factors with each patient, ED treatment is often approached with goal-directed management. A patient with or without his partner needs to make an informed selection on therapeutic options that best fits his personal needs. Evaluations of this approach have demonstrated its utility and typically show that patients therapeutic preferences are usually the least invasive forms of therapy (6).
Peyronie’s disease (PD) is a connective tissue disorder of penile tunica albuginea (TA) that is frequently accompanied by ED. Individual therapeutic options are more complex in PD patients with ED when contemplating treatment of both the deformity and erectile issues. The prevalence of ED in the PD population ranges from 40–60% (7). PD is relatively common and affects nearly 10% of adult men to some degree. PD is characterized by local changes in the collagen composition of the TA of the penis after trauma. The result is a fibrous plaque that contains an excessive amount of collagen, alterations in the elastin framework, and fibroblastic proliferation (8,9). The PD plaque is inelastic and therefore results in penile deformity including curvature, indentation, hinge effect, and shortening (10).
Arterial insufficiency is felt to be related to direct obstruction by fibrotic plaque growth into nearby cavernosal arteries thereby impeding blood flow. Similarly, in the chronic phase of The precise mechanism underlying the relationship between PD and ED is unknown and their remains controversy in the current literature. Previous studies have debated which of the two disease processes is actually the initiating factor, and it appears that both PD and ED are risk factors for each other. Penile microtrauma is thought to be the triggering event for the majority of PD (11). Udelson et al. have observed that during sexual intercourse, an erection with inadequate axial penile rigidity will result in penile buckling. Therefore, vaginal intromission with a semi-erect penis is likely primarily responsible for the buckling insult associated with PD plaque formation (12,13). In this fashion, men with early ED engaging in sexual activity may provoke their PD plaque formation by repetitive microtrauma. On the other hand, during healing, the dense layers of TA become compressed by edema and cell infiltration, whilst the influx of leukocytes and macrophages continues due to the unobstructed the arterial inflow. Early in the disease process inflammation and edema irritate nerve endings, thereby producing pain. As the inflammatory reaction matures and settles, the trapped nerve fibers may become ischemic and necrose. In the chronic phase of PD plaque formation, the fibrosis accelerates and impedes the erectile tissue, and therefore often results in ED (14). Also, imaging studies in men with PD and ED reveal at least one-third have arterial insufficiency and almost 60% have veno-occlusive disease. This result is significantly different than imaging of men with normal erectile function and PD, indicating that these two mechanisms are a common link between the two diseases. In the chronic phase of PD when plaques are stable, they often invade the smooth muscle architecture which results in veno-occlusive dysfunction (7). In addition to the PD physiologic factors resulting in ED, the psychological frustration of the disease and the relationship stress due to the inability for sexual interactions often leads to psychogenic ED. Since many patients with PD have no abnormal findings on hormonal screening, or Doppler ultrasound, psychological distress alone may be a major cause of ED in this patient population (7).
Recognizing PD in the earlier stages, before men develop the physical and psychological consequences should be our main goal for patient care. Treatment of PD in early stages has promising results for improvement of ED, and therefore this needs to be investigated further. Local oral therapies, and traction devices have shown some improvement in curvature, sexual function, and IIEF scores. On the contrary, although surgical correction of PD curvature is the gold-standard treatment, this is limited by significant morbidity including worsening ED. The devastating sexual and psychological impact PD has on patients, necessitates improved treatment and prevention strategies. Adequate therapeutic interventions need to be elucidated to maximize patient satisfaction and mental well-being. Our current therapies frequently do not meet expectations and therefore to further develop new strategies, we need a complete understanding of the precise cellular and pathophysiologic processes of PD and gather knowledge of how we can intervene at a molecular level (11,15).
Pathophysiology of PD: what we know
Fibrosis and abnormal wound healing
PD is a wound-healing disorder, similar to keloids, hypertrophic scars, or Dupuytren’s contractures, all of which may be coinciding findings (16). The dense plaques identified in PD are a result of an imbalance of fibrosis and fibrinolysis. Fibrosis is the result of chronic inflammation which can be induced by persistent infections, autoimmune reactions, allergic responses, chemical insults, radiation, and tissue injuries (17). Normal tissue healing restores the baseline levels and organization of extracellular matrix (ECM), whereas fibrosis involves the overgrowth, hardening, and scarring of tissues and is attributed to excess deposition of ECM components. Collagen is the most abundant protein in ECM and provides the structural and tensile strength in most human tissues (18,19). ECM accumulation occurs by two methods: increasing expression and deposition of connective tissue proteins and preventing the catabolism of ECM proteins (20). Excess production of ECM and the failure to degrade it are the hallmarks of fibrosis. This pathway can be broken down into four stages: (I) the injury or insult; (II) inflammatory response; (III) activation and differentiation of resident fibroblasts into myofibroblasts; and (IV) remodeling and resolution (14).
Mechanical trauma and oxidative stress are the key initiators of the fibrotic pathway. Stress in the form of free radicals such as superoxide, peroxynitrite and peroxide generated species result in lipid peroxidation and further tissue damage (21). In addition, free radicals stimulate connective tissue synthesis by fibroblasts and increase inflammatory activity by phagocytic cells such as neutrophils and macrophages (22). Penile microtrauma during sexual activity is the most common trigger for fibrosis associated with PD (11). Microtrauma occurs due to the buckling force experienced during the curving of an otherwise straight column when subjected to a sufficiently large axial compressive load, as experienced during repetitive pelvic thrusting (12,13). The TA is a laminated structure with two distinct layers, the outer longitudinal and the inner circular layer that fuse to form a median septum. Bending of the partially rigid penis can result in de-lamination of the TA in the area of stress, and cause microvascular trauma. In a partly rigid penis, destabilization of the axial erectile rigidity mechanism acts as a potential risk for buckling injury such trauma causes de-lamination of the septal fibers, with bleeding into the intraluminal spaces (17).
When epithelial or endothelial cells are damaged they release inflammatory mediators that initiate an anti-fibrinolytic coagulation cascade and trigger the formation of a provisional ECM (23). The regulation of collagen synthesis by many endogenous and exogenous peptides are key in the pathogenesis of PD. Tumor growth factor-beta (TGF-β) has recently received attention as a cytokine influencing the deposition of ECM, and inducing fibrosis in the TA (24,25). TGF-β is also implicated as a cause of chronic fibrotic conditions, and is involved in many vital processes, including inflammation, stimulating the formation of intracellular matrix, fibroblast production, and normal healing (26). The overexpression of TGF-β has been observed in PD plaques. In rat studies, TGF-β can induce PD scar formation, and is present in chronically formed scars (27). The ubiquitous presence of this cytokine implies that TGF-β plays an important role in all stages of PD fibrosis (28).
