
A narrative review: evaluation and surgical management of persistent and recurrent urinary incontinence after previous surgical treatment
Introduction
Urinary incontinence after prostate treatment (IPT) can be a devastating complication of radical prostatectomy or cavitating prostate surgery that has long-term ramifications on patient quality of life. Surgical treatments performed most commonly for IPT include insertion of a urethral sling or artificial urinary sphincter (AUS).
IPT prevalence following radical prostatectomy varies widely within the literature from 0.8–87%, partially due to definition variation as to what was considered urinary incontinence for the purposes of the studies (1-4). The American Urology Association (AUA) within their 2018 treatment guideline used a definition of continence as not requiring a pad to stay dry (1,5), however other definitions are commonly used in the literature, leading to wide heterogeneity between publications (2). It is generally understood that most men will have a degree of urinary incontinence on removal of their in-dwelling catheter after a radical prostatectomy, but the majority of men will regain continence by 12 months as described by the ProtecT trial reporting only 17% of men 6-year post radical prostatectomy were using continence pads (2,6). Guidelines recommend surgeons discuss options for operative treatment with patients if bothersome urinary incontinence persists beyond 6–12 months despite conservative measures (5,7,8).
Approximately 1–10% of patients with IPT proceed to surgical intervention (8). Quality of life outcomes are generally excellent after urethral sling and AUS insertion. However, revision surgery for persistent or recurrent urinary incontinence, device failure or less commonly device infection can occur and management thereafter in some cases may be quite complex. There is a paucity of high-level evidence in the literature to guide surgeons in reconstructive approaches (9-12).
In this narrative review, we outline recent evidence on the management of persistent and recurrent urinary incontinence following urethral sling or AUS insertion for men with IPT. We present this article in accordance with the Narrative Review reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-759/rc).
Methods
A comprehensive search of the literature was undertaken using online databases PubMed, MEDLINE, and Google Scholar to create this narrative review (Table 1). Publications from peer-reviewed journals written in English from years 2010–2023 were reviewed. The search strategy included the following MeSH terms: device, men, urinary incontinence, persistence, recurrence, and revision. A total of 140 articles were identified and 68 were included in this review. Abstracts were reviewed by the corresponding author for relevance, and summarised following peer-review performed by the authorship group.
Table 1
| Items | Specification |
|---|---|
| Date of search | 26/01/2022 |
| Databases and other sources searched | PubMed, MEDLINE, Google Scholar |
| Search terms used | Device, men, urinary incontinence, persistence, recurrence, and revision |
| Timeframe | 2010–2023 |
| Inclusion criteria | Meta-analysis, systematic reviews, randomised controlled trials, observational studies, narrative reviews. English language articles |
| Selection process | Search conducted by primary author independently with abstracts reviewed for relevance, followed by summary via consensus opinion amongst authorship group |
Incontinence post prostate treatment
IPT in males can be caused by bladder dysfunction, sphincter deficiency, or a combination of the two (7). Pre-operative risk factors for IPT include advanced age, increased body mass index, pre-existing lower urinary tract symptoms, increased prostate volume, oncological factors, and surgical factors (13,14). Surgical techniques used to help avoid risk of IPT following radical prostatectomy include preserving membranous urethral length, avoiding extensive dissection, and restoring pelvic anatomy (13).
Men presenting with IPT should undergo thorough clinical evaluation to determine the aetiology of their symptoms. IPT is due to external sphincter deficiency in over two thirds of patients, whereas isolated bladder dysfunction caused by detrusor overactivity/underactivity or poor compliance is found in less than 10% of cases (15). If IPT is due to stress urinary incontinence, the most common operative interventions performed include insertion of a urethral sling or placement of an AUS, the choice of which is determined by clinical factors in conjunction with patient preference. Whilst the AUS is well regarded as the gold-standard in management of IPT, the recently published MASTER trial completed by Abrams et al. identified similar continence outcomes for the AUS compared with male urethral slings (12). There are however circumstances in which one approach may be favoured over another. Within our center, an AUS insertion is preferred in men with 24-hour pad weights over 300 g and history of either pelvic radiotherapy or urethroplasty, whereas urethral slings are favoured for patients with lower volume leakage and/or manual dexterity that prohibits the use of a manually operated device.
Slings
Insertion of a sling is a surgical treatment option for mild-moderate stress urinary incontinence (5,7,8). Slings can be categorised as either fixed or adjustable.
Fixed slings can be further classified as repositioning (AdVancetm and AdVanceXP®) or compressive (Virtue® and iStop TOMStm) slings according to mechanism of action.
