The dangers of sexual enhancement supplements and counterfeit drugs to “treat” erectile dysfunction
What is a counterfeit drug?
The counterfeit drug market is a nebulous and constantly evolving entity that has become increasingly difficult to define and quantify. In 2009, the World Health Organization (WHO) defined a “counterfeit medicine” as one “which is deliberately and fraudulently mislabeled with respect to identity and/or source.” However, many in the organization have disputed this definition of counterfeit medicine, and there appears to be no universally agreed upon definition amongst member states of the WHO. Counterfeiting can apply to both branded and generic products. This can include products with bona fide ingredients, but with insufficient or excessive quantities, products with the wrong ingredients, or products with deceptive packaging. Currently, the WHO is using the term “Substandard, Spurious, Falsely labeled, Falsified, and Counterfeit (SSFFC)” for medical products until a new definition is agreed upon (1). For the purposes of this paper, the term “counterfeit” will be used to encompass all forms of SSFFC medications.
Is counterfeiting a significant problem?
Regardless of the definition, drug counterfeiting has become a global problem. In addition to the intellectual property rights that counterfeiters violate, the products they make can place consumers in danger. Legitimate pharmaceuticals must pass vigorous standards and undergo large, randomized trials before being formally approved for the public. Then, all sanctioned medications are manufactured under heavily monitored conditions. Counterfeit medications bypass all these safeguards (2).
Despite these potential dangers, the market for counterfeit products has grown astronomically. In 2006, the WHO estimated that 10% of drugs worldwide were counterfeit, with the figure at 25% in developing countries, and as high as 50% in certain African and Asian countries (3). However, the WHO has since become less definitive in estimating the scope of the problem, as there is an inherent difficulty in quantifying counterfeit products, which have become increasingly sophisticated and difficult to differentiate from genuine medicines. In a 2016 statement, the WHO stated that “there are many estimates of the scope and scale of the market in counterfeit medical products, but little validated evidence to underpin those estimates.” Furthermore, the WHO has withdrawn all of their previous estimations of the scale of the problem (1). Regardless, it is clear that counterfeit medications have reached an industrial level scale. In a private study, 2,177 incidents of pharmaceutical crime involving seizures or police raids were reported in 2015, with 53% of them classified as commercial involving greater than 1,000 dosage units (4). These numbers do not even account for individual distribution and consumption of illegal pharmaceuticals, not to mention the illegal operations that escape detection. Some sources estimate that the market for counterfeit medications ranges between 75 and 200 billion dollars (5,6).
The market for counterfeit phosphodiesterase-5 inhibitors (PDE-5i)
The large market for counterfeit medications is not equally distributed in size or class of medications worldwide. It is largest in poor and developing countries, with the size of the market being inversely proportional to the amount of regulation. The proportion of falsified drugs ranges from 1% in industrialized countries that have a well-regulated and controlled drug market to as high as 60% in some developing countries (6). However, even in these well-regulated countries, the market for counterfeit PDE-5i has grown. Globally, antibiotics comprise the largest class of counterfeit medications, but in Europe, PDE-5i are the most commonly counterfeited medicines (7). In fact, for PDE-5i, the illegal market in industrialized countries approaches the size of that in developing countries. Between 2004 and 2008, 35.8 million counterfeit sildenafil tablets were seized in Europe, which is 7 times greater than the amount of all other counterfeited Pfizer products combined. Two separate analyses in Europe estimated that 0.6 to 2.5 million men are being exposed to illicit sildenafil compared to approximately 2.5 million users of legal sildenafil (8). However, the true scope of illicit PDE-5i use is difficult to estimate. In one study attempting to estimate illicit sildenafil use, concentrations of sildenafil and sildenafil metabolites were measured in sewage treatment centers in the Netherlands. The total sewage load was back calculated to estimate total sildenafil consumption, and it was found that greater than 60% of identified sildenafil was not accounted for by legal prescriptions (9).
Why is there such a large market for counterfeit PDE-5i?