When platelets are exposed to ECM components, they trigger aggregation, clot formation and hemostasis. Platelet degranulation promotes vasodilation and increased blood vessel permeability. Simultaneously, fibroblasts and epithelial cells produce metalloproteinases (MMPs) which disrupt the basement membrane and allow inflammatory cells to be recruited to the site of injury. Growth factors (GF), cytokines and chemokines are produced and stimulate the proliferation and recruitment of leukocytes across the provisional ECM. Early responders including macrophages and neutrophils eliminate tissue debris, dead cells and invading organisms. During this period, lymphocytes become activated and begin secreting profibrotic cytokines and GF which further activate macrophages and fibroblasts (29-31).
Tissue injury provokes the extravasation of fibrinogen which is subsequently converted to fibrin with the aid of thrombin in the intraluminal space of the TA. Fibrin deposition is augmented by fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) (32). Fibrin deposition induces the expression of TGF-β1, which successively initiates the conversion of fibroblasts to myofibroblasts. Fibrin also increases plasminogen activator inhibitor 1 (PAI-1) to inhibit fibrinolysis and increases the reactive oxygen species (ROS) which subsequently cause oxidative stress and induction of the inducible nitric oxide synthase (iNOS). Elevation of iNOS appears to prolong wound healing, and worsen fibrosis. Dysregulation of the NO pathway may be an additional mechanism that links the pathophysiology of ED and PD (21). The lack of fibrinolytic enzymes and vascular structure in the TA, allow the protein itself to act as a strong chemo-attractant and therefore promote the ingrowth of cytokines, fibroblasts and inflammatory cells (33,34). Fibroblasts proliferate and are attracted to the trauma site as a result of mediators such as platelet-derived GF, tumor necrosis factor alpha (TNF-α), FGF and interleukin-1 (IL-1) (35). IL-1 and TNF-α originate from monocytes and macrophages, respectively, whereas FGF expression is primarily from the myofibroblasts in the plaque formations (36). Myofibroblasts are mesenchymal cells that combine contractile smooth muscle cells with collagen-synthesizing fibroblasts and are responsible for collagen deposition (33). Following activation, the myofibroblasts promote wound contraction, the process in which the edges of the wound migrate towards the center. In fibrotic conditions, myofibroblasts are pathologically present in abundance and are inhibited from undergoing apoptosis, resulting in the excessive accumulation of ECM and collagen types I and III (21). The mechanism of this prolonged myofibroblast activity is unknown but is responsible for pathologic fibrosis.
Wound healing is complete when cells divide and migrate over basal layers to regenerate damaged tissue. Chronic inflammation and excessive tissue repair trigger an accumulation of ECM components, which leads to the formation of a permanent fibrotic scar. These scars are responsible for the deformation seen in PD. During normal healing, collagen turnover and ECM remodeling is controlled by various MMPs and tissue inhibitors of metalloproteinases (TIMPs). MMP and TIMP expression are poorly regulated and can result in fibrosis. MMPs are zinc-dependent extracellular endopeptidases that modulate a range of biological processes related to immunity, tissue repair and tissue remodeling. Individual MMPs influence cellular proliferation and survival, gene expression, and can directly or indirectly influence the activation of myofibroblasts (37). There are four TIMPs, which as their name implies, inhibit the activity of MMPs to help regulate tissue healing. In healthy tissue the overexpression of MMPs allows for ECM catabolism, and therefore the prevention of excessive deposition of collagen. In fibrotic conditions however, the proteolytic effects of MMPs are disproportionately inhibited both directly and indirectly by the anabolic activity of TIMPs and therefore result in an abundance of disorganized ECM (38,39). Shifts in synthesis versus catabolism of the ECM regulate the net increase or decrease of collagen within the wound (40). Fibrosis occurs when the synthesis of new collagen by myofibroblasts exceeds the rate at which it is degraded, such that the total amount of collagen increases over time.
Autoimmunity and genetic theories
Mulhall et al. identified a strong association between PD and both Dupuytren’s disease and HLA B-27 (41). Other studies have explored immunological factors and demonstrated that patients with PD have abnormal immunological tests in up to 75% of cases. In addition, almost 50% of cases had alterations in cell-mediated immunity and nearly 40% showed an increase in markers for autoimmune diseases. It does appear that this genetic predisposition is localized to the TA rather than a systemic alteration given the lack of associated disease manifestations in PD patients (42). Another study has documented evidence of high anti-elastin antibodies in the serum of patients with PD, hypothesizing, in the same way, an autoimmune etiology (43).
Gonzalez-Cadavid et al. reported that the levels of TGF-β1 and certain gene products, as well as the ratio of nitric oxide to ROS in the TA, are necessary for the formation and progression of a PD plaque. Further assessment is possible with DNA-based chip arrays, and the results from the PD plaques are encouraging. The genes OSF-1 (osteoblast recruitment), MCP-1 (macrophage recruitment), and procollagenase IV (collagenase degradation), along with other fibrotic genes, were identified as being possible candidate regulatory genes for PD (44).
Oral therapies
To date, pharmacologic treatment of PD consists of oral, topical and injection therapies. Despite a wide variety of options, treatment outcomes have been disappointing. Multiple well-designed, prospective studies have evaluated oral agents however their clinical significance is currently limited by conflicting outcomes, single-center data, and small sample sizes which limits statistical power. Advances in our understanding of molecular mechanisms of PD pathogenesis have revealed promising molecular targets for anti-fibrotic treatments. Based on the best available evidence, there are currently no recommended oral agents for the routine treatment of PD (Table 1).
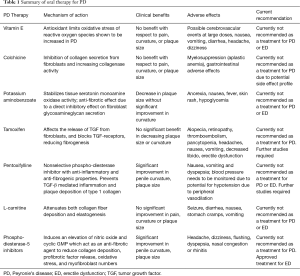
Full table
Vitamin E
Vitamin E has been extensively investigated for its potential use in the treatment of PD. This fat-soluble vitamin has antioxidant effects that are thought to limit the oxidative stress of ROS. There is an overexpression of ROS in elderly men with ED, as well as, during the acute and proliferative phases of PD plaque formation (45). This dual target makes vitamin E an ideal option for medical intervention. In 1983, Pryor and Farrell completed the first double-blind, placebo-controlled assessment of vitamin E in 40 PD patients and found no significant improvement in plaque size or penile curvature (46). Similarly, Gelbard et al. looked at 97 PD patients and demonstrated that vitamin E does not have an impact on the natural history of the disease (47). More recently, Safarinejad compared the efficacy and safety of oral vitamin E and propionyl-L-carnitine, separately, or in combination for the treatment of PD and found no significant difference in the improvement in pain, curvature or plaque size (48). Vitamin E has also been examined as a beneficial use in men with ED. Although this antioxidant appears to enhance relaxation in the corpus cavernosum and increase neuronal nitric oxide synthase (nNOS) expression, rat studies have failed to show a clinical benefit at this time (49). Vitamin E is not currently recommended for the use in treatment of PD or ED, but further studies are needed to explore the link.
