Off label therapies for testosterone replacement
Introduction
Hypogonadism, a disease state characterized by low testosterone levels, is typically treated with testosterone replacement when the etiology is secondary to testicular pathologies. As with any therapy, clinicians must weigh the risks and benefits of each agent at their disposal. Exogenous testosterone replacement is the most commonly studied treatment in adult onset hypogonadism. While the merits of testosterone therapy are hotly debated amidst the controversy related to the potential risks, the practicing clinician is challenged by the limited information available on alternative androgen replacement therapies in this disease process. There is an assortment of medications that act on a wide array of locations on the normal hypothalamic-gonadal axis that can alter testosterone production. These medications are used for a variety of reasons in medicine and since there is no specific FDA approval for use in hypogonadism, they are considered “off label” when prescribed for men with low testosterone. The most important distinction between this group of medications and testosterone replacement is that they alter endogenous testosterone production and thus can be considered restorative rather than replacement therapy.
Adult onset hypogonadism
The diagnosis of adult onset hypogonadism consists of clinical and biochemical deficiencies of testosterone. The dysfunction can be caused by testicular or hypothalamic-pituitary dysfunction. It is important to understand how the axis operates as our treatment options target various parts of the hormone axis. Primary hypogonadism is classically defined by low testosterone and elevated gonadotropins. This suggests a dysfunction or failure within the testicle itself. Causes of primary hypogonadism include testicular infection, infarction, testicular cancer, gonadotoxic medications including chemotherapy, orchiectomy, trauma, and Klinefelter’s syndrome. Precise diagnosis of these primary testicular disorders is critical since therapies for classic secondary hypogonadism that are aimed at boosting gonadotropins would characteristically not be beneficial in this subgroup.
According to the European Male Aging Study (EMAS), 85% of hypogonadal men are classified as secondary (1). Secondary hypogonadism, also referred to as centrally mediated hypogonadism, signifies a disruption in the hormonal axis classically at the hypothalamic and pituitary level. This results in low testosterone and low or inappropriately low gonadotropin levels. Interestingly, a majority of men diagnosed with secondary hypogonadism have an unknown etiology. In a study done by Corona et al. (2), it was discovered that 89% of secondary hypogonadal men had an unknown etiology and a majority also carried a diagnosis of metabolic disease which incorporated obesity, type 2 diabetes mellitus or metabolic syndrome.
Testosterone complications
As awareness continues to grow for testosterone deficiency in the media as well as the health care community, a clear trend demonstrating a rise in testosterone replacement treatment is seen. Between 2001 and 2011, prescriptions for TRT among men 40 years of age or older in the US increased more than threefold, from 0.81% in 2001 to 2.91% in 2011 (3,4). The use of testosterone replacement has been shown to increase serum testosterone to physiologic levels, improve libido, improve erectile dysfunction, improve overall sexual function, increase energy, improve mood, increase bone mineral density (BMD), decrease body fat mass, and increase lean body muscle mass (5). Testosterone effects on cardiovascular health continue to be debated and are controversial. The exact mechanism of testosterone effects on cardiovascular protection has yet to be elucidated, but a number of peer-reviewed articles have shown that reduced serum testosterone is associated with increased risk of cardiovascular death and adverse events (6,7). Questions regarding potential cardiovascular toxicity of testosterone replacement remain and recent literature has not been able to provide a definitive scientifically sound answer. Two meta-analyses found no differences in cardiovascular events between testosterone replacement and placebo groups (8,9). An additional meta-analysis has reported testosterone replacement to be significantly associated with an increased risk of cardiovascular disease (10). There are other controversies regarding the role of testosterone replacement in men with (or at risk for) prostate cancer and those with metabolic syndrome that are outside the scope of this review, although it is again noteworthy that all of these studies have been done with exogenous testosterone replacement. The medications reviewed in this manuscript raise endogenous testosterone levels through the hypothalamic pituitary axis and are considered off label use by the FDA. There is a paucity of reliable data on whether testosterone restoration is as effective as testosterone replacement in the resolution of hypogonadal symptoms.