Many factors contribute to the disproportionately large market of counterfeit PDE-5i. First, there are high economic gains to be made. Erectile dysfunction (ED) is a common problem and increases in incidence as men age (10). According to the Massachusetts Male Aging Study, 52% of men aged 50–70 have some degree of ED, a number that increases to upwards of 70% in men older than 70. As the world population ages, there is a larger potential market forming. Furthermore, the cost of a single PDE-5i pill is priced upwards of $20–30. With the low cost of base materials and the high cost of pills, the profit margin for creating sildenafil is approximately 2,000 times that of cocaine. Meanwhile, while the punishment for buying 1 kilo of cocaine would be a minimum of 5 years to a maximum of 40 years in jail, the punishment for buying 1 kilo of sildenafil is typically 3 years (11). With such significant profits to be made at a relatively low risk, there is a tremendous incentive for counterfeiters to tap into the growing market for PDE-5i.
Besides the high profit margins, the underlying characteristics of consumers of PDE-5i make it a prime target for counterfeiters. There is an associated embarrassment of the underlying condition of impotence that leads patients to seek alternative means of obtaining PDE-5i (12). In two large surveys, 23–32% of PDE-5i users had no prior interactions with healthcare professionals (13,14). Predictive factors for obtaining PDE-5i while avoiding interaction with healthcare professions were embarrassment and the perception that it would be cheaper to get the medicine through alternative means. Furthermore, many users of PDE-5i are recreational users. In those same surveys, 16–36% of reported users did not report ED (13,14). These statistics speak to the underlying idea that users believe PDE-5i are harmless and that they can be self-prescribed, with no negative consequences. Furthermore, PDE-5i use is a relative luxury. Increasing household income and availability of a sexual partner positively correlate with their legal use (15). These patients with higher socioeconomic statuses having the resources to pay for pharmaceuticals out-of-pocket, combined with the stigma of ED, creates an environment ripe for an illicit market.
Internet pharmacies have made it easier for PDE-5i users to bypass the healthcare system, as 16.5% of PDE-5i users obtained the medicine through the Internet (13). Of these users, 68% did so without a prescription, as patients often do not realize that they are getting false and possibly dangerous products, and 60% of these users believed they received the same product as from a legitimate pharmacy (13). A simple Internet search for “internet drug store” revealed 6.4 million hits and 7,000 Internet pharmacies. Of these “pharmacies,” only 4% were in compliance of the Verified Internet Pharmacy Practice Sites (VIPPS) program (16). Many of these pharmacies are based in foreign countries and are poorly regulated. In a random search of Internet pharmacies for Viagra (Pfizer, New York City, New York, USA), 90% of these Internet pharmacies offered illegal “generic Viagra.” No pharmacy required a prescription or a health screening survey for purchase, and the products lacked product information, appropriate safety warnings, and genuine Viagra formulations. In fact, only 18% of Internet-ordered “Viagra” was genuine (17). The ease and convenience of purchasing counterfeit PDE-5i products via the Internet have contributed to the growth of the illicit market.
While the market for counterfeit pro-erectile medications is partially driven by the cost of prescription drugs, this reality could soon change. As the patents for PDE-5i will be expiring soon, it is conceivable that the cost of these drugs may be reduced significantly. This in turn could lead to a decreased demand for cheaper alternative counterfeit options. These dynamics are already evident with many pharmacies advertising for generic sildenafil (20 mg), used for pulmonary hypertension, at a significantly lower cost. Furthermore, as mentioned earlier, embarrassment with potency issues leads patients to avoid physician visits. PDE-5i have been shown to be safe medications and may ultimately be available over the counter. This strategy could help ease patient concerns and re-invigorate the PDE-5i market.
Why are counterfeit PDE-5i dangerous?
In order to understand the dangers of PDE-5i, it is important to first understand how PDE-5i are used. PDE-5i are effective in treating ED of many etiologies, and are currently both the first line and mainstay treatment option. Studies have suggested that PDE-5i are also effective in treating premature ejaculation, decreasing refractory time post-ejaculation, and diminishing lower urinary tract symptoms (18). They are an inhibitor of phosphodiesterase, which is an enzyme in the biochemical cascade of erection initiation. In the normal erection process, vascular endothelium releases nitrous oxide, which activates guanalyl cyclase to convert guanosine triphosphate to cyclic guanosine monophosphate (cGMP). cGMP then acts on protein kinase G to open calcium channels that leads to vasodilation and an erection. Phosphodiesterase-5 acts to convert cGMP to GMP, which interrupts this chain and leads to detumescence (19,20). Thus, PDE-5i prevent cGMP from being metabolized, leading to sustained vasodilation.