Colchicine
Colchicine is commonly prescribed for the treatment of gout, but has also been explored for its potential benefit in the primary and secondary treatment of PD. The mechanism of action of colchicine involves the binding of tubulin to inhibit microtubule polymerization. Through inhibition of neutrophil microtubules, colchicine has been shown to prevent fibrosis and collagen deposition (50). The depolymerization of tubulin results in the inhibition of cell mitosis, leukocyte adhesion, and transcellular collagen transport. Colchicine also had a role in the activation of collagenases and therefore in promoting fibrinolysis and plaque breakdown (51,52). Initial studies using colchicine as a treatment for PD were promising, but had small populations and no randomization. These studies did show improvement in curvature, but did not reveal any significant clinical benefit (53,54). In 2004, Safarinejad et al. performed the first, and only, randomized, placebo-controlled trial using colchicine in 84 men with PD. The results of this study were disappointing and demonstrated no improvement in penile pain, plaque size, or curvature (55). Due to the undesirable gastrointestinal and hematologic side effects of colchicine, further work exploring this medication as a treatment for PD have been abandoned. There is no evidence to suggest a role for colchicine in men with isolated ED.
Potaba
Potassium aminobenzoate (Potaba®, Glenwood, LLC, Englewood, NJ, USA) has both anti-inflammatory and anti-fibrotic effects and is used to treat fibrotic conditions such as Dupuytren’s contracture. Potaba stabilizes tissue serotonin–monoamine oxidase activity and has a direct inhibitory effect on fibroblast glycosaminoglycan secretion. The increase in activity of monoamine oxidase in tissues decreases local levels of serotonin and therefore decreases fibrogenesis (56). In 2005, Weidner proposed that the use of Potaba may have a protective benefit against the progression of PD plaques (57). Their multi-center group performed a double-blind randomized-control trial comparing 3 g Potaba 4 times a day for 1 year to placebo. Although this study found a significant reduction in plaque size and stabilization of the plaque at one year in the treatment group, there was no clinical decrease in penile curvature. Potaba may be useful in the acute phase of PD to prevent scar progression, but this has not been thoroughly investigated (57). There is currently no evidence to support a clinically beneficial role for Potaba in PD or ED and therefore it is not recommended for use in these patients.
Tamoxifen
Tamoxifen citrate is a selective estrogen receptor used in the treatment of breast cancer. This medication has been explored as a therapy option for PD due to its effect on TGF release from fibroblasts and its blockade of TGF-receptors. The combined mechanism of action of tamoxifen was felt to be potentially useful in the reduction of the pathological fibrosis in PD plaque formation (58,59). Studies with small patient populations have been unable to confirm a benefit of tamoxifen in reducing PD plaque or clinical curvature compared to placebo (60). Adverse reactions of oral tamoxifen include hot flashes, ED, rashes, and gastrointestinal upset.
Pentoxifylline (PTX)
PTX is a nonspecific phosphodiesterase (PDE) inhibitor with anti-inflammatory and anti-fibrogenic properties. PTX down regulates TGF-β and TNF and increases fibrinolytic activity. In vitro, studies have shown that PTX prevents TA fibroblast proliferation, attenuates TGF-β mediated deposition of collagen type I and reduces the deposition of elastin (61,62). In animal models, administration of PTX initiates apoptosis of TA fibroblasts, resulting in a decrease in plaque size (63). In 2010, Safarinejad’s group demonstrated that compared to placebo, PD patients with previous failed therapy taking 400 mg PTX twice daily had improvement in their penile curvature and plaque volume. Side effects of PTX seem mild and early results are promising, however larger, multi-centered studies are needed to better determine its potential use as an accepted PD treatment option (64). There has been a lack of good quality studies looking at PTX in treatment of ED, but recently Kumar et al. did examine this in combination with tadalafil. They observed a slight improvement in IIEF scores in men with severe ED when compared to tadalafil alone. The clinical relevance is unclear and further studies need to be done to look at this further (65).
Carnitine
Carnitine is a naturally occurring ammonium compound biosynthesized from amino acids. This metabolite facilitates the entry of long chain fatty acids into muscle mitochondria and allows them to be used as an energy substrate. Carnitine is an inhibitor of acetyl coenzyme-A, which helps repair damaged DNA and prevents the formation of free radicals during cell stress (66). In 2001, Biagiotti and Cavallini examined the potential of acetyl-L-carnitine in correction of PD. Their randomized trial involved carnitine and vitamin E alone or in combination, versus placebo. They revealed that men taking carnitine had an improvement in pain and curvature (67). Follow-up studies comparing carnitine to placebo failed to demonstrate any significant improvement in pain, curvature or plaque size in any group and therefore this medication is still not recommended (48). A Chinese study in 2014 did some some benefit in ED in hypogonad men when treated with oral carnitine and phosphodiesterase-5 inhibitors (PDE5is). IIEF symptom score in the study group improved significantly compared to PDE5i use alone (68).
PDE5is
Regular use of PDE5is is a standard of care for men with ED. They have also been proposed for men suffering from PD without coexisting ED. Activation of the nitric oxide and cyclic GMP pathway by PDE5is not only has a role in improving erectile function but also in suppressing collagen synthesis and initiating myofibroblast apoptosis (69,70). Long-term administration of PDE5is in PD rat models demonstrate an increase in collagen and decrease in fibroblasts in TA cells (63,71). PDE5is have therefore been investigated for their implications in human PD plaque formation and remodeling. Ozturk and colleagues compared 39 PD patients with ED by randomizing them to either daily sildenafil or vitamin E for 3 months. They showed a similar reduction in plaque size in both groups, but the PDE5i group had a statistically significant improvement in IIEF scores and pain reduction (72). A study by Chung et al. provides evidence of scar remodeling in patients with isolated septal scars without evidence of penile deformity taking daily tadalafil for 6 months. In this study, patients did not have visual deformities and therefore the PDE5i played a role in the acute phase rather than degradation of an ossified plaque (73). If we start treating men earlier with low dose daily PDE5i to help treat their ED, we will not only avoid the microtrauma endured by the buckling force, but should injury occur, the PDE5i would prevent scar formation before deformity occurs. The dual mechanism of PDE5is would make these medications an ideal treatment option for patients with ED and PD. Larger scale, placebo-controlled trials for the use of PDE5is need to performed to help support their potential use as a treatment option for PD.