Clomiphene citrate (CC)
Though not FDA approved for treatment of male hypogonadism selective estrogen receptor modulators have gradually made their way into the mainstream of treatment modalities for male infertility and hypogonadism. Originally an agent for female infertility and hyper estrogen states, CC exerts its effect on the hypothalamus and the pituitary gland. Acting as an antagonist on these target organs, it will increase endogenous hormones such as gonadotropin-releasing hormones (GnRH), luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Observation of these relatively consistent and reproducible properties in female infertility patients led to studies evaluating the role of CC as a potential treatment for infertile men. CC has a favorable side effect profile even after two years of use and is increasingly evaluated in more long-term studies. Adverse events on BMD were an early concern, but one study of patients on CC greater than 12 months showed improved bone densitometry scores throughout the 3-year follow up period (11). In contrast to pure exogenous testosterone therapy, CC offers the advantage of not adversely affecting seminal parameters in symptomatic hypogonadal men seeking to maintain fertility. Demonstrating a potential knowledge gap and need for further educational projects, it is indeed alarming to note that a recent American Urological Association study indicated that 25% of urologists responding to a survey use testosterone as a treatment modality for infertility (12).
The ability of CC to improve hormone panels is well supported in the literature. Shabsigh et al. demonstrated that low dose CC (25 mg daily) significantly raised the testosterone level in young men presenting with a testosterone of less than 300 ng/dL (13). The biochemical efficacy is replicated by other studies (11,14). Clomiphene actually is a mixture of two diastereoisomers: zuclomiphene and enclomiphene. Recent studies looking at the trans isomer, enclomiphene, have shown its ability to increase testosterone levels while preserving sperm concentrations at a normal level (15,16). Support for CC and its isomers as potential therapies for addressing hypogonadal symptoms has been documented in observational and retrospective studies. Subjective symptom relief documented as an improvement in the androgen deficiency in aging males (ADAM) questionnaire has been used as a clinical guide to evaluate symptoms of hypogonadism. Developed by Morley in 2000, recent studies have demonstrated a lower sensitivity and specificity than originally estimated (17), but the questionnaire is nonetheless used widely in clinical practice. Taylor and Levine observed that men taking clomiphene had a significant improvement in their ADAM score. In this study, the authors further demonstrated that the improvement in biochemical parameters were similar to that of exogenous testosterone at a much lower cost to the patient (14). In a prospective study done by Katz et al., ADAM scores improved in all but one question (i.e., change in height) (18). There was improvement among 90% of participants in at least one symptom and among 60% in at least three symptoms. The design and questionnaire nature of the studies notwithstanding, the findings of these studies are still noteworthy both from a clinical efficacy as well as economic/financial standpoint.
Aromatase inhibitors (AI)
Men with estrogen deficiency caused by a mutation in the CYP19 gene suffer from low BMD, unfused epiphyses, and have high gonadotropin and testosterone levels. Conversely, estrogen excess has been associated with premature closure of the epiphyses and gynecomastia as well as low gonadotropin and testosterone levels (19). Estrogen effects on the gonadotropins and testosterone have led to the evaluation of estrogen as a potential target for treating hypogonadism.
Estradiol is the most potent form of estrogen and its synthesis is a result of the enzymatic activity of aromatase. Aromatase activity has been found in the gonads, placenta, brain, fat, hair, bone, muscle and vascular tissue (19). The conversion of testosterone to estradiol in the gonads has been predicted to be the cause of an increased testosterone/estradiol (T/E) ratio. This ratio has been implicated as one of the causes of infertility. Decreased seminal T/E levels have been shown to be a good indicator for identifying the absence of sperm (20).
AIs are classified as steroidal or nonsteroidal and by their generation. Anastrozole and letrozole are examples of third generation non-steroidal AIs originally used in breast cancer therapy that are generally well tolerated. Much of the data on side effects is reserved for female use and breast cancer. Although long term data are lacking, AIs do not appear to adversely affect lipid profiles, inflammatory markers of cardiovascular risk or insulin resistance when used to enhance androgen levels in hypogonadal men (21). One area that has been well studied is the effect of AIs on bone health. Bone health may benefit from increased testosterone, but may also deteriorate from lack of estradiol. The literature is unclear as to which plays a more prominent role in men (22-24). It has been shown that a modest decrease in estradiol and an increase in androgen are associated with a decrease in posterior-anterior spine BMD as measured by dual energy X-ray absorptiometry (DEXA) (22). It is unclear at this point whether higher increases in testosterone via aromatase inhibition would help counteract the negative consequences of lower estradiol.
