Erectile dysfunction in fit and healthy young men: psychological or pathological?
Introduction
Erectile dysfunction (ED) is defined as a consistent or recurrent inability to attain and/or maintain penile erection sufficient for sexual satisfaction. This definition, which has been recently endorsed by the Fourth International Consultation on Sexual Medicine (ICSM) (1), is based on a clinical principle which leaves room to the judgement of patients, being widely affected by their self-perception of normality. Furthermore, rather than focusing on possible causes of the dysfunction, it hinges on the sexual distress which it causes. This is consistent with the philosophy of Sexual Medicine, according to which, only symptoms creating despair are worthy of medical care. On the other hand, it carries the risk of over- or under-estimating a medical condition that does not have objective medical parameters of definition. This is particularly the case for young and apparently healthy men whose complaint of ED can be perceived by medical practitioners as excessive or overrated thus, minimized without even performing an adequate screening of possible associated or causing conditions. This review is aimed at summarizing the available evidence on the organic and non-organic disorders that can be associated with ED in young men, underlining the importance of recognition and assessment of a symptom, which can lead to a unique opportunity for performing a high quality preventive medicine intervention.
Epidemiology
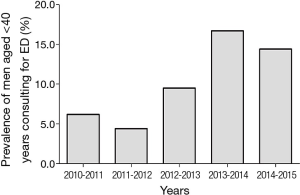
Describing the epidemiology of ED in young men requires, first of all, defining what it is meant by youth. While the definition of old age is matter of discussion and a precise threshold does not exist, the most shared definition in Western Countries is age above 65 years (http://www.who.int/healthinfo/survey/ageingdefnolder/en/). Considering that most of the epidemiological studies on general populations aimed at studying health changes with age, enrol men more than 40 years, it seems reasonable to define young age as below 40 years. Epidemiological studies on erectile function, which considered the prevalence of ED according to age bands, consistently find a significant increase with ageing. Advancing age remains one of the most important unmodifiable risk factors for ED (1). Studies on ED mostly involve middle-aged and older men, with younger aged men often overlooked. In a multi-centre worldwide study, involving more than 27,000 men from eight countries, Rosen et al. (2) showed an ED prevalence of 8% among men aged 20–29 years and 11% among those aged 30–39 years. Most of the studies involving younger men and conducting age-stratified analyses have been performed in Europe, where the prevalence of ED in men younger than 40 years ranges between 1% to 10% (3-10). The prevalence reported in these studies is highly variable due to different methodologies used in defining ED, population accrual, acquisition of data and choice of tools for investigating erectile function. A smaller number of studies on this topic have been conducted outside Europe. Both in Australia (11,12) and in America (13-15), the available information suggests a similar range of prevalence of ED among young subjects, with the same extent of variability among studies. According to these data, ED in younger men, although still not extensively studied and largely overlooked by the scientific community, is a quite common condition. In a recent study conducted in a Urology Clinic, it has been observed that one out of four men seeking medical care for ED was younger than 40 years (16). In our Sexual Medicine and Andrology Unit, established in an Endocrinology setting at the University of Florence, medical consultations for younger men are infrequent, with a prevalence of men aged less than 40 years at only 14.1% of more than 3,000 men complaining of ED. However, when considering the new referrals to our Unit during the last 6 years, we can notice a progressive increase in prevalence of men below 40 years seeking medical care for ED (Figure 1). According to these data, ED is becoming a common concern even among young men, and the clinical practitioner in sexual medicine must become aware of how to manage the problem and avoid underestimating a symptom. The identification of ED in a young man may potentially provide a great deal of useful information that can help improve their quality and even length of life.
Pathogenetic components of ED in young patients
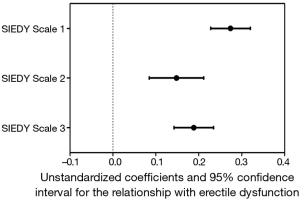
ED has been for long time considered a problem mainly related to psychological conditions and distress. Accordingly, until phosphodiesterase type 5 inhibitors (PDE5i) were introduced, psychoanalysis and cognitive-behavioural therapy were the only option for ED. In the last few decades, ED has been recognized as a clinical consequence of several different organic diseases and the importance of vascular health in erectile function has been so emphasized that ED is now considered not only the result of vascular impairment, but also a harbinger of forthcoming cardiovascular (CV) events (17). Despite the increasing attention of research towards organic mechanisms and conditions leading to ED, it is now known that considering this symptom as entirely due to organic disorders, is as imprecise as considering it only secondary to psychological conditions. In fact, this pathogenetic dichotomy is now obsolete (1,18,19), because it is now known that ED is a multidimensional disorder deriving from the interaction of different components related to organic conditions, relational context and psychological status (20,21). Even when only one of these components is involved in the initial development of erectile impairment, eventually the other ones will appear, thus further worsening ED (21-23). The multidimensional nature of ED is still not fully accepted by health care professionals when dealing with young patients. In fact, complaints of ED in young men is often underestimated and attributed to transient and self-limiting psychological conditions, such as performance anxiety. Young patients are often reassured without any further medical investigations, including physical exam. However, organic disorders, as well as relational and psychological or psychiatric conditions, can be meaningful in determining ED in younger men. In a population of subjects seeking medical care at the Sexual Medicine and Andrology Unit of the University of Florence for sexual dysfunction, the first tertile of age (n=1,873 subjects) represents younger subjects (18–44 years). Pathogenetic components of ED in our sample are investigated by the Structured Interview on Erectile Dysfunction (SIEDY), a structured interview including 13 questions, whose answers, organized in a Likert scale, provide three scales, one for the organic subdomain [(SIEDY Scale 1); (22)], one for the relational subdomain [(SIEDY Scale 2); (23)] and one for the intrapsychic subdomain [(SIEDY Scale 3); (21)]. According to these scale scores, organic, relational and intrapsychic conditions are all significant risk factors for ED in younger patients of our population (Figure 2).