Intralesional therapies
Intralesional injection therapy has been used for years to treat PD. Compared to oral medications these choices help ensure adequate drug concentrations reach the ECM affected by PD at the level of the TA. Adequate drug penetration may significantly slow, prevent, or reverse PD plaque formation. Higher concentrations immediately into cells should hopefully negate the need for prolonged treatment as seen with some oral medications. Unfortunately results have been limited and many medications are riddled with local side effects including pain, bruising, and local inflammation (Table 2). Clostridium collagenase is the only FDA approved treatment for PD.
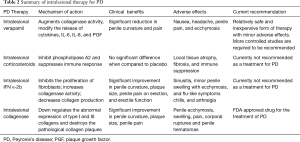
Full table
Intralesional corticosteroids
In the 1950s, Urologists began using intralesional injections as a treatment option for PD. Due to their anti-inflammatory and fibrinolytic properties, corticosteroids were an intriguing potential option for an intralesional therapy directed at reducing plaque size and penile curvature (74). Multiple studies have looked at corticosteroids injections in PD plaques, without much success. There has been no significant observed benefit in penile curvature, plaque size, disease progression or sexual function (75). In 2010, Dickstein reported that intralesional injections of corticosteroids did improve penile pain in patients with PD (76). Significant side effects including worsening fibrosis, immunosuppression, thinning of penile skin, and tissue atrophy have precluded further research into intralesional steroids as a potential therapy option for PD (77).
Calcium channel blockers
Verapamil, a common calcium channel blocker, has shown promising data for the treatment of PD when used intralesionally. In vitro studies reveal that verapamil inhibits local ECM production by reducing fibroblast proliferation, increasing local collagenase activity, and altering the cytokine environment of fibroblasts (78,79). Calcium channel blockers modify the release of cytokines, IL-6, IL-8, and plaque growth factor (PGF) and therefore reduce fibrinogenesis and the formation of a stable plaque. Verapamil was first used as an intralesional treatment for PD by Levine and colleagues in 1994. Their non-randomized study used intralesional verapamil bi-weekly in 14 men for 6 months and reported outcomes of dose escalation efficacy and toxicity. Intralesional verapamil in these men had no significant side effects and provided a significant improvement in plaque-associated narrowing, curvature, volume, and plaque firmness (80). Rehman et al. published the first randomized, single-blind trial using intralesional verapamil for PD treatment and revealed improvement in erection quality and softening of plaque (81). Randomized placebo-controlled trials followed this study comparing verapamil to saline injections intralesionally. A total of 80 patients with PD were treated, but the verapamil group failed to demonstrate any significant improvements in penile deformity, pain, plaque softening or sexual function (82). Overall, there have been inconsistent results regarding the use of intralesional verapamil in studies. Discrepancies in patient selection, plaque size, plaque calcification, injection technique, and drug concentration are noted. Verapamil likely has a role as a non-surgical intervention for PD, but further randomized studies need to be undertaken to discover which patients will achieve the maximum benefit.
More recently, nicardipine has been under investigation as another potential calcium antagonist as an intralesional treatment for PD. Soh et al. published a randomized placebo-controlled trial focusing on the impact of nicardipine injections as a conservative treatment modality for PD in the transition period of acute phase to chronic plaque formation. The results of this study demonstrated significant improvement in IIEF-5 score and in plaque size in the nicardipine group. Reduction in penile curvature was seen in both groups. Although this needs further investigation, intralesional nicardipine may be a viable alternative to verapamil as a treatment for PD in the transition period from acute to chronic phase (83).
Intralesional interferon (IFN)
Initial reports on the impact of IFN as an intralesional treatment modality for PD were encouraging. In 1991, Duncan et al. demonstrated that in vitro IFN promotes fibrinolysis by decreasing fibroblast proliferation, decreasing ECM collagen, and increasing collagenase within PD plaques (84). In 2006, Hellstrom et al. published their data of a placebo-controlled, multicenter trial of 117 PD patients who had bi-weekly injections of 5×106 units of IFN-2α for a total of 12 weeks. Results showed an average improvement of penile deviation of 13° compared to only 4° change with placebo. Pain resolution was observed in 67% of the treatment group versus 28% in the placebo group (85). Wegner and colleagues demonstrated low rates of improvement and a high incidence of side effects, including myalgia and fever (86). The limited improvements in curvature observed in these studies are unlikely to have a significant clinical benefit and therefore intralesional IFN is currently not recommended as a treatment for PD.
Newer data focus on the role of IFN gamma (IFNγ). IFNγ is an important agent controlling TGF-β signaling and is used as a treatment option for other fibrotic diseases, such as lung fibrosis. In search for a potential anti-fibrotic effect mediated by IFNγ, Haag and associates examined the effects of IFNγ on TGF-β1-stimulated fibroblasts in patients with PD. They showed an enhancement of the profibrotic effect of TGF-β1 by IFNγ in fibroblasts. An inhibitory effect of IFNγ on the TGF-β pathway could not be found in PD. The authors concluded that IFNγ cannot be taken as a useful tool in the therapy of PD (87).
Intralesional collagenase
The newest intralesional treatment of PD is collagenase Clostridium histolyticum (CCH). Collagenases are enzymes which catalyze the breakdown of collagen. This natural enzyme degrades type I and III collagen, which are the most abundant types found in the plaques formed in PD. CCH has also been found to directly increase apoptosis of fibroblasts to prevent tissue fibrosis. Direct injection of CCH is marketed under Xiaflex® (Auxilium Pharmaceuticals, Malvern, PA, USA) as a novel local therapy for PD. The impact of collagenase as a potential intralesional agent for PD treatment was first examined by Gelbard and colleagues in the 1980s. Intralesional injection of CCH in 31 men showed clinical improvement in plaque size and resolution of penile pain within 4 weeks of treatment and without significant adverse reactions (88). A decade ago Gelbard’s group published a prospective double-blind placebo-controlled trial of intralesional CCH in 49 patients suffering from PD. Results confirmed their previous findings and demonstrated significant reduction in penile curvature and plaque size, especially in patients with a less than 60-degree curve and plaques less than 4 cm (89). More recently, the IMPRESS I and II trials were published in the United States and Australia respectively to support the use of CCH for PD. These large multi-institutional randomized-control trials included patients in a 6-week cycle of two intralesional CCH or saline injections followed by manual plaque remodeling. Enrolled patients had primarily dorsal curvatures and were in the chronic phase PD with a mean penile curvature of 50-degrees. Patients were analyzed on penile length, plaque size, pain, IIEF scores, and PDQ values. Significant results at 1-year follow-up include a mean improvement in curvature of 17-degree, and an improvement of IIEF and PDQ scores. CCH is associated with minor local adverse events including ecchymosis, swelling, and penile pain. Serious adverse events did occur in six patients including three corporeal ruptures requiring surgical repair and three penile hematomas and therefore it has been recommended that patients should avoid intercourse for at least 2 weeks after their injection (90-92). Since the IMPRESS trials, the safety and efficacy of CCH has been supported by a phase-3 open-label trial and is now FDA approved for the intralesional use for PD treatment.