The lowering of estradiol by AIs has been shown to increase the levels of LH, FSH and testosterone (25). Several placebo controlled studies have been documented showing statistically significant increases in biochemical parameters (25,26). While the literature is robust with improvement of hormone levels, there is a lack of data showing clinical improvement. One randomized, placebo controlled study by Loves et al looked at clinical and psychologic changes with obese, hypogonadal men who were give placebo or letrozole. In this study, the authors confirmed increases in testosterone but failed to reach any of their other clinical endpoints. These endpoints include psychological and metabolic parameters, including HbA1c, insulin sensitivities, body composition and exercise capacity (26). It was thus hypothesized that the lack of change in clinical parameters may be the result of a decrease in estradiol with AIs. The clinical significance of estradiol is most strongly elucidated by observing men with aromatase deficiency, who by nature of the condition have undetectable estradiol. These men will exhibit non-fusion of the epiphyses, have excess abdominal fat, reduced BMD, elevated triglyceride levels, low HDL, hepatic steatosis, and insulin resistance (27). This highlights the fundamental difference between AIs vs. exogenous testosterone, as the latter will cause a rise in both estradiol and testosterone levels.
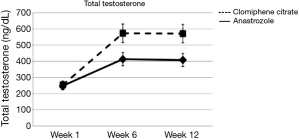
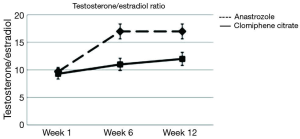
There has been very limited data on comparing selective estrogen receptor modulators and AIs for hypogonadal men. In a randomized, double blinded study the researchers demonstrated that clomiphene resulted in significantly higher testosterone levels than anastrozole (28). Increase in testosterone was 130% in the clomiphene group vs. 69% in the anastrozole group (Figure 1). Only the anastrozole group showed a significant increase in the T/E ratio although the clomiphene group did show improvement in this ratio (Figure 2). When it comes to subjective clinical improvement the quantitative ADAM scores showed no overall change but there was a small positive trend to improvement in both arms of treatment.
Human chorionic gonadotropins (HCG)
HCG was originally used for the treatment of female infertility by promoting follicular maturation and the progression of the immature oocyte. As adult onset hypogonadism is due to disruption in the central endocrine axis, it has been shown that HCG acts similarly to LH in this pathway. These observations have been confirmed by Kaufmann et al when it was demonstrated that the pituitaries of older men still have the ability to respond to gonadotropins despite lower baseline levels and surges from the hypothalamus (29). Reasons to use HCG over exogenous testosterone have been studied, but the most predominant application appears to be in the hypogonadal man seeking to maintain fertility. HCG along with human menopausal gonadotropin (HMG) has been found to induce and maintain spermatogenesis by increasing intratesticular testosterone. Researchers have discovered that HCG therapy alone would lead to decreasing sperm counts indicating FSH is essential for normal spermatogenesis (30). HCG has also been one of the few combatants in iatrogenic azoospermia. Anabolic steroid induced male infertility is a frequently observed problem in some bodybuilders and young men seeking to enhance muscle bulk. Turek et al. first reported on the use of HCG as a successful treatment modality in an azoospermia anabolic steroid abuser (31). Another area where HCG has shown promise is in concomitant use with exogenous testosterone. Coviello et al. performed a study in young healthy men where they observed that men placed on exogenous testosterone and HCG was able to maintain normal levels of intratesticular testosterone (32). Taking this further, Hsieh et al showed that men treated with concomitant low dose HCG (500 IU every other day) and exogenous testosterone maintained normal semen parameters (33). Nine of the 26 subjects contributed to pregnancy during the study follow up.