Organic component
As shown in Figure 2, in younger patients consulting for ED, the organic component plays the predominant role. These data provide evidence for the need to adequately investigate possible organic causes of ED in younger and apparently fit men. The organic causes of ED can be classified into three categories: metabolic and CV, endocrine and neurologic conditions.
Metabolic and CV conditions
Ageing is one of the most important unmodifiable risk factors for the development of metabolic disorders and CV diseases. Accordingly, the common algorithms for the estimation of risk of forthcoming diabetes or CV events include age as a factor of the equations (24-29). The weight attributed to age for estimating the risk in these equations is often so significant that younger men are automatically considered at low risk, irrespective of the other possible risk factors. However, even in younger subjects, overlooking the contribution of cardio-metabolic factors to pathogenesis of ED is a mistake that can lead to the loss of the opportunity of early recognition of patients who deserve a change in life-style or a pharmacological correction of risk factors. ED, besides being considered one of the clinical manifestations of metabolic and cardiovascular diseases (CVD), is regarded as an early marker of CV events (17). In fact, according to Montorsi’s hypothesis (30), impairment of penile artery blood flow occurs before that of coronary or carotid arteries, whose diameter is greater and needs longer time to acquire a clinically relevant damage. The clinical consequence of this pathological event is that ED often manifests earlier than myocardial infarction or stroke. In particular, it has been demonstrated that ED occurs on average three years before the first major adverse CV event (MACE) (31). Quite surprisingly, although CV risk increases with ageing, the role of ED as a harbinger of forthcoming MACE becomes progressively less evident. Data derived from almost 2,500 community-dwelling men aged 40–79 years, involved in the Olmsted County study show that ED is associated with an almost 50-fold higher risk of incident heart diseases in men aged 40–49 years, whereas the difference in risk between ED and non-ED men progressively declines in older men (32). The different CV risk associated with ED in different age bands has been confirmed by the meta-analysis of the available longitudinal studies (33). These observations suggest that, in younger men, the role of ED as a marker of CV risk is even more dramatic than in older ones and as a consequence, investigating the presence of metabolic or CV conditions in younger ED patients is pivotal for identifying men in whom an early life-style modification may avoid serious CV consequences. Even more than erection during sexual intercourse, erection during masturbation is considered a physiologic function that mirrors metabolic and CV health. In fact, erections during masturbation are far less affected by relational and psychological components than sex-related ones (34). In a population of subjects attending the Sexual Medicine and Andrology Unit of the University of Florence for sexual dysfunction, more than 2,500 men reported autoeroticism in the previous 3 months. Among these men, the impairment of erection during masturbation was associated with family and personal history of CVD (35), as well as with impaired response to the test with the intracavernous injection (ICI) of prostaglandin E1, which suggests an arteriogenic damage of penile arteries and predicts forthcoming MACE (36). For a subset of these men (n=862), information on the occurrence of MACE during a mean follow-up of 4.3 years was available and those who reported impaired erections during masturbation had a significantly higher incidence of MACE (35). However, when considering separately younger and older men, this association was confirmed only in younger ones, and it was still significant after excluding men reporting severe ED during masturbation (35). This suggests that the impairment of erection during masturbation is a symptom not completely overlapping with sex-related ED and that it can provide different and supplementary information, in particular when assessed in younger and apparently healthy men. Similarly to what is observed for erection during masturbation, acceleration of blood in penile arteries, as measured by the colour Doppler ultrasound in flaccid conditions, is associated with an adverse CV profile in men consulting for ED. A reduction in flaccid acceleration, which can be used by clinicians to objectively verify the arteriogenic origin of ED and to characterize the extent of a self-reported symptom, has been also associated with a future risk of CV events, with the association being significant in younger but not in older men (37).
These findings demonstrate the importance of recognizing a possible organic component of ED even in younger men. In fact, in younger, more than in older men, who are by definition at high CV risk, searching for signs of metabolic or CV disorders can help identify those men who apparently healthy, have subtle and subclinical conditions that can be treated before the damage becomes clinically overt.