Non-pharmacologic therapies
Penile traction devices (PTD)
PTD have been studied as a treatment for straightening the curvature inflicted on men with PD. Specifically, researchers have been looking at PTDs as a treatment during the acute phase of PD. Some studies have shown as high as a 25-degree reduction in curvature, an improvement in sexual function, and a significant lower risk of requiring surgery (93). The pathophysiology of these devices is not completely understood. Recently, rat studies have investigated PTDs and have revealed a redistribution of MMP and TIMPs, indicating their potential role in altering the pathophysiology of this disease. Further studies are being completed. It is likely the PTD will play a more important role in the future as part of combination therapy for early PD treatment.
Extracorporeal shock wave therapy (ESWT)
In recent years many investigators have examined the effects of ESWT for its potential effects on PD. The molecular mechanism of action has not been clearly defined, however, shock waves are used to disrupt the dense scar tissue (94). No improvement in penile curvature deformity or plaque size has been shown in placebo-controlled trials using ESWT. Compared to baseline, after ESWT patients had no significant change in mean plaque size or mean penile curvature deformity (94,95). Even with modern modifications, the most recent studies have not observed any significant improvement in this treatment option (85). ESWT is not currently used as a treatment option for PD, although alterations to the technique and further studies may show some benefit in chronic plaques. A few studies have recently been investigating lithotripsy as a treatment option for men with ED who do not respond to PDE5is. These studies, with small patient populations, have deemed this a safe option with minimal complications. The mechanism behind lithotripsy as an ED treatment option is somewhat unclear, but is felt to be related to an improvement in penile hemodynamics and endothelial dysfunction. Short-term evidence is promising, but larger studies need to be done to further elucidate whether this is a viable option (96,97).
Stem cell treatment
Innovative treatment strategies are looking at alternative ways to treat PD. Lin and Lue have been using stem cells to improve PD (98). Similarly, Castiglione’s group have been using stem cells to assess improvement of both PD and ED in rat models. Although there are no long-term results, they have shown that in their models, then can induce PD and ED with TGF-β injections and when treated with human adipose tissue-derived stem cells, they see a reduction in fibrosis and improvement in erectile function (99). Early treatment with stem cell therapy seems to have a positive effect on diseased TA cells (100). This is very promising for human treatment of PD. Stem cell therapy could be a minimally invasive approach to treat PD and ED simultaneously.
Conclusions
Currently medical treatment options for PD are suboptimal and can leave patients with physical and psychological morbidity. One of the biggest consequences of leaving PD untreated is ED (8,9). To determine a more effective treatment option, one must first fully understand the complete cellular basis to the pathophysiology of this disease. Many treatments discussed above should provide more benefit given their proposed mechanism of action. Clearly, there is a discrepancy in what scientists believe occurs at a molecular level and the actual fibrotic process. We need to direct our therapies at the specific cytokines responsible for the pathologic scarring resulting in PD. The precise mechanisms are still under investigation, although we are getting close. Specific MMPs, TIMPs, and cytokines have been identified and interventions that target these are currently under development. Understanding the early causative factors of this disease will allow us to intervene earlier and prevent long-term complications and worsening ED. Gene-targeted therapy and treatment with anti-fibrotic compounds such as MMPs or inhibitors of TGF-β. There are no approved medications currently that target these pathways for PD, but have been studied for cardiac medications and this pathway is a potential target for future drugs (101).
A common link between ED and PD may also be hypogonadism. We now know that testosterone deficiency is present in up to 70% of men with PD and associated with worse curvature compared to men with normal testosterone concentrations (102,103). It is now standard practice to treat hypogonadal men with testosterone replacement to improve their ED. Treating men with PD and hypogonadism with testosterone replacement may improve their curvature and potentially improve or prevent their ED as well. Future studies need to address this common link.
There has been a recent increase in the utilization and practice of preventative medicine for many diseases. PD should not be any different. Identifying at risk patients and counseling them about micro trauma may identify these patients earlier. Given what we know about buckling forces in men with ED, all of these patients should be counseled on the risk of PD. In this sense, we can identify the active phase of the disease process and apply our therapies during the fibrosis process, rather than in the chronic phase. Preventing plaque formation would be a much more effective approach than attempting to reduce the size of an already calcified scar. This strategy would allow us to use non-invasive techniques to optimize care.
In the age of individualized medicine, the time has come to treat PD according to each patients’ specific needs, subtype of disease and likelihood of progression. Ideal therapies for the medical management of PD will be those that are administered orally, are well tolerated with chronic use, prevent the progression of fibrosis, stabilize tissue, and improve ED if present (104). Understanding concerns regarding ED as a consequence of PD treatment, should encourage physicians to treat in a stepwise manner: from least invasive to most. The morbidity associated with surgical procedures can be detrimental to a patient’s psychological well-being and often pushes them towards conservative systemic and local therapies. Instead of ongoing trials with failed oral therapies, we need to direct research at exploring the specific pathophysiologic factors involved in PD progression, so we can provide individual patients with reliable prognostic information. For a disease that has been described for over 250 years, we have a very poor understanding of its natural history and an inability to provide individuals with strong outcome data (47). Further research needs to investigate human cells at a molecular level and novel fibrotic pathway inhibitors. When this is accomplished, the early recognition of patients with their disease-specific issues will allow for selection of their optimal treatment approach.