The synthetic transobturator sling is a repositioning sling that aims to relocate the urethral bulb 2–4 cm proximally into the pelvis allowing for dynamic compression as opposed to urethral obstruction (see Figure 1), which has been appreciated on ultrasound (16,17). As hypothesised by Rehder et al. support of the urethral bulb may also indirectly contribute to continence following sling insertion (18). The AdVanceXP® is the newer product brought to market (Boston Scientific, Marlborough, USA) with additional features including a chevron tissue anchoring mechanism aimed at improving tissue fixation and preventing sling migration and longer mesh arms for ease of use in larger patients (19,20). A prospective, multicenter study by Grabbert et al. with extended follow-up beyond 4 years of 115 patients identified a cure rate of 71.7%, and only 13.3% reported no improvement post sling insertion (21).
Urethral sling placement in the context of previous pelvic radiotherapy is associated with decreased efficacy and increased rates of complication compared to sling insertion in patients without prior history of pelvic radiotherapy (17). Patients within our center that have received pelvic radiotherapy are counselled accordingly and encouraged to consider alternative options such as an AUS, which is advised by the AUA and EAU guidelines (5,8), unless there are factors that preclude the AUS as a suitable option.
Adjustable slings (Argus®, Remix®, and ATOMS®) provide patients and surgeons the theoretical benefit of altering the degree of urethral compression to rectify persisting or recurrent urinary incontinence post insertion (see Figure 2). Each of the above listed sling devices is unique with varying insertion procedures and mechanisms of action—as such, whilst they are each marketed as an adjustable sling, they should not be viewed as the same product. At this time, adjustable slings are not associated with a strong level of evidence due to a smaller volume of observational studies and as such, they are not currently recommended in AUA or EAU guidelines (5,8).
Only a single, small volume RCT has been completed on adjustable slings during which the Argus® device was randomised against the AdVancetm sling (22). Cure rates were similar between the two groups, however pad volume was significantly reduced within the Argus® arm (22). Systematic review of the ATOMS® involving 1,393 patients from 20 studies (13 retrospective and 7 prospective) has been conducted by Esquinas et al. and identified a mean dryness rate of 67% for patients (23). Complication rates within this meta-analysis identified an overall complication rate of 16% and a major complication rate of 3% (23). Whilst studies have demonstrated safety and efficacy with the ATOMS® device in the post-radiotherapy population, efficacy remains decreased within this patient subgroup (23).
A recently published systematic review and meta-analysis by Choiniere et al. identified 20 trials of fixed and adjustable male urethral slings including 1,956 patients, which identified an overall cure rate (defined as using <1 pad per day) of 58.6% (24). These results are similar to a review by Meisterhofer et al. comparing fixed and adjustable slings, which identified that whilst adjustable slings may yield a higher cure rate, they may be associated with higher rates of complication and explantation (25).
Overall, current AUA and EAU guidelines recommend fixed male urethral slings in the management of mild-moderate stress urinary incontinence (5,8). Slings are a viable option for patients with reasonable efficacy and safety profile, and at this stage no fixed sling is recommended in favour over another. For appropriately selected patients that are seeking to gain more durable results than those provided by urethral bulking agents, but do not wish or are unable to operate the scrotal pump of an AUS, the urethral sling may be a suitable management choice.
Artificial urinary sphincter
The AUS remains the standard of treatment in men with moderate-severe SUI (5,8). Originally suggested by Foley in 1947 (26), the first commercially available AUS, the AMS 721 (American Medical Systems, subsidiary of Boston Scientific), came to market in the 1970s (26). Continued refinement led towards production of the AMS 800, which is a three-component system of inflatable cuff, pressure regulating balloon (PRB), and control pump (see Figure 3). The AMS 800 remains the most commonly used AUS, has high volume, long-term observational data, and has only had minimal design change over the past few decades (26,27). One such innovation has been InhibiZone (Boston Scientific), which is a combination of minocycline and rifampin, being impregnated into the device components. However, unlike the penile prosthesis, evidence to date for decreased device infection has not been found (28).

Systematic review performed by Chen et al. identified 8 observational studies assessing AUS insertion for SUI and found a cure rate of 29–90.9% (29). Whilst continence rates vary widely across studies due to the heterogeneity of study protocol, outcome definition and data collection techniques, patient reported outcomes of satisfaction are consistently high (5).
Alternative options for AUS device exist including the two component ZSI 375 (Zephyr Surgical Implants, Geneva, Switzerland), which does not include a reservoir balloon (8,30). Limited publications are available within the literature to date for the ZSI 375, with a retrospective review reporting a cure rate (0–1 pads/day) of 84.4% (30). There are no randomised trials within the literature comparing different AUS devices.
The AUS is the gold standard surgical treatment for moderate to severe IPT with excellent continence and patient satisfaction rates. However, surgical revision rates are not low, due to potential for mechanical failure and longer term complications, particularly in patients who have had previous pelvic radiotherapy.
