Analysis of semen parameters during 2 weeks of daily ejaculation: a first in humans study
Introduction
The effect of frequency of ejaculation on semen characteristics is an important clinical question with obvious connections to conception rates. The 1999 WHO guidelines recommended a 2–7 days period of abstinence prior to providing a semen sample for laboratory analysis (1) but does not otherwise comment on how the frequency of ejaculation may affect semen analysis parameters or conception outcomes. In the joint committee opinion paper from the American Society of Reproductive Medicine and Society for Reproductive Endocrinology and Infertility timed and frequent intercourse around the time of female ovulation is recommended but does not make more specific comments (2).
In their influential report from the 1990s, investigators found that conception occurred most frequently when intercourse occurred on either the 2 days before ovulation or the day of ovulation (3). Interestingly, authors did not find that frequency of intercourse decreased the probability of conception (3) despite older studies that suggest overly frequent ejaculation may be detrimental to sperm counts (4,5). One would suspect that if sperm counts remain stable with daily ejaculation during these optimal days of conception, then pregnancy rates could be optimized by frequent intercourse, as more total sperm would be available for conception. In other older works increasing sexual frequency did appear to increase fecundability in patients recording conception and basal body temperature, although semen characteristics were not assessed in these studies (6,7).
While previous literature has primarily focused on the length of abstinence and the ultimate effect on semen quality, little is known about the effects of frequent ejaculation on semen analysis parameters. The clinical relevance of this question is tied to optimizing the number of available sperm during the days when conception is most likely. If decreasing sperm counts are found with each subsequent ejaculation after the initial period of abstinence, then theoretically overly frequent ejaculation during the optimal conception period could be tied to decreasing numbers of available sperm for conception and decreasing pregnancy rates.
To our knowledge, this is the first study to assess the effect of prolonged, daily ejaculation on conventional semen parameters and measures of DNA integrity. We hypothesized that men would have a progressive decrease in sperm concentration and semen volume during the study period with improvements in DNA damage indicators.
Methods
Internal review board approval (IRB # 13-691) was obtained at our institution before beginning the study. Men were recruited and compensated ($100) for this prospective study. Informed consent was obtained from all subjects. Subject exclusion criteria included active smoking and a known history of infertility.
After 3–5 days abstinence, daily ejaculations were performed by the study population of men with semen samples collected on days 1 (baseline), 3, 7 and 14. Adherence to the prescribed ejaculation schedule was reiterated during the study with participants signing an attestation at each semen collection and study conclusion that they abided by the daily ejaculation schedule. The daily ejaculations that were not collected for study purpose could be completed via masturbation or intercourse.
Semen analysis was performed on each study sample within 30 minutes of collection at our infertility center by trained, certified technologists. Assessed parameters included those in the standard semen analysis panel [volume, concentration, motility, pH, total motile count (TMC), morphology] and determinants of DNA integrity (described below).
Morphology was determined using the strict (Tygerberg) criteria after staining (Diff Quick, Thermo Fisher, Waltham, MA, USA) of air-dried semen smears. Both manual morphology analyses were performed in a blinded manner following completion of all sample collections. Manual analyses were performed by a single investigator with 25+ years of experience in sperm morphology analysis. Computer assisted morphology analysis was performed using the Sperm Class Analyzer (Microptic, Barcelona, Spain) programmed for morphology.
Sperm DNA integrity was assessed by measuring the DNA fragmentation index (DFI) via TUNEL assay and high DNA stainability (HDS) was evaluated using flow cytometry/acridine orange (SDFA test, Reprosource Inc., Woburn, MA, USA) based on the method developed by Evenson (8). The oxidative stress adduct (OSA) test (ReproSource Inc.) was used to quantify molecules produced from covalent reactions between sperm structures with free radicals and reactive oxygen species (9,10). DFI was considered abnormal above 30%, OSA above 4.4 µM and HDS above 15%.