This is an exciting time for PD research, and with further investigations, greater cellular understanding and novel therapeutic approaches we can hope to find a treatment that decreases curvature, improves patient and partner sexual satisfaction and helps to prevent the devastating effects of PD on quality of life.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bella AJ, Lee JC, Carrier S, et al. 2015 CUA Practice guidelines for erectile dysfunction. Can Urol Assoc J 2015;9:23-9. [Crossref] [PubMed]
- Lewis RW, Fugl-Meyer KS, Corona G, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med 2010;7:1598-607. [Crossref] [PubMed]
- McVary KT. Clinical practice. Erectile dysfunction. N Engl J Med 2007;357:2472-81. [Crossref] [PubMed]
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007;120:151-7. [Crossref] [PubMed]
- Inman BA, Sauver JL, Jacobson DJ, et al. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc 2009;84:108-13. [Crossref] [PubMed]
- Hanash KA. Comparative results of goal oriented therapy for erectile dysfunction. J Urol 1997;157:2135-8. [Crossref] [PubMed]
- Lopez JA, Jarow JP. Penile vascular evaluation of men with Peyronie's disease. J Urol 1993;149:53-5. [PubMed]
- Mulhall JP, Creech SD, Boorjian SA, et al. Subjective and objective analysis of the prevalence of Peyronie's disease in a population of men presenting for prostate cancer screening. J Urol 2004;171:2350-3. [Crossref] [PubMed]
- Schwarzer U, Sommer F, Klotz T, et al. The prevalence of Peyronie's disease: results of a large survey. BJU Int 2001;88:727-30. [Crossref] [PubMed]
- Del Carlo M, Cole AA, Levine LA. Differential calcium independent regulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases by interleukin-1beta and transforming growth factor-beta in Peyronie's plaque fibroblasts. J Urol 2008;179:2447-55. [Crossref] [PubMed]
- Nehra A, Alterowitz R, Culkin DJ, et al. Peyronie's Disease: AUA Guideline. J Urol 2015;194:745-53. [Crossref] [PubMed]
- Udelson D, Park K, Sadeghi-Nejad H, et al. Axial penile buckling forces vs Rigiscan radial rigidity as a function of intracavernosal pressure: why Rigiscan does not predict functional erections in individual patients. Int J Impot Res 1999;11:327-37; discusion 337-9.
- Udelson D, Nehra A, Hatzichristou DG, et al. Engineering analysis of penile hemodynamic and structural-dynamic relationships: Part II--Clinical implications of penile buckling. Int J Impot Res 1998;10:25-35. [Crossref] [PubMed]
- Lue TF. Peyronie's disease: an anatomically-based hypothesis and beyond. Int J Impot Res 2002;14:411-3. [Crossref] [PubMed]
- Hatzimouratidis K, Eardley I, Giuliano F, et al. EAU guidelines on penile curvature. Eur Urol 2012;62:543-52. [Crossref] [PubMed]
- Hellstrom WJ, Bivalacqua TJ. Peyronie's disease: etiology, medical, and surgical therapy. J Androl 2000;21:347-54. [PubMed]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199-210. [Crossref] [PubMed]
- Somers KD, Dawson DM. Fibrin deposition in Peyronie's disease plaque. J Urol 1997;157:311-5. [Crossref] [PubMed]
- Davila HH, Ferrini MG, Rajfer J, et al. Fibrin as an inducer of fibrosis in the tunica albuginea of the rat: a new animal model of Peyronie's disease. BJU Int 2003;91:830-8. [Crossref] [PubMed]
- Gonzalez-Cadavid NF, Rajfer J. Mechanisms of Disease: new insights into the cellular and molecular pathology of Peyronie's disease. Nat Clin Pract Urol 2005;2:291-7. [Crossref] [PubMed]
- Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med 1997;22:287-305. [Crossref] [PubMed]
- El-Sakka AI, Hassan MU, Nunes L, et al. Histological and ultrastructural alterations in an animal model of Peyronie's disease. Br J Urol 1998;81:445-52. [Crossref] [PubMed]
- Kumar V, Abbas AK, Aster JC. Robbins and Contran Pathologic Basis of Disease, 8th edition. Philadelphia, PA: Elsevier Saunders, 2010:87-118.
- El-Sakka AI, Hassoba HM, Pillarisetty RJ, et al. Peyronie's disease is associated with an increase in transforming growth factor-beta protein expression. J Urol 1997;158:1391-4. [Crossref] [PubMed]
- El-Sakka AI, Hassoba HM, Chui RM, et al. An animal model of Peyronie's-like condition associated with an increase of transforming growth factor beta mRNA and protein expression. J Urol 1997;158:2284-90. [Crossref] [PubMed]
- Sporn MB, Roberts AB, Wakefield LM, et al. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol 1987;105:1039-45. [Crossref] [PubMed]
- Cantini LP, Ferrini MG, Vernet D, et al. Profibrotic role of myostatin in Peyronie's disease. J Sex Med 2008;5:1607-22. [Crossref] [PubMed]
- Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest 1992;90:1-7. [Crossref] [PubMed]
- Li MO, Wan YY, Sanjabi S, et al. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006;24:99-146. [Crossref] [PubMed]
- Wynn TA. IL-13 effector functions. Annu Rev Immunol 2003;21:425-56. [Crossref] [PubMed]
- Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol 2007;22 Suppl 1:S79-84. [Crossref] [PubMed]
- Costa WS, Rebello SB, Cardoso LE, et al. Stereological and biochemical analysis of muscular and connective tissue components in the penile corpus cavernosum adjacent to the fibrous plaque of Peyronie's disease. BJU Int 2009;103:212-6. [Crossref] [PubMed]
- Diegelmann RF. Cellular and biochemical aspects of normal and abnormal wound healing: an overview. J Urol 1997;157:298-302. [Crossref] [PubMed]
- Van de Water L. Mechanisms by which fibrin and fibronectin appear in healing wounds: implications for Peyronie's disease. J Urol 1997;157:306-10. [Crossref] [PubMed]
- Moreland RB, Nehra A. Pathophysiology of Peyronie's disease. Int J Impot Res 2002;14:406-10. [Crossref] [PubMed]
- Mulhall JP, Thom J, Lubrano T, et al. Basic fibroblast growth factor expression in Peyronie's disease. J Urol 2001;165:419-23. [Crossref] [PubMed]
- Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech 2014;7:193-203. [Crossref] [PubMed]
- Rosenbloom J, Mendoza FA, Jimenez SA. Strategies for anti-fibrotic therapies. Biochim Biophys Acta 2013;1832:1088-103.
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028-40. [Crossref] [PubMed]
- Pardo A, Selman M. Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc Am Thorac Soc 2006;3:383-8. [Crossref] [PubMed]
- Mulhall JP. Expanding the paradigm for plaque development in Peyronie's disease. Int J Impot Res 2003;15 Suppl 5:S93-102. [Crossref] [PubMed]
- Schiavino D, Sasso F, Nucera E, et al. Immunologic findings in Peyronie's disease: a controlled study. Urology 1997;50:764-8. [Crossref] [PubMed]
- Stewart S, Malto M, Sandberg L, et al. Increased serum levels of anti-elastin antibodies in patients with Peyronie's disease. J Urol 1994;152:105-6. [PubMed]
- Gonzalez-Cadavid NF, Magee TR, Ferrini M, et al. Gene expression in Peyronie's disease. Int J Impot Res 2002;14:361-74. [Crossref] [PubMed]
- Sikka SC, Hellstrom WJ. Role of oxidative stress and antioxidants in Peyronie's disease. Int J Impot Res 2002;14:353-60. [Crossref] [PubMed]
- Pryor JP, Farrell CF. Controlled clinical trial of vitamin E in Peyronie’s disease. Prog Reprod Biol 1983;9:41-5.