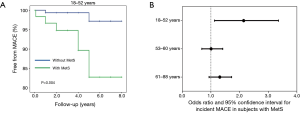
Unconventional CV risk factors, such as impaired erections during masturbation and reduced flaccid acceleration, are interesting parameters to implement in Sexual Medicine context, because they can help fill the gap of information on CV risk, left by the conventional risk factors (the so-called residual risk) (38). However, it should be recognized that not all the healthcare professionals who deal with the complaint of ED (i.e., general practitioners, diabetologists, cardiologists, sport physicians, nurses, etc.) have the facilities or competence for the specific assessment of these parameters. In contexts different than Sexual Medicine and Andrology, the assessment of conventional risk factors is certainly more convenient. Metabolic syndrome (MetS) represents a cluster of metabolic derangements easily and commonly evaluated in several different medical contexts. In a population of more than 600 subjects attending the Sexual Medicine and Andrology Unit of the University of Florence for ED, the presence of MetS was associated with an increased incidence of MACE during 4.3 years of follow-up in younger (first tertile of age: 18–52 years) (Figure 3, Panel A and B) but not in middle aged and older men (second and third tertiles of age: 53–60 and 61–88 years, respectively) (Figure 3, Panel B). Similar to MetS, the algorithms for estimating the risk of developing MACE are easily computed and they take into account factors largely available in a clinical setting. In Europe, the most commonly used algorithm is the SCORE, which takes into account age, smoking habits, systolic blood pressure and total cholesterol (26). These parameters are introduced in a calculation tool that returns the 10-year risk of developing the first MACE. The same estimated risk rate can obviously be derived from different combinations and extent of the single risk factors and, as aforementioned, age has a heavy weight in the amount of risk, even when the other parameters are normal. For overcoming this overestimation, the concept of vascular age, based on the predicted CV risk, has been introduced. Vascular age of a subjects with a specific CV risk profile corresponds to the chronological age of a subjects who has the same estimated risk but only due to chronological age, because of the absence of the other modifiable risk factors (i.e., a non-smoker, normotensive and normocholesterolemic subject) (39,40). Vascular age carries the advantage of easily and directly communicating the concept of high relative risk to patients, in particular to younger ones, who are by definition at low absolute risk (“Your CV risk is the same of a man that is 15 years older than you”). Based on this interesting and useful concept of vascular age, we recently studied the clinical consequences of having a high discrepancy between the estimated vascular and the actual chronological age in our population of men consulting for ED. In our sample, a greater difference between vascular and chronological age was associated with higher glucose and triglyceride levels as well as with impaired penile colour Doppler ultrasound parameters, suggesting a CV impairment (41). When evaluating the subset of men for whom information on incident MACE during a mean follow-up of 4.3 years was available, a greater difference between vascular and chronological age was associated with the incidence of MACE in younger, but not in older men (42).
Once observed that the organic component of ED is the most important one in younger patients (Figure 2), the summarized evidence underlines that metabolic and CV risk must not be underestimated in younger men even when they are apparently healthy. In fact, it is particularly in these men that recognizing the presence of risk factors can help in changing life-style, thus really changing the natural history of metabolic and CV diseases. In older men the damage is often already established and the identification of further risk factors usually does not add information to the estimation of CV risk. ED is a symptom that can provide a chance for both the patients and physicians to unearth the presence of CV risk factors and improve both the quality and length of life of these men.
Endocrine disorders
Erectile function can be impaired in several endocrine disorders and treating these conditions can improve ED (43). This is the case of adrenal insufficiency, whose treatment with glucocorticoid and mineralocorticoid replacement is able to improve erectile function (44). Similarly, an adequate control of thyroid function in hyper- and hypothyroid patients is associated with an improvement in ED (45,46). However, although ED is a common complaint in subjects with Addison’s disease, hypo- and even more hyperthyroidism (45-48), the prevalence of these disorders is subjects with ED is not so high for recommending the routine screening of adrenal and thyroid hormone in these men (49). In contrast with the low prevalence of adrenal or thyroid disturbances in ED subjects, testosterone (T) deficiency is frequently found in subjects with ED (49,50) and, in turn, low T is frequently associated with the occurrence of sexual dysfunctions, including ED, even in general population (51). Accordingly, the Fourth ICSM recommends the routine assessment of T levels in patients with ED (43). The assessment of prolactin (PRL) in ED patients is controversial because an actual pathological increase in PRL levels (severe hyperprolactinemia: prolactin ≥735 mU/L or 35 ng/mL) is rarely found in ED men (52). Furthermore, the role of PRL in inducing ED is still not clarified. Hyperprolactinemia has been consistently associated with loss of sexual desire (43,53) and development of hypogonadotropic hypogonadism, both conditions that can in turn induce ED. However, a direct role of high PRL levels in inducing an impairment of erectile function is not consistently proven (52,54) and, conversely, more recent evidence suggests that lower, rather than higher, PRL levels are associated with impaired erectile function (55-57). For these reasons, at present, the assessment of PRL levels in subjects with ED is not routinely recommended (43) and it could be advisable only in men with hypogonadotropic hypogonadism, as a possible cause of this condition.
In summary, in subjects with ED, T is the only hormone whose measurement is recommended. T levels progressively decline with ageing (58) and the clinical significance of this decline is still uncertain (59). Conversely, low T levels in young men, although less frequent, are of particular importance.
Similar to the general population (58), in subjects consulting for sexual dysfunction, T deficiency is progressively more prevalent as a function of age (50). In a series of 4,890 subjects consulting our Sexual Medicine and Andrology Unit for sexual dysfunction, one in five (19.6%) and one in three (29.4%) patients have total T below 10.4 and 12 nmol/L, respectively (60). Clinical correlates of T deficiency show different figures according to patient’s age. In fact, we previously demonstrated that in the youngest quartile (17–42 years old), but not in the oldest one (62–88 years old), severity of reported ED and penile blood flow impairment (dynamic peak systolic velocity) were not associated to decreasing testosterone levels (50). It is possible to speculate that, in young individuals, intercourse-related penile erection is such a complex phenomenon that other determinants (i.e., intrapsychic or relational) might mask its androgen regulation and that T deficiency produces greater sexual disturbances in subjects with greater frailty, such as older individuals. However, reported frequency of spontaneous erection and sexual thoughts were significantly decreased as a function of T decline even in younger subjects (50). Moreover, in young individuals low T was associated with a worse metabolic profile, including hypertriglyceridemia and increased waist circumference (50). Accordingly, the prevalence of MetS in the youngest quartile was clearly associated with T deficiency, as it was in the older quartiles (50). Therefore, T deficiency must be accurately verified in all subjects consulting for sexual dysfunction, even in the youngest ones.
Diagnosing T deficiency in young individuals is not difficult; however, blood sampling must be performed in the early morning, because of a circadian decline later on during the day (61).