Statistical analysis was performed using Minitab version 17 (Minitab Inc., State College, PA, USA). Statistical significance was defined by p values of less than 0.05. Linear trends were assessed using classic repeated measures ANOVA with a Tukey’s post hoc test to determine variable independence. When analyzing men with increasing or decreasing DFI, the Student’s t-test was used to compare groups. McNemar’s test was used on paired nominal data sets (day 1 to day 3, day 1 to day 7 and day 1 to day 14) to determine if changes from normal to abnormal values in DFI, OSA and HDS were statistically significant.
Results
Twenty-one healthy men, 23–33 years of age (mean age 25 years) were consecutively recruited. One subject failed to complete the study due to scheduling difficulties and his data is not included leaving 20 men with complete data; 3 of these men had proven past fertility with other study participants having no known inability to conceive. All had normal baseline semen parameters according to 2010 WHO criteria (11) except one man who was oligospermic. Data from this oligospermic man were included in analysis.
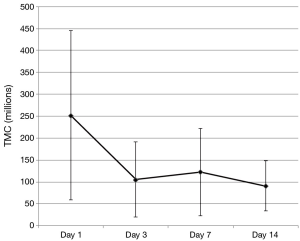
Significant decreases were observed in mean semen volume, TMC and sperm concentration (Table 1). Motility and number of morphologically normal sperm displayed no significant trends over the study period as a whole with some point to point variation that was not statistically different by Tukey’s post hoc test. Contrary to our hypothesis, changes in DNA integrity measures (DFI, OSA, HDS) did not change in a statistically or clinically meaningful way.
Full table
Initial decreases were noted in some standard semen parameters between day 1 (baseline) and day 3 and trends for these values from day 3 to day 14 were then assessed using the Tukey’s post hoc test. In the cases of TMC and semen volume, day 1 was found to be statistically different from all other study period points (P<0.001) with days 3–14 not being statistically independent from each other. The overall trend for sperm concentration was statistically significant (downward) but only day 1 and day 14 were statistically independent using Tukey’s post hoc test (P=0.0099).
The examination of damage due to oxidative stress using OSA revealed that at baseline, 90% of men fell within two standard deviations of normal (range, 0–3.8 µM). However, 11 patients had at least one value above 3.8 µM, with 7 men having excursions above 4.4 µM.
Only one subject in the study had HDS above 15%. He was also noted to have an elevated OSA and the highest DFI values.
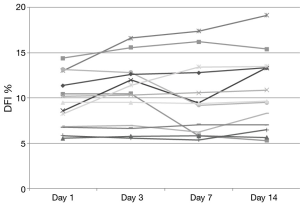
DNA integrity results demonstrated that 13 of 20 patients had a DFI <15% at baseline. None of these exceeded 20% during the study and only 2 of these 13 men increased their DFI above 15% (Figure 1). Seven subjects began the study with a DFI>15% at baseline. All but 2 of these 7 subjects maintained a DFI greater than 15 during the study. Additionally, 2 men reached a DFI >30% during the study period. However, they both began with a DFI close to this value at baseline (Figure 2).
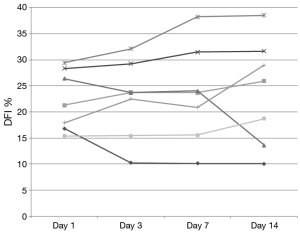
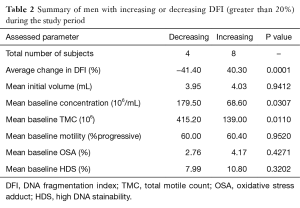
Changes in DFI were calculated and men were then grouped according to change in DFI. The established groups were those consisting of men with a greater than 20% increase from baseline (n=8), greater than 20% decrease (n=4) and change less than 20% in either direction (n=8). In men with a worsening DFI over the course of the study (DFI increasing more than 20%), a lower initial concentration and TMC was noted without significant between-group differences in initial motility, volume, OSA and HDS (Table 2).
Full table
McNemar’s test found that the number of patients moving from normal to abnormal values for DFI, OSA and HDS was not statistically significant.