- Gelbard MK, Dorey F, James K. The natural history of Peyronie's disease. J Urol 1990;144:1376-9. [PubMed]
- Safarinejad MR, Hosseini SY, Kolahi AA. Comparison of vitamin E and propionyl-L-carnitine, separately or in combination, in patients with early chronic Peyronie's disease: a double-blind, placebo controlled, randomized study. J Urol 2007;178:1398-403; discussion 1403. [Crossref] [PubMed]
- Kovac JR, DeYoung L, Lehmann KJ, et al. The effects of combined free radical scavenger and sildenafil therapy on age-associated erectile dysfunction: An animal model. Urol Ann 2014;6:314-20. [Crossref] [PubMed]
- Furst DE, Munster T. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics & drugs used in gout. In: Bertram G. editor. Basic and clinical pharmacology. New York: Katzung Lange, 2001:596.
- Ehrlich HP, Bornstein P. Microtubules in transcellular movement of procollagen. Nat New Biol 1972;238:257-60. [Crossref] [PubMed]
- Taylor EW. The mechanism of colchicine inhibition of mitosis. I. Kinetics of inhibition and the binding of H3-colchicine. J Cell Biol 1965;25 Suppl:145-60. [Crossref] [PubMed]
- Akkus E, Carrier S, Rehman J, et al. Is colchicine effective in Peyronie's disease? A pilot study. Urology 1994;44:291-5. [Crossref] [PubMed]
- Kadioglu A, Tefekli A, Köksal T, et al. Treatment of Peyronie's disease with oral colchicine: long-term results and predictive parameters of successful outcome. Int J Impot Res 2000;12:169-75. [Crossref] [PubMed]
- Safarinejad MR. Therapeutic effects of colchicine in the management of Peyronie's disease: a randomized double-blind, placebo-controlled study. Int J Impot Res 2004;16:238-43. [Crossref] [PubMed]
- Zarafonetis CJ, Horrax TM. Treatment of Peyronie's disease with potassium para-aminobenzoate (potaba). J Urol 1959;81:770-2. [PubMed]
- Weidner W, Hauck EW, Schnitker J, et al. Potassium paraaminobenzoate (POTABA) in the treatment of Peyronie's disease: a prospective, placebo-controlled, randomized study. Eur Urol 2005;47:530-5; discussion 535-6. [Crossref] [PubMed]
- Ralph DJ, Brooks MD, Bottazzo GF, et al. The treatment of Peyronie's disease with tamoxifen. Br J Urol 1992;70:648-51. [Crossref] [PubMed]
- Colletta AA, Wakefield LM, Howell FV, et al. Anti-oestrogens induce the secretion of active transforming growth factor beta from human fetal fibroblasts. Br J Cancer 1990;62:405-9. [Crossref] [PubMed]
- Teloken C, Rhoden EL, Grazziotin TM, et al. Tamoxifen versus placebo in the treatment of Peyronie's disease. J Urol 1999;162:2003-5. [Crossref] [PubMed]
- Shindel AW, Lin G, Ning H, et al. Pentoxifylline attenuates transforming growth factor-β1-stimulated collagen deposition and elastogenesis in human tunica albuginea-derived fibroblasts part 1: impact on extracellular matrix. J Sex Med 2010;7:2077-85. [Crossref] [PubMed]
- Lin G, Shindel AW, Banie L, et al. Pentoxifylline attenuates transforming growth factor-beta1-stimulated elastogenesis in human tunica albuginea-derived fibroblasts part 2: Interference in a TGF-beta1/Smad-dependent mechanism and downregulation of AAT1. J Sex Med 2010;7:1787-97. [Crossref] [PubMed]
- Valente EG, Vernet D, Ferrini MG, et al. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie's fibrotic plaque and related fibroblast cultures. Nitric Oxide 2003;9:229-44. [Crossref] [PubMed]
- Safarinejad MR, Asgari MA, Hosseini SY, et al. A double-blind placebo-controlled study of the efficacy and safety of pentoxifylline in early chronic Peyronie's disease. BJU Int 2010;106:240-8. [Crossref] [PubMed]
- Kumar S, Roat R, Agrawal S, et al. Combination Therapy Of Tadalafil And Pentoxifylline In Severe Erectile Dysfunction; A Prospective Randomized Trial. Pol Przegl Chir 2015;87:377-83. [Crossref] [PubMed]
- Bremer J. Carnitine--metabolism and functions. Physiol Rev 1983;63:1420-80. [PubMed]
- Biagiotti G, Cavallini G. Acetyl-L-carnitine vs tamoxifen in the oral therapy of Peyronie's disease: a preliminary report. BJU Int 2001;88:63-7. [Crossref] [PubMed]
- Zhang W, Li P, Cai ZK, et al. Safety and efficacy of L-carnitine and tadalafil for late-onset hypogonadism with ED: a randomized controlled multicenter clinical trial. Zhonghua Nan Ke Xue 2014;20:133-7. [PubMed]
- Vernet D, Ferrini MG, Valente EG, et al. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie's fibrotic plaque and in its rat model. Nitric Oxide 2002;7:262-76. [Crossref] [PubMed]
- Gonzalez-Cadavid NF, Rajfer J. Treatment of Peyronie's disease with PDE5 inhibitors: an antifibrotic strategy. Nat Rev Urol 2010;7:215-21. [Crossref] [PubMed]
- Ferrini MG, Kovanecz I, Nolazco G, et al. Effects of long-term vardenafil treatment on the development of fibrotic plaques in a rat model of Peyronie's disease. BJU Int 2006;97:625-33. [Crossref] [PubMed]
- Ozturk U, Yesil S, Goktug HN, et al. Effects of sildenafil treatment on patients with Peyronie's disease and erectile dysfunction. Ir J Med Sci 2014;183:449-53. [Crossref] [PubMed]
- Chung E, Deyoung L, Brock GB. The role of PDE5 inhibitors in penile septal scar remodeling: assessment of clinical and radiological outcomes. J Sex Med 2011;8:1472-7. [Crossref] [PubMed]
- Sherer BA, Godlewski KF, Levine LA. Pharmacologic therapy for Peyronie's disease: what should we prescribe? Expert Opin Pharmacother 2015;16:1299-311. [Crossref] [PubMed]
- Larsen SM, Levine LA. Review of non-surgical treatment options for Peyronie's disease. Int J Impot Res 2012;24:1-10. [Crossref] [PubMed]