Since the decrease in T levels is often a consequence of obesity or weight gain (51), the milestone of treating testosterone deficiency in obese men is encouraging substantial lifestyle changes, including physical activity and weight loss. In fact, it is universally recognized that a low calorie diet or bariatric surgery can induce a significant increase in T plasma levels, reaching 10 nmol/L with the most invasive surgical procedures (62). Weight loss-induced T rise is more evident in young individuals (62), and, therefore, it must be strongly recommended in this age band.
Pharmacological treatment of T deficiency in the young essentially relies on the site of origin of the dysfunction: the testis (primary hypogonadism) or the hypothalamic-pituitary region (secondary hypogonadism). In the case of primary hypogonadism, the only available treatment is T replacement therapy (TRT). In secondary hypogonadism, patient needs dictate the therapy. If fertility is requested, gonadotropin is the only option, with the caveat of anti-estrogens in selected cases. If fertility is not an issue, TRT is again the primary choice (63).
Neurologic conditions
Neurologic illnesses leading to ED have been recently reviewed (64). The most common of them (i.e., consequences of prostatic surgery, stroke and Parkinson’s disease) are not typical of younger age and, similarly to conditions less common but more typical of younger men, such as spinal cord injury, multiple sclerosis and spina bifida, the clinical features of the underlying disease are clearly apparent, being ED one of the multiple manifestations, rather than a harbinger of a subtle condition. Diagnosis of neurologic origin of ED is often quite simple, based on medical history and physical exam. The clinical management is a multidimensional and coordinated work of rehabilitation and medical therapy, which includes ICI injection of vaso-relaxant drugs, vacuum device and surgery (64).
Intrapsychic component
The association between psychiatric conditions and sexual dysfunctions, including ED, is well known. Data from population-based studies demonstrate a cross-sectional association between depressive symptoms and ED (65-68) and, among men seeking medical care for ED, depression is significantly associated with a greater severity of the impairment in erectile function (69,70). A meta-analysis of the available prospective studies has shown the role of depression as a significant risk factor for development of ED (71). However, the relationship seems to be bidirectional, as also ED has been associated with the occurrence of depression (72). In addition, treatment with PDE5i is related with an improvement in depressive symptoms (72). Most of this evidence comes from studies not specifically designed for the assessment of this relationship in younger men. However, few studies available in younger populations seem to confirm these results. In an internet-based survey, involving more than 800 North American medical students with a mean age of 25.7 years, ED was reported by 13% of them and it showed a significant association with depressive symptoms, whose frequency got higher as a function of ED severity (73). In a population of more than 2,500 very young Swiss men, aged 18–25 years, participating to a survey on sexual function while attending the medical screening for the evaluation of military capability, ED had a prevalence of 30%. Among the possible correlated conditions, mental health showed an independent association, besides the use of medications without medical prescription, a shorter sexual lifespan and impaired physical health (74). The results from this Swiss study were then prospectively extended on a sample of 3,700 men evaluated at baseline and 15.5 months later (75). Among a number of different possible predictors, including life-style, drug abuse, perceived physical fitness and BMI, only perceived impairment in mental health and depression, either newly occurred or continuously present, were associated with both persistence and development of ED (75). In a retrospective population-based study from Finland, involving almost 3,500 men aged 18–48 years, the role of depression as a significant predictor for ED was confirmed, but this study also showed that anxiety plays a significant role and that ED is significantly less frequent in men with a longer lasting sexual life, thus underlining the positive role of sexual experience and self-confidence (76). Anxiety is often involved in the pathogenesis of ED at the beginning of sexual life. In fact, anxiety can lead to an excessive focus on quality of erection, thus providing a cognitive distraction that negatively affects the arousal and consequently the erection itself (77-79). On the other hand, anxiety can result from one or more sexual failures, with loss of sexual confidence, increasing fears and avoidance for sexual experiences that, in the end, lead to an increased probability of new failures, thus creating a vicious circle (77). Cognitive distraction could be also provided by excessive worry for physical, and in particular genital, self-image. In fact, it has been proposed that when most mental energy is focused on monitoring body, psychological resources are distracted from sex, resulting in an impaired functioning (80,81). In line with this cognitive explanation, a recent study conducted on 367 military personnel younger than 40 years showed that a deteriorated genital self-image is associated with sexual anxiety which, in turn, is associated with a higher probability of sexual dysfunction (82).
If on one hand, depression and anxiety can lead to ED, drugs commonly used for their treatment can cause ED, as well. Sexual dysfunctions are common side effects of several psychotropic drugs that can disrupt sexual health by several different mechanisms (83). In particular, ED has been reported in subjects using serotonin selective re-uptake inhibitors (SSRI), lithium and benzodiazepines (83). SSRI are associated with a broad spectrum of sexual dysfunctions, but the most commonly reported complaints are delayed ejaculation or anorgasmia and reduced sexual desire (84). Several mechanisms could be advocated including the agonist effect on serotonin receptors type 2 and the increase in PRL levels. ED is a frequent complaint as well (84,85). The relationship between the use of SSRI and ED can be secondary to loss of sexual desire but SSRI, in particular paroxetine, are also able to inhibit cholinergic receptors and nitric oxide synthase (86). In addition, it has been observed that SSRIs might down-regulate hypothalamic-pituitary-testis axis in depressed men (87).