Discussion
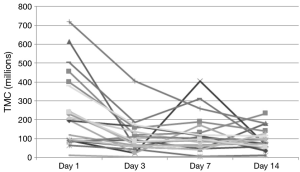
This pilot study examines the effects of daily ejaculation on sperm quality and availability for conception. We found that semen volume and available sperm for contraception (TMC) decreased with essentially all of this decrease seen between the initial collection (day 1) and next scheduled collection (day 3). After day 3, these values then plateaued (were not statistically independent) showing no additional loss in available sperm for conception with repeated ejaculation after this initial decrease (Figure 3). This finding is clinically relevant as it provides quantitative data that can be used to counsel couples on sexual frequency around the time of ovulation to optimize conception. A definitive conclusion on whether daily or every other day intercourse in the conception window cannot be convincingly determined from this data as we did not measure values on day 2. However, we can say that there is no continued loss to important semen analysis values after 3 days of daily ejaculation as a plateau was noted in these outcomes starting as early as day 3.
Previous works have convincingly shown that length of abstinence affects semen analysis parameters. Significantly lower semen volume has been demonstrated in men who abstained for less than 5 days; however there was no difference in sperm concentration (12). De Jonge et al. demonstrated that longer abstinence increased both sperm count and sample volume. However, measures like pH, viability, morphology, motility and DNA fragmentation were not affected (13). In a large retrospective study on this subject, Levitas et al. reported on abstinence effects with general improvements in TMC with longer abstinence and the largest gains seen with abstinence from 3–6 days. In a subgroup of men with low concentration at baseline (<20×106 sperm/mL) declines in sample quality were noted after 2 days of abstinence (14).
While changes in semen analysis characteristics with number of days of abstinence have been extensively studied, the effects of frequent ejaculation are unknown in humans. An extensive literature search by these authors found no similar studies in humans. However, similar studies have occurred in an equine population. In a study looking at 15 stallions, semen samples were quantified daily. They found that there was a decrease of 1.54 billion sperm per day (average initial count was approximately 12.5 billion sperm) for the first 4.71 days. As four trials were conducted on each of these 15 stallions, they were able to produce mathematically predictive models based on their results. Similar to our study in humans, there is a large initial decrease with a transition point after which daily sperm output remained unchanged until the end of the study (15). Interestingly, another equine study looking at pregnancy rates found no difference in per mating pregnancy rate with an increase in daily matings (sometimes up to 5 matings in a day) but did find that a decrease in per mating pregnancy rate when a day of abstinence was incorporated into the cycles (56.3% compared to 60.4%, P=0.024) (16).
Examination of the figures of the TMC from our subjects (Figures 3,4), we found that it looks strikingly similar to the daily sperm output of the equine study population. The net effect of consecutive ejaculation in both humans and horses is a decrease in TMC (humans) or total number of available sperm (stallions) to about 40% of the initial result (humans 252×106 to 106×106, stallions 12.5×109 to 5.28×109 sperm). In the absence of day 2 data in humans, comparisons of the slope during this initial decrease cannot be mathematically assessed but there is clearly a large initial decrease in TMC with a plateau thereafter.
For decades, traditional semen analysis outcomes have included quantitative measures (sperm concentration, semen volume) and other measures to assess sperm quality (motility, morphology). More recent advances have included a molecular assessment of the sperm that includes examination of the contained DNA. There is a growing body of evidence on the importance of the DNA integrity on fertility.
The metrics that assessed sperm DNA quality in this study were statistically and clinically unchanged over the study period, a finding contrary to our initial hypothesis. Previous works have convincingly shown that poor DNA integrity in men has been correlated with recurrent pregnancy loss (17-20) and infertility (21-23). While authors have examined that the period of abstinence before ejaculation may have effects on DNA integrity (24,25), there is no previous work examining the effects of daily ejaculation. This is somewhat surprising as it is hypothesized that once the sperm leave their favorable antioxidant residence in the testis (20) they are exposed to increasing DNA damage in the reproductive tract (26). A more rapid transit—with repeat ejaculation—may be of benefit as mature sperm have been shown to acquire oxidative damage as they undergo transport (27).