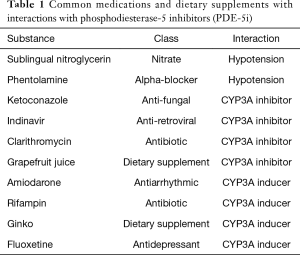
There are numerous known side effects of PDE-5i, some of which may be life threatening. As PDE-5i are active in the vasodilation pathway, there is a risk of profound hypotension and syncope with concurrent use of nitrates and alpha-blockers. The use of nitrates is an absolute contra-indication to PDE-5i use and the use of alpha-blockers is a relative contraindication. There is no current pharmacologic antidote to these interactions (21). Other side effects include headache, dyspepsia, flushing, myalgia/back pain, visual disturbances, and nasal congestion. Many of these side effects occur because there is cross-reaction with other phosphodiesterase types located throughout the body (18,22). PDE-5i are metabolized almost exclusively by the cytochrome P 450 system in the liver, specifically Cytochrome P4503A. Concurrent use of CYP 3A inhibitors or inducers can significantly alter the concentration of serum PDE-5i changing the side-effect profile, effectiveness of the medication, or severity of drug interactions (21). Examples of commonly prescribed medications that have interactions with PDE-5i are included in Table 1. Before PDE-5i are prescribed, patients must be evaluated for potential drug interactions and warned of the absolute contra-indications.
Full table
There is also an indirect risk of missing potential medical conditions in patients that bypass the healthcare system to purchase illicit PDE-5i. It has been well documented that ED is associated with significant medical comorbidities including cardiovascular disease, diabetes, metabolic syndrome, hypertension, and hyperlipidemia (23). The Second Princeton Consensus on sexual dysfunction and cardiac risk stated that all men with ED, even in the absence of manifesting cardiac symptoms should be regarded as having potential risks for cardiovascular disease (24). Currently, evidence suggests that ED is not only correlated with cardiovascular disease due to shared medical comorbidities, but is, in fact, an independent risk factor for cardiovascular disease. Thus, all men with vasculogenic ED should undergo cardiac risk stratification and risk factor management (25). In an analysis of 24,708 patients receiving a PDE-5i, 70% had an underlying medical diagnosis, with 50% of these patients having vasculogenic disease. Furthermore, 11.5% of patients being evaluated for a PDE-5i prescription had a new underlying disease detected (26).
What is in counterfeit PDE-5i?
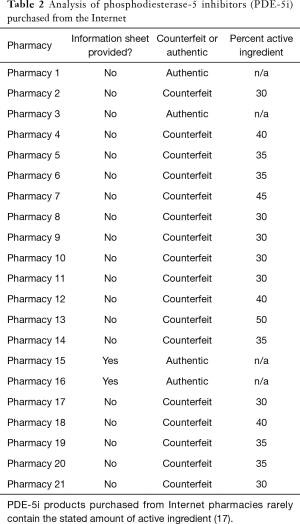
The most detrimental problem with counterfeit PDE-5i is that the content is unregulated. In 2009, United Kingdom authorities seized 2,383 samples of counterfeit Viagra and forwarded them to Pfizer laboratories for analysis. The concentration of active sildenafil ranged from 0–200% of indicated strength, and only 10% of the samples contained an active ingredient within 10% of what was advertised on the packaging (27). Similar results were found in an analysis of counterfeit ED drugs sold in Italy, Austria, and Canada (28). These problems are magnified in developing countries with less regulation. In Indonesia, 100% of “Viagra” sold by street peddlers, 56% purchased in “drug stores”, and 13% acquired from legitimate pharmacies was counterfeit Viagra marked as authentic (29). In pills marked as 100 mg, 64% contained <50 mg, 25.5% contained between 50–95 mg, and only 4.7% contained between 95 to 105 mg; 5.7% contained >105 mg (30). Quantities of active ingredients are also highly variable, as seen in Table 2. The effects of widely variable amounts of active ingredient range from low efficacy at a minimum to severe side effects and negative drug interactions at the worst. As stated, counterfeit PDE-5i rarely are packaged with the appropriate warning labels.
Full table
In addition, counterfeit PDE-5i often contain contaminants. These are used either as bulking agents to lower production costs or to imitate the appearance and physical qualities of the genuine product. In those samples seized in the United Kingdom, Italy, and Indonesia, there were contaminants such as gypsum, non-purified talc, amphetamine, commercial grade paints, paracetamol, and metronidazole (27,28,30). These non-pharmaceutical ingredients can have toxicities of their own. Non-declared pharmaceuticals can have drug interactions and side effects such as gastrointestinal symptoms and nausea when combining metronidazole and alcohol. Counterfeiters do not declare these ingredients or warnings of possible deleterious interactions on their packaging.