Relational component
The relationship between ED and couple relation impairment is well documented. In our population of subjects consulting for sexual dysfunction, subjects reporting conflicts within the couple were characterized by a broad spectrum of sexual symptoms, including a severe extent of ED, and they had a higher SIEDY Scale 2 score, indicating a strong relational component in the pathogenesis of ED (88). If on one hand, it is easy to understand that problems in couple relationship can cause ED, the other way around is also feasible. In the Female Experience of Men’s Attitudes to Life Events and Sexuality (FEMALES) study, 292 female partners of men aged more than 20 years complaining for ED were involved in a survey assessing the quality of their sexual experience (89). In this study, women reported a significant deterioration of satisfaction for sexual intercourse after the onset of ED in their partners. The satisfaction, sexual desire, arousal and orgasm were then improved in women whose partner used PDE5i (89). The role of ED as a risk factor for female dysfunction, including impairment in arousal, orgasm, sexual satisfaction and sexual pain, has been also confirmed in a study involving 632 sexually active couples, whose male partner age ranged 18–80 years (90).
The improvement of both man and woman sexual function in couples whose male partner is treated with PDE5i has been further confirmed by randomized clinical trials (RCTs) comparing the effectiveness of PDE5i vs. placebo in improving couple sexual function (91-95). In a more recent RCT, the use of vardenafil 10 mg oral dispersible tablets has been compared to the use of the drug itself in association with cognitive behavioral sexual therapy (CBST) for 10 weeks in 30 couples with ED male partners, randomly assigned to one study arm (96). Results from this RCT showed that vardenafil is able to improve male sexual function, but this improvement is maintained only in patients receiving both vardenafil and CBST. Furthermore, female sexual function and satisfaction are enhanced only in the arm with vardenafil and CBST combined therapy, thus suggesting that a therapy healing the couple is more effective and has a longer-lasting efficacy than the use of a medication focusing only on ED.
Unfortunately, studies specifically considering the relationship between couple liaison and ED in younger men are not available. Although the aforementioned studies include also young men, thus making their results theoretically applicable even in this specific group, it should be recognized that mean age of men enrolled is usually shifted toward the middle-age, rather than younger age. It is conceivable that couple relationship can act differently in younger men because it could show peculiar characteristics likely affecting ED onset, maintenance, resolution or responsiveness to therapies, including the short duration, lack of experience in both the partners, limited privacy, fears for emotional involvement or worry for undesired pregnancies.
Conclusions
Although few studies specifically evaluated the clinical characteristics of ED in younger men, this problem is increasingly frequent. Healthcare professionals both inside and outside of Sexual Medicine are likely to deal with young men complaining for ED and it is important that basic knowledge on this topic is available. In fact, young men reporting ED risk being dismissed without any specific medical assessment, including medical history or physical exam, owing to the assumption that ED in younger is a self-limiting condition, without any clinical consequence. However, evidence shows that, similar to middle-aged or older men, ED can be the consequence of the combination of organic, psychological and relational factors and all these components must be assessed for a correct clinical management. In particular, ED in younger, even more than in older men, can be considered a harbinger of CVD and it offers the unique opportunity to unearth the presence of CV risk factors, thus allowing effective and high quality preventive interventions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- McCabe MP, Sharlip ID, Atalla E, et al. Definitions of Sexual Dysfunctions in Women and Men: A Consensus Statement From the Fourth International Consultation on Sexual Medicine 2015. J Sex Med 2016;13:135-43. [Crossref] [PubMed]
- Rosen RC, Fisher WA, Eardley I, et al. The multinational Men's Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin 2004;20:607-17. [Crossref] [PubMed]
- Kontula O, Haavio-Mannila E. Sexual Pleasures. Enhancement of Sex Life in Finland, 1971-1992. Aldershot: Dartmouth, 1995.
- Parazzini F, Menchini Fabris F, Bortolotti A, et al. Frequency and determinants of erectile dysfunction in Italy. Eur Urol 2000;37:43-9. [Crossref] [PubMed]
- Braun M, Wassmer G, Klotz T, et al. Epidemiology of erectile dysfunction: results of the ‘Cologne Male Survey’. Int J Impot Res 2000;12:305-11. [Crossref] [PubMed]
- de Boer BJ, Bots ML. Erectile dysfunction in primary care: prevalence and patient characteristics. The ENIGMA study. Int J Impot Res 2004;16:358-64. [Crossref] [PubMed]
- May M, Gralla O, Knoll N, et al. Erectile dysfunction, discrepancy between high prevalence and low utilization of treatment options: results from the 'Cottbus Survey' with 10 000 men. BJU Int 2007;100:1110-5. [PubMed]
- Stulhofer A, Baji Z. Prevalence of erectile and ejaculatory difficulties among men in Croatia. Croat Med J 2006;47:114-24. [PubMed]
- Andersen I, Heitmann BL, Wagner G. Obesity and sexual dysfunction in younger Danish men. J Sex Med 2008;5:2053-60. [Crossref] [PubMed]
- Fugl-Meyer AR, Sjögren Fugl-Meyer K. Sexual disabilities, problems, and satisfaction in 18-74 year old Swedes. Scand J Sexol 1999;3:79-105.