As with other off label medications for testosterone replacement, the efficacy of HCG is not well replicated in treating symptomatic adult onset hypogonadism. One study demonstrating support for HCG as a treatment modality for hypogonadism was conducted on healthy Australian men. In this randomized double blind placebo study, 40 men received either 250 µg (5,000 IU) r-hCG or placebo. Their primary endpoint was a 20% increase in muscle mass measured as peak torque (PT) by isokinetic dynamometry in the knee and shoulder joints. The investigators were unable to reach the study’s primary end point, but they demonstrated a significant increase in lean body mass, testosterone and estradiol. There was no difference in groups when it came to sexual function, PSA or International Prostate Symptoms Score (IPSS) (34). In a recent comparison study done on HCG and different forms of testosterone, it was noted that HCG leads to a lesser increase in estrogen than exogenous testosterone. The authors also noted an increase in vitamin D which they hypothesized may be the reason for maintenance of semen parameters. In this study there were significant changes in the International Index of Erectile Function (IIEF-5) and Aging Male Symptom Score (AMS) but no difference between the groups. IPSS scores were not changed with exogenous testosterone or HCG preparations (35). As there are definite changes seen with the use of HCG, its use is tempered by the lack of large, well-designed efficacy studies.
Summary
The prevalence of hypogonadism (23.3%) is higher than previously suspected (36). As our ability to better diagnose and recognize this complex disease process improves, clinicians will understandably seek to improve the safety and efficacy of the existing treatment modalities. The ability to restore testosterone rather than replace it may change the way the aging male will pursue his health in the golden years. We have reiterated that these therapies are not currently FDA approved for primary or adult-onset hypogonadism. The studies reviewed here show efficacy and safety, but the effects of long term use remain to be elucidated. These treatments add to the armamentarium of andrologists for treating male hypogonadism when there are concerns about exogenous testosterone therapy such as the scenario in the case of a hypogonadal male seeking to preserve spermatogenic potential. Novel agents and future research on safety and efficacy will hopefully provide more options and a brighter future for our patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 2008;93:2737-45. [Crossref] [PubMed]
- Corona G, Vignozzi L, Sforza A, et al. Risks and benefits of late onset hypogonadism treatment: an expert opinion. World J Mens Health 2013;31:103-25. [Crossref] [PubMed]
- Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 2013;173:1465-6. [Crossref] [PubMed]
- Grech A, Breck J, Heidelbh J. Adverse effects of testosterone replacement therapy: an update on the evidence and controversy. Ther Adv Drug Saf 2014;5:190-200. [Crossref] [PubMed]
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536-59. [Crossref] [PubMed]
- Corona G, Rastrelli G, Vignozzi L, et al. Testosterone, cardiovascular disease and the metabolic syndrome. Best Pract Res Clin Endocrinol Metab 2011;25:337-53. [Crossref] [PubMed]
- Jackson G, Montorsi P, Adams MA, et al. Cardiovascular aspects of sexual medicine. J Sex Med 2010;7:1608-26. [Crossref] [PubMed]
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010;95:2560-75. [Crossref] [PubMed]
- Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 2005;60:1451-7. [Crossref] [PubMed]
- Xu L, Freeman G, Cowling BJ, et al. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013;11:108. [Crossref] [PubMed]
- Moskovic DJ, Katz DJ, Akhavan A, et al. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int 2012;110:1524-8. [Crossref] [PubMed]
- Ko EY, Siddiqi K, Brannigan RE, et al. Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol 2012;187:973-8. [Crossref] [PubMed]
- Shabsigh A, Kang Y, Shabsign R, et al. Clomiphene citrate effects on testosterone/estrogen ratio in male hypogonadism. J Sex Med 2005;2:716-21. [Crossref] [PubMed]
- Taylor F, Levine L. Clomiphene citrate and testosterone gel replacement therapy for male hypogonadism: efficacy and treatment cost. J Sex Med 2010;7:269-76. [Crossref] [PubMed]
- Kim ED, McCullough A, Kaminetsky J. Oral enclomiphene citrate raises testosterone and preserves sperm counts in obese hypogonadal men, unlike topical testosterone: restoration instead of replacement. BJU Int 2016;117:677-85. [Crossref] [PubMed]
- Kaminetsky J, Werner M, Fontenot G, et al. Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone gel. J Sex Med 2013;10:1628-35. [Crossref] [PubMed]
- tínez-Jabaloyas JM, Queipo-Zaragozá A, Rodríguez-Navarro R, et al. Relationship between the Saint Louis University ADAM questionnaire and sexual hormonal levels in a male outpatient population over 50 years of age. Eur Urol 2007;52:1760-7.