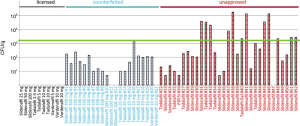
Furthermore, the manufacturing conditions of counterfeiters cannot match the sterile processing conditions of legitimate pharmaceuticals. In an analysis of microbial loads of various illicit ED drugs, 23% were contaminated with more than 103 colony-forming units (CFU), and 69% had elevated levels considered within acceptable limits. Not a single CFU was detected in any of the approved PDE-5i obtained legally (29) (Figure 1). These results are not surprising, when considering the strict regulations and inspections that legitimate pharmaceutical producers must pass, compared with the unsterile conditions in which counterfeiters may work. Many laboratories of counterfeiters are exposed to the open air and use unsterile water that would not be safe for drinking. Contamination with either adulterants or bacteria poses risks to consumers of counterfeit PDE-5i.
What about alternative ED medicines?
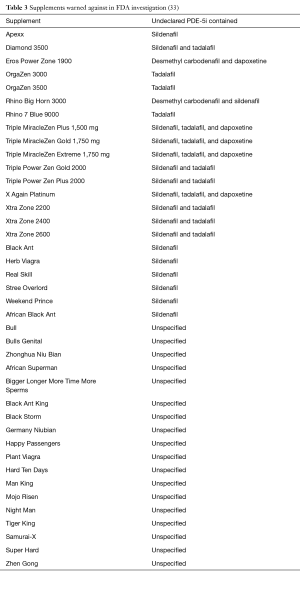
Perhaps even more dangerous than the counterfeit PDE-5i that is marketed as legitimate pharmaceuticals, however, are those marketed as “natural” supplements. Unlike pharmaceuticals requiring prescriptions, there is little Food and Drug Administration (FDA) regulation of health supplements. As a result, many so-called “natural” supplements, in fact, contain active ingredients of prescription strength drugs that could be potentially harmful. In a well-known example, 150 non-diabetic patients were hospitalized with hypoglycemia in Singapore. Seven patients fell into a comatose state, and four subsequently died. The common link between these patients was the use of an ED supplement that contained glyburide (a sulfonylurea used to treat diabetes), as well as illicit PDE-5i (31). In one study, the ingredients of 58 products available for the treatment of ED without a prescription were analyzed. Even though 57 of 58 products were labeled as “all natural” and no sample claimed to include synthetic substances, 81% contained PDE-5i. Several contained higher-than-approved amounts of PDE-5i, and others contained PDE-5i analogs that are not approved for use. One even contained phentolamine, an alpha-blocker, with which concurrent use with PDE-5i is contraindicated. Packaging and labeling were inadequate, and only 14 samples warned against concomitant nitrate use, a potentially fatal drug interaction (32). Recently, in January of 2016, the FDA announced warnings against 40 products marketed as dietary supplements that contained undeclared PDE-5i (33) (Table 3). Unsuspecting patients often seek alternative treatments of ED due to embarrassment and lower cost, but also because of the perception that “all natural” products are somehow safer than synthetic medications. However, by doing so, many of these patients are unknowingly subjecting themselves to significant health dangers.
What is being done to limit counterfeiting?
Strategies to limit counterfeiting should be multi-faceted and target prescribing healthcare professionals, pharmaceutical companies, regulatory authorities, and patients. As such, in 2006, the WHO created a global coalition of stakeholders called International Medical Products Anti-Counterfeiting Taskforce (IMPACT), which aims to build coordinated networks across and between countries in order to halt the production, trading, and selling of fake medicines around the globe (34). Similarly, PDE-5i manufacturers have started working with regulatory and law enforcement authorities, as well as providing distinctive packaging using holographic security foil, 2D barcodes, and radio frequency identification (RFID) tags. The problem with RFID is the unfunded cost of implementation that has been estimated to range from $84,000 for individual pharmacies to $1.3 billion for large-chain pharmacies and may present significant barriers to RFID adoption (35).
Similarly, the FDA has launched significant public education campaigns through magazine public service announcements, education leaflets, news articles, a consumer website (www.fda.gov/counterfeit), and a pharmacist education program (36).