Previous studies examining clinical outcomes with DFI have chosen different differentiation points for DNA fragmentation. While variety exists in where studies define a cut point, most have established a DFI of greater than 25% or 30% as a poor prognostic factor (19). For our analysis, DFI of less than 15% was considered good fertility potential with over 30% exhibiting poor fertility potential. While we did note a small and statistically insignificant increase in DFI during the study time, examination of Figures 1,2 reveal that our normal men kept a relatively stable value over time with men infrequently crossing over this 15% threshold. Only 25% (5/20) of men in this study experienced a decrease in DFI with increased ejaculation, strongly refuting our hypothesis. The biggest net change in DFI occurred in one subject with a decrease from 27% (near poor prognostic category) to 14% (normal).
The OSA is a biomarker which quantifies reactive oxygen species damage by measuring covalently bonded modifications in semen. These bonded adducts may take the form of lipids, DNA and proteins. Previous work funded by Reprosource has shown an increased value in infertile men (9) with OSA scores correlating with DFI values in infertile men (10).
HDS is the least studied of the DNA integrity tests examined in this publication. This is a calculated value which identifies sperm with immature chromatin and has historically been correlated with immature sperm. Others have defined a normal cut off of less than 7.5% (28) we used our laboratory’s reference range of 15%. During our study a statistically insignificant trend towards worsening (increasing) HDS was observed, but only one man achieved a value above 15%, and he began the study above this level.
When men were grouped according to changes in DFI with daily ejaculation, we found that men who had a worsening DFI during the study period were more likely to have a lower initial concentration and TMC (Table 2). While the ramifications and causality of this finding is unclear, further study could investigate if men with low sperm counts should be considered to avoid frequent ejaculation, not just for maintenance of adequate sperm counts, but also to maintain DNA integrity.
The primary limitation of this study is the overall small sample size and correspondingly lower statistical power this generates. We undertook this study as a pilot study (proof of concept) and certainly studies with more subjects and more elaborate end points could be justified based on this data. While subjects were carefully counseled on the study design with attestation to daily ejaculation, we could not absolutely assure daily ejaculation and daily semen analyses during the study would have excessively increased the study cost. Future similar studies with larger numbers of enrollees, especially in oligospermic men, including measurement of day 2 semen parameters are needed to solidify clinically meaningful recommendations to couples.
Conclusions
While a small study, this represents the first examinations of semen quality with daily ejaculations. Initial decreases were seen in TMC along with a decrease in semen volume and sperm concentration with a subsequent plateau. There were no changes in DNA integrity markers with daily ejaculation. In context, these findings generally support an approach of a short period of abstinence followed by daily copulation around ovulation to maximize the number of sperm available and optimize conception.
Acknowledgements
The authors would like to thank the Division of Urology at Southern Illinois University School of Medicine for providing the patient compensation and funding for running the semen analyses. Markers of DNA integrity were processed pro bono by ReproSource Inc.
Footnote
Conflicts of Interest: Charles Welliver received honoraria from American Society of Andrology, and is a consultant of Coloplast, an investigator of Antares, NexMed, Auxilium, Sophiris, Procept BioRobotics, an employee (brother) of Bristol-Meyers Squib. Benjamin Leader and Edna Tirado are employees of Reprosource, Inc. Tobias S. Köhler is a consultant of American Medical Systems, Coloplast, Lipocine, Abbvie, a speaker of Auxilium, Allergan, an investigator of AbbVie, AMS, Hollister. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Internal Review Board of #13-691 and written informed consent was obtained from all patients.
References
- World Health Organization. WHO Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press, 1999.