- Katz DJ, Nabulsi O, Tal R, et al. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int 2012;110:573-8. [Crossref] [PubMed]
- de Ronde W, de Jong FH. Aromatase inhibitors in men: effects and therapeutic options. Reprod Biol Endocrinol 2011;9:93. [Crossref] [PubMed]
- Zhang Q, Bai Q, Yuan Y, et al. Assessment of seminal estradiol and testosterone levels as predictors of human spermatogenesis. J Androl 2010;31:215-20. [Crossref] [PubMed]
- Dougherty RH, Rohrer JL, Hayden D, et al. Effect of aromatase inhibition on lipids and inflammatory kers of cardiovascular disease in elderly men with low testosterone levels. Clin Endocrinol (Oxf) 2005;62:228-35. [Crossref] [PubMed]
- Burnett-Bowie SA, McKay EA, Lee H, et al. Effects of aromatase inhibition on bone mineral density and bone turer in older men with low testosterone levels. J Clin Endocrinol Metab 2009;94:4785-92. [Crossref] [PubMed]
- Center JR, Nguyen TV, Sambrook PN, et al. Hormonal and biochemical parameters in the determination of osteoporosis in elderly men. J Clin Endocrinol Metab 1999;84:3626-35. [PubMed]
- Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 1997;12:1833-43. [Crossref] [PubMed]
- T'Sjoen GG, Giagulli VA, Delva H, et al. Comparative assessment in young and elderly men of the gonadotropin response to aromatase inhibition. J Clin Endocrinol Metab 2005;90:5717-22. [Crossref] [PubMed]
- Loves S, de Jong J, van Sorge A, et al. Somatic and psychological effects of low-dose aromatase inhibition in men with obesity-related hypogonadotropic hypotestosteronemia. Eur J Endocrinol 2013;169:705-14. [Crossref] [PubMed]
- Jones ME, Boon WC, Proietto J, et al. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab 2006;17:55-64. [Crossref] [PubMed]
- Helo S, Ellen J, Mechlin C, et al. A Randomized Prospective Double-Blind Comparison Trial of Clomiphene Citrate and Anastrozole in Raising Testosterone in Hypogonadal Infertile Men. J Sex Med 2015;12:1761-9. [Crossref] [PubMed]
- Kaufman JM, Giri M, Deslypere JM, et al. Influence of age on the responsiveness of the gonadotrophs to luteinizing hormone-releasing hormone in males. J Clin Endocrinol Metab 1991;72:1255-60. [Crossref] [PubMed]
- Depenbusch M, von Eckardstein S, Simoni M, et al. Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with human chorionic gonadotropin alone. Eur J Endocrinol 2002;147:617-24. [Crossref] [PubMed]
- Turek PJ, Williams RH, Gilbh JH 3rd, et al. The reversibility of anabolic steroid-induced azoospermia. J Urol 1995;153:1628-30. [Crossref] [PubMed]
- Coviello AD, Matsumoto AM, Bremner WJ, et al. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab 2005;90:2595-602. [Crossref] [PubMed]
- Hsieh TC, Pastuszak AW, Hwang K, et al. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol 2013;189:647-50. [Crossref] [PubMed]
- Liu PY, Wishart SM, Handelsman DJ. A double-blind, placebo-controlled, randomized clinical trial of recombinant human chorionic gonadotropin on muscle strength and physical function and activity in older men with partial age-related androgen deficiency. J Clin Endocrinol Metab 2002;87:3125-35. [Crossref] [PubMed]
- La Vignera S, Condorelli RA, Cimino L, et al. Late-onset hypogonadism: the advantages of treatment with human chorionic gonadotropin rather than testosterone. Aging Male 2016;19:34-9. [Crossref] [PubMed]
- Tajar A, Forti G, O'Neill TW, et al. Characteristics of secondary, priy, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010;95:1810-8. [Crossref] [PubMed]