Finally, it is the healthcare provider’s responsibility to continue to support efforts to maintain consumer access to potency drugs, educate patients on the risks of counterfeit and other non-FDA approved products, remind them to only purchase from a VIPPS-certified pharmacy if they want to fill their prescription online, and report any suspected cases of counterfeit medicines to the FDA (37).
Conclusions
Counterfeit PDE-5i pose many—possibly serious—risks to patients. As the population ages, and the market for PDE-5i grow, so does the illicit market for PDE-5i. Counterfeit PDE-5i have become a worldwide problem that comprises a large percentage of PDE-5i use in both developing and well-developed countries. Patients embarrassed by their condition or seeking less expensive alternatives to legitimate pharmaceuticals have fueled the market, and the growth of Internet pharmaceuticals have made counterfeit pharmaceuticals easy to obtain. However, ED is a medical condition that needs to be treated as such. By bypassing the legitimate healthcare system, users of counterfeit PDE-5i bypass screening for concurrent medical comorbidities, as well as proper education and warnings of PDE-5i use. Furthermore, counterfeit PDE-5i often contain improper dosing and contaminants that may place patients at direct risk. Many “natural supplements” contain illicit PDE-5i, subjecting users to the same risks with even less warning. Physicians who treat ED should warn patients against purchasing PDE-5i via alternative means, especially the Internet. The use of dietary supplements for treatment of ED should be screened for and given due precautions.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. WJ Hellstrom has been an advisor or consultant to Pfizer. The other authors have no conflicts of interest to declare.
References
- World Health Organization. Definitions of SSFFC Medical Products. Accessed January 23, 2016. Available online: http://www.who.int/medicines/regulation/ssffc/definitions/en/
- Hellstrom WJ. The growing concerns regarding counterfeit medications. J Sex Med 2011;8:1-3. [Crossref] [PubMed]
- World Health Organization. Counterfeit medicines. Available online: https://www.gphf.org/images/downloads/library/who_factsheet275.pdf
- Pharmaceutical Security Institute. Counterfeit situation. Incident trend. Accessed January 23, 2016. Available online: http://www.psi-inc.org/incidentTrends.cfm
- The Economist. Poison pills. Accessed February 26, 2016. Available online: http://www.economist.com/node/16943895
- Taverriti-Fortier C, Pape E, Scala-Bertola J, et al. Counterfeit and Falsified Drugs: an Overview. Therapie 2015;70:455-64. [Crossref] [PubMed]
- Jack A. Counterfeit medicines. Bitter pills. BMJ 2007;335:1120-1. [Crossref] [PubMed]
- Jackson G, Arver S, Banks I, et al. Counterfeit phosphodiesterase type 5 inhibitors pose significant safety risks. Int J Clin Pract 2010;64:497-504. [Crossref] [PubMed]
- Venhuis BJ, de Voogt P, Emke E, et al. Success of rogue online pharmacies: sewage study of sildenafil in the Netherlands. BMJ 2014;349:g4317. [Crossref] [PubMed]
- Prins J, Blanker MH, Bohnen AM, et al. Prevalence of erectile dysfunction: a systematic review of population-based studies. Int J Impot Res 2002;14:422-32. [Crossref] [PubMed]
- Bingham J. Drug dealers 'switching from cocaine to fake Viagra'. The Telegraph 2009. Accessed January 24, 2016. Available online: http://www.telegraph.co.uk/news/uknews/law-and-order/4925836/Drug-dealers-switching-from-cocaine-to-fake-Viagra.html
- Dorsey PJ, Hellstrom WJ. The Illicit Sale of Medications for the Treatment of Erectile Dysfunction. Accessed January 24, 2016. Available online: http://www.medscape.com/viewarticle/566897_3
- Shaeer O. The Global Online Sexuality Survey (GOSS): the United States of America in 2011 chapter II: phosphodiesterase inhibitors utilization among English speakers. J Sex Med 2013;10:532-40. [Crossref] [PubMed]
- Schnetzler G, Banks I, Kirby M, et al. Characteristics, behaviors, and attitudes of men bypassing the healthcare system when obtaining phosphodiesterase type 5 inhibitors. J Sex Med 2010;7:1237-46. [Crossref] [PubMed]