- Richters J, Grulich AE, de Visser RO, et al. Sex in Australia: sexual difficulties in a representative sample of adults. Aust N Z J Public Health 2003;27:164-70. [Crossref] [PubMed]
- Chew KK, Stuckey B, Bremner A, et al. Male erectile dysfunction: Its prevalence in Western Australia and associated sociodemographic factors. J Sex Med 2008;5:60-9. [Crossref] [PubMed]
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA 1999;281:537-44. [Crossref] [PubMed]
- Moreira ED Jr, Abdo CH, Torres ED, et al. Prevalence and correlates of erectile dysfunction: results of the Brazilian study of sexual behavior. Urology 2001;58:583-8. [Crossref] [PubMed]
- De Almeida Claro J, Kaufmann OG, Alarcon G, et al. Could a rural lifestyle decrease the prevalence of erectile dysfunction BJU Int 2007;99:127-9. [Crossref] [PubMed]
- Capogrosso P, Colicchia M, Ventimiglia E, et al. One patient out of four with newly diagnosed erectile dysfunction is a young man--worrisome picture from the everyday clinical practice. J Sex Med 2013;10:1833-41. [Crossref] [PubMed]
- Corona G, Monami M, Boddi V, et al. Male sexuality and cardiovascular risk. A cohort study in patients with erectile dysfunction. J Sex Med 2010;7:1918-27. [Crossref] [PubMed]
- Sachs BD. The false organic-psychogenic distinction and related problems in the classification of erectile dysfunction. Int J Impot Res 2003;15:72-8. [Crossref] [PubMed]
- Jannini EA, McCabe MP, Salonia A, et al. Organic vs. psychogenic? The Manichean diagnosis in sexual medicine. J Sex Med 2010;7:1726-33. [Crossref] [PubMed]
- Balon R. Human sexuality: An intimate connection of psyche and soma—Neglected area of psychosomatics? Psychother Psychosom 2009;78:69-72. [Crossref] [PubMed]
- Corona G, Ricca V, Bandini E, et al. SIEDY scale 3, a new instrument to detect psychological component in subjects with erectile dysfunction. J Sex Med 2012;9:2017-26. [Crossref] [PubMed]
- Petrone L, Mannucci E, Corona G, et al. Structured interview on erectile dysfunction (SIEDY): a new, multidimensional instrument for quantification of pathogenetic issues on erectile dysfunction. Int J Impot Res 2003;15:210-20. [Crossref] [PubMed]
- Boddi V, Corona G, Fisher AD, et al. "It takes two to tango": the relational domain in a cohort of subjects with erectile dysfunction (ED). J Sex Med 2012;9:3126-36. [Crossref] [PubMed]
- Schmidt MI, Duncan BB, Bang H, et al. Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care 2005;28:2013-8. [Crossref] [PubMed]
- Corona G, Rastrelli G, Silverii A, et al. The identification of prediabetes condition with ARIC algorithm predicts long-term CV events in patients with erectile dysfunction. J Sex Med 2013;10:1114-23. [Crossref] [PubMed]
- Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987-1003. [Crossref] [PubMed]
- Palmieri L, Panico S, Vanuzzo D, et al. Evaluation of the global cardiovascular absolute risk: the Progetto CUORE individual score. Ann Ist Super Sanita 2004;40:393-9. [PubMed]
- Assmann G, Schulte H, Cullen P, et al. Assessing risk of myocardial infarction and stroke: new data from the Prospective Cardiovascular Münster (PROCAM) study. Eur J Clin Invest 2007;37:925-32. [Crossref] [PubMed]
- D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743-53. [Crossref] [PubMed]
- Montorsi P, Ravagnani PM, Galli S, et al. The artery size hypothesis: a macrovascular link between erectile dysfunction and coronary artery disease. Am J Cardiol 2005;96:19M-23M. [Crossref] [PubMed]
- Montorsi P, Ravagnani PM, Galli S, et al. Association between erectile dysfunction and coronary artery disease. Role of coronary clinical presentation and extent of coronary vessels involvement: the COBRA trial. Eur Heart J 2006;27:2632-9. [Crossref] [PubMed]
- Inman BA, Sauver JL, Jacobson DJ, et al. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc 2009;84:108-13. [Crossref] [PubMed]
- Vlachopoulos CV, Terentes-Printzios DG, Ioakeimidis NK, et al. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systematic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes 2013;6:99-109. [Crossref] [PubMed]
- Corona G, Ricca V, Boddi V, et al. Autoeroticism, mental health, and organic disturbances in patients with erectile dysfunction. J Sex Med 2010;7:182-91. [Crossref] [PubMed]
- Rastrelli G, Boddi V, Corona G, et al. Impaired masturbation induced erections: A new cardiovascular risk factor for male subjects with sexual dysfunction. J Sex Med 2013;10:1100-13. [Crossref] [PubMed]
- Rastrelli G, Corona G, Monami M, et al. Poor response to alprostadil ICI test is associated with arteriogenic erectile dysfunction and higher risk of major adverse cardiovascular events. J Sex Med 2011;8:3433-45. [Crossref] [PubMed]
- Rastrelli G, Corona G, Lotti F, et al. Flaccid penile acceleration as a marker of cardiovascular risk in men without classical risk factors. J Sex Med 2014;11:173-86. [Crossref] [PubMed]
- Corona G, Maggi M. Conventional and unconventional cardiovascular risk factors in men with erectile dysfunction. J Sex Med 2013;10:305-8. [Crossref] [PubMed]
- D’Agostino RB Sr, Grundy S, Sullivan LM, et al. Validation of the Framingham coronary heart disease prediction scores: Results of a multiple ethnic groups investigation. JAMA 2001;286:180-7. [Crossref] [PubMed]