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2013;100:631-7. [Crossref] [PubMed]
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995;333:1517-21. [Crossref] [PubMed]
- Poland ML, Moghissi KS, Giblin PT, et al. Variation of semen measures within normal men. Fertil Steril 1985;44:396-400. [Crossref] [PubMed]
- Freund M. Interrelationships among the characteristics of human semen and factors affecting semen-specimen quality. J Reprod Fertil 1962;4:143-59. [Crossref] [PubMed]
- Barrett JC. Fecundability and coital frequency. Popul Stud (Camb) 1971;25:309-13. [Crossref] [PubMed]
- Schwartz D, Macdonald PD, Heuchel V. Fecundability, coital frequency and the viability of Ova. Popul Stud (Camb) 1980;34:397-400. [Crossref] [PubMed]
- Evenson DP. Flow cytometric analysis of male germ cell quality. Methods Cell Biol 1990;33:401-10. [Crossref] [PubMed]
- Berookhim B, Leader B, Copperman A, et al. Characterization of a novel marker of oxidative stress in men from 774 infertile couples. Fertil Steril 2011;96:S165. [Crossref]
- Berookhim B, Leader B, McElyea B, et al. 2189 The oxidative stress adduct (OSA) test correlates with DNA fragmentation index in men from infertile couples. J Urol 2011;185:e877-878. [Crossref]
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231-45. [Crossref] [PubMed]
- Padova G, Tita P, Briguglia G, et al. Influence of abstinence length on ejaculate characteristics. Acta Eur Fertil 1988;19:29-31. [PubMed]
- De Jonge C, LaFromboise M, Bosmans E, et al. Influence of the abstinence period on human sperm quality. Fertil Steril 2004;82:57-65. [Crossref] [PubMed]
- Levitas E, Lunenfeld E, Weiss N, et al. Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples. Fertil Steril 2005;83:1680-6. [Crossref] [PubMed]
- Thompson JA, Love CC, Stich KL, et al. A Bayesian approach to prediction of stallion daily sperm output. Theriogenology 2004;62:1607-17. [Crossref] [PubMed]
- Allen WR, Wilsher S. The influence of mare numbers, ejaculation frequency and month on the fertility of Thoroughbred stallions. Equine Vet J 2012;44:535-41. [Crossref] [PubMed]
- Carrell DT, Liu L, Peterson CM, et al. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch Androl 2003;49:49-55. [Crossref] [PubMed]
- Brahem S, Mehdi M, Landolsi H, et al. Semen parameters and sperm DNA fragmentation as causes of recurrent pregnancy loss. Urology 2011;78:792-6. [Crossref] [PubMed]
- Robinson L, Gallos ID, Conner SJ, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod 2012;27:2908-17. [Crossref] [PubMed]
- Zini A, Boman JM, Belzile E, et al. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod 2008;23:2663-8. [Crossref] [PubMed]
- Spanò M, Bonde JP, Hjøllund HI, et al. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril 2000;73:43-50. [PubMed]
- Feijó CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril 2014;101:58-63.e3. [Crossref] [PubMed]
- Kodama H, Yamaguchi R, Fukuda J, et al. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril 1997;68:519-24. [Crossref] [PubMed]
- Raziel A, Friedler S, Schachter M, et al. Influence of a short or long abstinence period on semen parameters in the ejaculate of patients with nonobstructive azoospermia. Fertil Steril 2001;76:485-90. [Crossref] [PubMed]
- Gosálvez J, González-Martínez M, López-Fernández C, et al. Shorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculate. Fertil Steril 2011;96:1083-6. [Crossref] [PubMed]
- Suganuma R, Yanagimachi R, Meistrich ML. Decline in fertility of mouse sperm with abnormal chromatin during epididymal passage as revealed by ICSI. Hum Reprod 2005;20:3101-8. [Crossref] [PubMed]
- Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 2003;79:829-43. [Crossref] [PubMed]
- Bujan L, Walschaerts M, Moinard N, et al. Impact of chemotherapy and radiotherapy for testicular germ cell tumors on spermatogenesis and sperm DNA: a multicenter prospective study from the CECOS network. Fertil Steril 2013;100:673-80. [Crossref] [PubMed]