- Dickstein R, Uberoi J, Munarriz R. Severe, disabling, and/or chronic penile pain associated with Peyronie disease: management with subcutaneous steroid injection. J Androl 2010;31:445-9. [Crossref] [PubMed]
- Serefoglu EC, Hellstrom WJ. Treatment of Peyronie's disease: 2012 update. Curr Urol Rep 2011;12:444-52. [Crossref] [PubMed]
- Mulhall JP, Anderson MS, Lubrano T, et al. Peyronie's disease cell culture models: phenotypic, genotypic and functional analyses. Int J Impot Res 2002;14:397-405. [Crossref] [PubMed]
- Roth M, Eickelberg O, Kohler E, et al. Ca2+ channel blockers modulate metabolism of collagens within the extracellular matrix. Proc Natl Acad Sci U S A 1996;93:5478-82. [Crossref] [PubMed]
- Levine LA, Merrick PF, Lee RC. Intralesional verapamil injection for the treatment of Peyronie's disease. J Urol 1994;151:1522-4. [PubMed]
- Rehman J, Benet A, Melman A. Use of intralesional verapamil to dissolve Peyronie's disease plaque: a long-term single-blind study. Urology 1998;51:620-6. [Crossref] [PubMed]
- Shirazi M, Haghpanah AR, Badiee M, et al. Effect of intralesional verapamil for treatment of Peyronie's disease: a randomized single-blind, placebo-controlled study. Int Urol Nephrol 2009;41:467-71. [Crossref] [PubMed]
- Soh J, Kawauchi A, Kanemitsu N, et al. Nicardipine vs. saline injection as treatment for Peyronie's disease: a prospective, randomized, single-blind trial. J Sex Med 2010;7:3743-9. [Crossref] [PubMed]
- Duncan MR, Berman B, Nseyo UO. Regulation of the proliferation and biosynthetic activities of cultured human Peyronie's disease fibroblasts by interferons-alpha, -beta and -gamma. Scand J Urol Nephrol 1991;25:89-94. [Crossref] [PubMed]
- Hellstrom WJ, Kendirci M, Matern R, et al. Single-blind, multicenter, placebo controlled, parallel study to assess the safety and efficacy of intralesional interferon alpha-2B for minimally invasive treatment for Peyronie's disease. J Urol 2006;176:394-8. [Crossref] [PubMed]
- Wegner HE, Andresen R, Knispel HH, et al. Local interferon-alpha 2b is not an effective treatment in early-stage Peyronie's disease. Eur Urol 1997;32:190-3. [PubMed]
- Haag SM, Hauck EW, Eickelberg O, et al. Investigation of the antifibrotic effect of IFN-gamma on fibroblasts in a cell culture model of Peyronie's disease. Eur Urol 2008;53:425-30. [Crossref] [PubMed]
- Gelbard MK, Lindner A, Kaufman JJ. The use of collagenase in the treatment of Peyronie's disease. J Urol 1985;134:280-3. [PubMed]
- Gelbard MK, James K, Riach P, et al. Collagenase versus placebo in the treatment of Peyronie's disease: a double-blind study. J Urol 1993;149:56-8. [PubMed]
- Gelbard M, Hellstrom WJ, McMahon CG, et al. Baseline characteristics from an ongoing phase 3 study of collagenase clostridium histolyticum in patients with Peyronie's disease. J Sex Med 2013;10:2822-31. [Crossref] [PubMed]
- Lipshultz LI, Goldstein I, Seftel AD, et al. Clinical efficacy of collagenase Clostridium histolyticum in the treatment of Peyronie's disease by subgroup: results from two large, double-blind, randomized, placebo-controlled, phase III studies. BJU Int 2015;116:650-6. [Crossref] [PubMed]
- Gelbard M, Goldstein I, Hellstrom WJ, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol 2013;190:199-207. [Crossref] [PubMed]
- Martínez-Salamanca JI, Egui A, Moncada I, et al. Acute phase Peyronie's disease management with traction device: a nonrandomized prospective controlled trial with ultrasound correlation. J Sex Med 2014;11:506-15. [Crossref] [PubMed]
- Palmieri A, Imbimbo C, Longo N, et al. A first prospective, randomized, double-blind, placebo-controlled clinical trial evaluating extracorporeal shock wave therapy for the treatment of Peyronie's disease. Eur Urol 2009;56:363-9. [Crossref] [PubMed]
- Hauck EW, Diemer T, Schmelz HU, et al. A critical analysis of nonsurgical treatment of Peyronie's disease. Eur Urol 2006;49:987-97. [Crossref] [PubMed]
- Lei H, Liu J, Li H, et al. Low-intensity shock wave therapy and its application to erectile dysfunction. World J Mens Health 2013;31:208-14. [Crossref] [PubMed]
- Ruffo A, Capece M, Prezioso D, et al. Safety and efficacy of low intensity shockwave (LISW) treatment in patients with erectile dysfunction. Int Braz J Urol 2015;41:967-74. [Crossref] [PubMed]
- Lin CS, Lue TF. Adipose-Derived Stem Cells: Characterization and Application in Urology. In: Illouz YG, Sterodimas A. editors. Adipose Stem Cells and Regenerative Medicine. New York, NY: Springer, 2011:193-207.
- Castiglione F, Hedlund P, Van der Aa F, et al. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie's disease. Eur Urol 2013;63:551-60. [Crossref] [PubMed]
- Lin CS, Xin ZC, Wang Z, et al. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev 2012;21:343-51. [Crossref] [PubMed]
- Ceron CS, Castro MM, Rizzi E, et al. Spironolactone and hydrochlorothiazide exert antioxidant effects and reduce vascular matrix metalloproteinase-2 activity and expression in a model of renovascular hypertension. Br J Pharmacol 2010;160:77-87. [Crossref] [PubMed]
- Moreno SA, Morgentaler A. Testosterone deficiency and Peyronie's disease: pilot data suggesting a significant relationship. J Sex Med 2009;6:1729-35. [Crossref] [PubMed]
- Karavitakis M, Komninos C, Simaioforidis V, et al. The relationship between androgens, regulators of collagen metabolism, and Peyronie's disease: a case control study. J Sex Med 2010;7:4011-7. [Crossref] [PubMed]
- Lee CH, Shin JH, Ahn GJ, et al. Udenafil enhances the recovery of erectile function and ameliorates the pathophysiological consequences of cavernous nerve resection. J Sex Med 2010;7:2564-71. [Crossref] [PubMed]