- Cuende JI, Cuende N, Calaveras-Lagartos J. How to calculate vascular age with the SCORE project scales: a new method of cardiovascular risk evaluation. Eur Heart J 2010;31:2351-8. [Crossref] [PubMed]
- Rastrelli G, Corona G, Mannucci E, et al. Vascular and chronological age in subjects with erectile dysfunction: a cross-sectional study. J Sex Med 2015;12:2303-12. [Crossref] [PubMed]
- Rastrelli G, Corona G, Mannucci E, et al. Vascular and Chronological Age in Men With Erectile Dysfunction: a Longitudinal Study. J Sex Med 2016;13:200-8. [Crossref] [PubMed]
- Corona G, Isidori AM, Aversa A, et al. Endocrinologic Control of Men's Sexual Desire and Arousal/Erection. J Sex Med 2016;13:317-37. [Crossref] [PubMed]
- Granata A, Tirabassi G, Pugni V, et al. Sexual dysfunctions in men affected by autoimmune Addison’s disease before and after short-term gluco- and mineralocorticoid replacement therapy. J Sex Med 2013;10:2036-43. [Crossref] [PubMed]
- Carani C, Isidori AM, Granata A, et al. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab 2005;90:6472-9. [Crossref] [PubMed]
- Krassas GE, Tziomalos K, Papadopoulou F, et al. Erectile dysfunction in patients with hyper- and hypothyroidism: how commonand should we treat? J Clin Endocrinol Metab 2008;93:1815-9. [Crossref] [PubMed]
- Veronelli A, Masu A, Ranieri R, et al. Prevalence of erectile dysfunction in thyroid disorders: comparison with control subjects and with obese and diabetic patients. Int J Impot Res 2006;18:111-4. [Crossref] [PubMed]
- Corona G, Wu FC, Forti G, et al. Thyroid hormones and male sexual function. Int J Androl 2012;35:668-79. [Crossref] [PubMed]
- Maseroli E, Corona G, Rastrelli G, et al. Prevalence of endocrine and metabolic disorders in subjects with erectile dysfunction: a comparative study. J Sex Med 2015;12:956-65. [Crossref] [PubMed]
- Corona G, Mannucci E, Ricca V, et al. The age-related decline of testosterone is associated with different specific symptoms and signs in patients with sexual dysfunction. Int J Androl 2009;32:720-8. [Crossref] [PubMed]
- Rastrelli G, Carter EL, Ahern T, et al. Development of and Recovery from Secondary Hypogonadism in Aging Men: Prospective Results from the EMAS. J Clin Endocrinol Metab 2015;100:3172-82. [Crossref] [PubMed]
- Corona G, Mannucci E, Fisher AD, et al. Effect of hyperprolactinemia in male patients consulting for sexual dysfunction. J Sex Med 2007;4:1485-93. [Crossref] [PubMed]
- Corona G, Rastrelli G, Ricca V, et al. Risk factors associated with primary and secondary reduced libido in male patients with sexual dysfunction. J Sex Med 2013;10:1074-89. [Crossref] [PubMed]
- Maggi M, Buvat J, Corona G, et al. Hormonal causes of male sexual dysfunctions and their management (hyperprolactinemia, thyroid disorders, GH disorders, and DHEA). J Sex Med 2013;10:661-77. [Crossref] [PubMed]
- Rastrelli G, Corona G, Maggi M. The role of prolactin in andrology: what is new? Rev Endocr Metab Disord 2015;16:233-48. [Crossref] [PubMed]
- Corona G, Wu FC, Rastrelli G, et al. Low prolactin is associated with sexual dysfunction and psychological or metabolic disturbances in middle-aged and elderly men: the European Male Aging Study (EMAS). J Sex Med 2014;11:240-53. [Crossref] [PubMed]
- Corona G, Mannucci E, Jannini EA, et al. Hypoprolactinemia: a new clinical syndrome in patients with sexual dysfunction. J Sex Med 2009;6:1457-66. [Crossref] [PubMed]
- Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 2008;93:2737-45. [Crossref] [PubMed]
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123-35. [Crossref] [PubMed]
- Rastrelli G, Corona G, Tarocchi M, et al. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J Endocrinol Invest 2016;39:473-84. [Crossref] [PubMed]
- Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab 1983;56:1278-81. [Crossref] [PubMed]
- Corona G, Rastrelli G, Monami M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol 2013;168:829-43. [Crossref] [PubMed]
- Corona G, Rastrelli G, Vignozzi L, et al. Emerging medication for the treatment of male hypogonadism. Expert Opin Emerg Drugs 2012;17:239-59. [Crossref] [PubMed]
- Shridharani AN, Brant WO. The treatment of erectile dysfunction in patients with neurogenic disease. Transl Androl Urol 2016;5:88-101. [PubMed]
- Araujo AB, Durante R, Feldman HA, et al. The relationship between depressive symptoms and male erectile dysfunction: Cross sectional results from the Massachusetts Male Aging Study. Psychosom Med 1998;60:458-65. [Crossref] [PubMed]
- Mak R, De Backer G, Kornitzer M, et al. Prevalence and correlates of erectile dysfunction in a population-based study in Belgium. Eur Urol 2002;41:132-8. [Crossref] [PubMed]
- Nicolosi A, Moreira ED Jr, Villa M, et al. A population study of the association between sexual function, sexual satisfaction and depressive symptoms in men. J Affect Disord 2004;82:235-43. [Crossref] [PubMed]
- Sugimori H, Yoshida K, Tanaka T, et al. Relationships between erectile dysfunction, depression, and anxiety in Japanese subjects. J Sex Med 2005;2:390-6. [Crossref] [PubMed]
- Corona G, Ricca V, Bandini E, et al. Association between psychiatric symptoms and erectile dysfunction. J Sex Med 2008;5:458-68. [Crossref] [PubMed]