- Travison TG, Hall SA, Fisher WA, et al. Correlates of PDE5i use among subjects with erectile dysfunction in two population-based surveys. J Sex Med 2011;8:3051-7. [Crossref] [PubMed]
- Lowe G, Costabile R. Phosphodiesterase type 5 inhibitor abuse: a critical review. Curr Drug Abuse Rev 2011;4:87-94. [Crossref] [PubMed]
- Campbell N, Clark JP, Stecher VJ, et al. Internet-ordered viagra (sildenafil citrate) is rarely genuine. J Sex Med 2012;9:2943-51. [Crossref] [PubMed]
- Dorsey P, Keel C, Klavens M, et al. Phosphodiesterase type 5 (PDE5) inhibitors for the treatment of erectile dysfunction. Expert Opin Pharmacother 2010;11:1109-22. [Crossref] [PubMed]
- Ignarro LJ. Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacol Toxicol 1990;67:1-7. [Crossref] [PubMed]
- Rajfer J, Aronson WJ, Bush PA, et al. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med 1992;326:90-4. [Crossref] [PubMed]
- Gur S, Kadowitz PJ, Gokce A, et al. Update on drug interactions with phosphodiesterase-5 inhibitors prescribed as first-line therapy for patients with erectile dysfunction or pulmonary hypertension. Curr Drug Metab 2013;14:265-9. [PubMed]
- Taylor J, Baldo OB, Storey A, et al. Differences in side-effect duration and related bother levels between phosphodiesterase type 5 inhibitors. BJU Int 2009;103:1392-5. [Crossref] [PubMed]
- Kubin M, Wagner G, Fugl-Meyer AR. Epidemiology of erectile dysfunction. Int J Impot Res 2003;15:63-71. [Crossref] [PubMed]
- Kostis JB, Jackson G, Rosen R, et al. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference). Am J Cardiol 2005;96:85M-93M. [Crossref] [PubMed]
- Miner M, Nehra A, Jackson G, et al. All men with vasculogenic erectile dysfunction require a cardiovascular workup. Am J Med 2014;127:174-82. [Crossref] [PubMed]
- Kirby MG, Schnetzler G, Zou KH, et al. Prevalence and detection rate of underlying disease in men with erectile dysfunction receiving phosphodiesterase type 5 inhibitors in the United Kingdom: a retrospective database study. Int J Clin Pract 2011;65:797-806. [Crossref] [PubMed]
- Stecher VJ, Jackson G, Banks I, et al. Analysis of Pharmaceuticals Seized by Authorities for Suspicion of Being Counterfeit Viagra® (Sildenafil Citrate). Lyon, France: European Society for Sexual Medicine, 2009.
- Gaudiano MC, Manna L, Rodomonte AL, et al. A survey on illegal and counterfeit medicines for the treatment of erectile dysfunctions in Italy. J Sex Med 2012;9:2130-7. [Crossref] [PubMed]
- Pullirsch D, Bellemare J, Hackl A, et al. Microbiological contamination in counterfeit and unapproved drugs. BMC Pharmacol Toxicol 2014;15:34. [Crossref] [PubMed]
- Taher A, Setiawati A. VICTORY project: a study of counterfeit PDE5 inhibitor (sildenafil) in Indonesia. Acta Med Indones 2013;45:290-4. [PubMed]
- Kao SL, Chan CL, Tan B, et al. An unusual outbreak of hypoglycemia. N Engl J Med 2009;360:734-6. [Crossref] [PubMed]
- Campbell N, Clark JP, Stecher VJ, et al. Adulteration of purported herbal and natural sexual performance enhancement dietary supplements with synthetic phosphodiesterase type 5 inhibitors. J Sex Med 2013;10:1842-9. [Crossref] [PubMed]
- FDA Announces New Supplement Recall. Accessed March 9th, 2016. Available online: http://community.auanet.org/blogs/policy-brief/2016/01/19/fda-announces-new-supplement-recall?utm_term=ttitus@auanet.org&utm_content=buffer22d4d&utm_medium=social&utm_source=facebook.com&utm_campaign=buffer
- World Health Organization. Health Impact Assessment. Available online: http://www.who.int/hia/en/
- Paxton M. Current challenges with supply-chain integrity and the threat to the quality of marketed drugs. Clin Pharmacol Ther 2011;89:316-9. [Crossref] [PubMed]
- U.S. Food & Drug Administration. MedWatch: The FDA Safety Information and Adverse Event Reporting Program. Availble online: http://www.fda.gov/medwatch
- U.S. Food & Drug Administration. Counterfeit Medicine. Availble online: http://www.fda.gov /Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/CounterfeitMedicine/