- Bandini E, Fisher AD, Corona G, et al. Severe depressive symptoms and cardiovascular risk in subjects with erectile dysfunction. J Sex Med 2010;7:3477-86. [Crossref] [PubMed]
- Atlantis E, Sullivan T. Bidirectional association between depression and sexual dysfunction: A systematic review and meta-analysis. J Sex Med 2012;9:1497-507. [Crossref] [PubMed]
- McCabe MP, Althof SE. A systematic review of the psychosocial outcomes associated with erectile dysfunction: Does the impact of erectile dysfunction extend beyond a man's inability to have sex? J Sex Med 2014;11:347-63. [Crossref] [PubMed]
- Smith JF, Breyer BN, Eisenberg ML, et al. Sexual function and depressive symptoms among male North American medical students. J Sex Med 2010;7:3909-17. [Crossref] [PubMed]
- Mialon A, Berchtold A, Michaud PA, et al. Sexual dysfunctions among young men: prevalence and associated factors. J Adolesc Health 2012;51:25-31. [Crossref] [PubMed]
- Akre C, Berchtold A, Gmel G, et al. The evolution of sexual dysfunction in young men aged 18-25 years. J Adolesc Health 2014;55:736-43. [Crossref] [PubMed]
- Jern P, Gunst A, Sandnabba K, et al. Are early and current erectile problems associated with anxiety and depression in young men? A retrospective self-report study. J Sex Marital Ther 2012;38:349-64. [Crossref] [PubMed]
- Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers 2016;2:16003. [Crossref] [PubMed]
- Hale VE, Strassberg DS. The role of anxiety on sexual arousal. Arch Sex Behav 1990;19:569-81. [Crossref] [PubMed]
- Beck JG, Barlow DH. The effects of anxiety and attentional focus on sexual responding — II: cognitive and affective patterns in erectile dysfunction. Behav Res Ther 1986;24:19-26. [Crossref] [PubMed]
- Fredrickson BL, Roberts TA, Noll SM, et al. That swimsuit becomes you: Sex differences in self objectification, restrained eating, and math performance. J Pers Soc Psychol 1998;75:269-84. [Crossref] [PubMed]
- Aubrey JS. The impact of sexually objectifying media exposure on negative body emotions and sexual self-perceptions: Investigating the mediating role of body self-consciousness. Mass Commun Soc 2007;10:1-23. [Crossref]
- Wilcox SL, Redmond S, Davis TL. Genital image, sexual anxiety, and erectile dysfunction among young male military personnel. J Sex Med 2015;12:1389-97. [Crossref] [PubMed]
- Kennedy SH, Rizvi S. Sexual dysfunction, depression, and the impact of antidepressants. J Clin Psychopharmacol 2009;29:157-64. [Crossref] [PubMed]
- Corona G, Ricca V, Bandini E, et al. Selective serotonin reuptake inhibitor-induced sexual dysfunction. J Sex Med 2009;6:1259-69. [Crossref] [PubMed]
- Montejo AL, Llorca G, Izquierdo JA, et al. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry 2001;62 Suppl 3:10-21. [PubMed]
- Pollack MH, Reiter S, Hammerness P. Genitourinary and sexual adverse effects of psychotropic medication. Int J Psychiatry Med 1992;22:305-27. [Crossref] [PubMed]
- Safarinejad MR. Evaluation of endocrine profile and hypothalamic-pituitary-testis axis in selective serotonin reuptake inhibitor-induced male sexual dysfunction. J Clin Psychopharmacol 2008;28:418-23. [Crossref] [PubMed]
- Boddi V, Fanni E, Castellini G, et al. Conflicts within the family and within the couple as contextual factors in the determinism of male sexual dysfunction. J Sex Med 2015;12:2425-35. [Crossref] [PubMed]
- Fisher WA, Rosen RC, Eardley I, et al. Sexual experience of female partners of men with erectile dysfunction: the Female Experience of Men's Attitudes to Life Events and Sexuality (FEMALES) study. J Sex Med 2005;2:675-84. [Crossref] [PubMed]
- Jiann BP, Su CC, Tsai JY. Is female sexual function related to the male partners' erectile function? J Sex Med 2013;10:420-9. [Crossref] [PubMed]
- Heiman JR, Talley DR, Bailen JL, et al. Sexual function and satisfaction in heterosexual couples when men are administered sildenafil citrate (Viagra) for erectile dysfunction: A multicentre, randomised, double-blind, placebo-controlled trial. BJOG 2007;114:437-47. [Crossref] [PubMed]
- Fisher WA, Rosen RC, Mollen M, et al. Improving the sexual quality of life of couples affected by erectile dysfunction: A double-blind, randomized, placebo-controlled trial of vardenafil. J Sex Med 2005;2:699-708. [Crossref] [PubMed]
- Goldstein I, Fisher WA, Sand M, et al. Women's sexual function improves when partners are administered vardenafil for erectile dysfunction: A prospective, randomized, double-blind, placebo-controlled trial. J Sex Med 2005;2:819-32. [Crossref] [PubMed]
- Rosen R, Goldstein I, Huang XY, et al. The Treatment Satisfaction Scale (TSS) is a sensitive measure of treatment effectiveness for both patients and partners: Results of a randomized controlled trial with vardenafil. J Sex Med 2007;4:1009-21. [Crossref] [PubMed]
- Martín-Morales A, Graziottin A, Jaoudé GB, et al. Improvement in sexual quality of life of the female partner following vardenafil treatment of men with erectile dysfunction: a randomized, double-blind, placebo-controlled study. J Sex Med 2011;8:2831-40. [Crossref] [PubMed]
- Boddi V, Castellini G, Casale H, et al. An integrated approach with vardenafil orodispersible tablet and cognitive behavioral sex therapy for treatment of erectile dysfunction: a randomized controlled pilot study. Andrology 2015;3:909-18. [Crossref] [PubMed]