Urinary incontinence after previous sling or AUS surgery
Urinary incontinence following sling or AUS surgery can be classified as persistent (early) or recurrent (late). If persistent, the clinician should consider whether the cause is due to either undertreated SUI, mechanical device failure, post-operative complication, or storage issues such as detrusor overactivity.
Clinical evaluation includes history to differentiate between stress and urgency symptoms, urine microscopy and culture, imaging, and consideration of urodynamics and cystourethroscopy. Imaging for incontinence after sling insertion can include dynamic ultrasound to exclude sling migration (16,17), and following AUS can include ultrasound or computed tomography imaging to assess PRB and cuff volume. Some surgeons also elect to use contrast solution when inflating AUS components, in which case plain radiographs can also be used to assess device fluid volume. Cystourethroscopy can be performed to assess coaptation of the urethra and exclude device erosion.
Following evaluation, if urgency urinary incontinence is suspected, management should follow guidelines for overactive bladder (31), with the caveat of care regarding the use of intradetrusor botulinum toxin injection in patients with an AUS. Urethral instrumentation is a risk factor for urethral erosion in patients with an AUS, and we would suggest that if botulinum toxin injections are considered, they should be carried out with the AUS deactivated with as small a calibre cystoscope as possible. The authorship group would suggest use of flexible cystoscopy in this setting.
Urinary incontinence after urethral sling insertion
Persistent or recurrent urinary incontinence after sling insertion occurs in 20–65% of men in observational studies (12,32).
Urine microscopy and culture should be performed to exclude urinary tract infection. Urodynamics assessment should be considered for evaluation of UUI to assess for possibility of detrusor overactivity or poor compliance and cystourethroscopic evaluation should be performed to exclude urethral erosion, which has rarely been reported post male urethral sling insertion (17).
The management of SUI incontinence after sling placement is dependent on degree of incontinence, timing of device failure, and patient factors. If only mild incontinence is diagnosed, then consideration can be given to conservative strategies as well as periurethral bulking agents or insertion of the ProACT balloon device, for which there are small observational studies within the literature (17,33-35). Otherwise moderate to severe urinary incontinence would be better treated with either repeat sling or insertion of AUS.
There are no randomised controlled studies or systematic reviews providing consensus on treatment of incontinence after sling failure; observational studies provide data on a range of treatments which may be used (36-40). Some studies report significant benefit of AUS insertion (36), and AUS insertion is considered the gold standard treatment for severe SUI. Whereas other studies report similar outcomes can be achieved between repeat sling insertion and AUS in carefully selected patients. Patients who are more likely to have similar outcomes with repeat sling compared with AUS are patients with a mild to moderate degree of SUI, and patients who had initial success with sling, but later deterioration in sling efficacy, in comparison to patients who never had success or had early failure post sling insertion (37-40).
Urinary incontinence after artificial urinary sphincter insertion
Persistent incontinence after AUS insertion is most often associated with improper device use or accidental deactivation of the cuff (41), which may lead to overflow incontinence or inadequate coaptation of the urethra. Whilst this can be managed with patient re-education, if the patient is using the device appropriately other causes must be sought.
Procedural complications including insufficient device fluid volume and oversized cuff placement require consideration. Intraoperative flexible cystoscopy after AUS insertion to ensure appropriate coaptation of the urethra on device cycling can assist in preventing such issues. Persistent incontinence secondary to the cuff size being too large can be treated by downsizing the cuff and/or repositioning the cuff. Inadvertent placement of a cuff size too large for the diameter of the urethra has decreased significantly in the advent of the 3.5 cm cuff in 2010 (42). The pressure regulating balloon (PRB) will occasionally provide insufficient pressure to cause adequate urethral coaptation, which may occur if it is underfilled, and this can be assessed by imaging of the PRB to assess fluid volume or less commonly by pressure profilometry (43).
De novo detrusor overactivity is possible following radical prostatectomy (15). However prevalence of de novo overactive bladder following AUS insertion is not well documented in the literature. If overactive bladder is considered following clinical evaluation, pharmacotherapy can be trialed and if ongoing concern a urodynamics study can be performed to evaluate for UUI.
Urethral erosion at the AUS cuff site is a complication which can present as persistent or recurrent urinary incontinence; when it occurs early causing persistent incontinence, it is usually as a result of inadvertent urethral trauma during cuff placement (41,43). Urethral trauma should be suspected post-operatively if there is haematuria or blood emerging from the urethral meatus. Diagnosis of urethral trauma is made by urethroscopy, following which device explantation is required with urethral repair, and a period of urethral healing with an IDC.
Recurrent incontinence post artificial urinary sphincter
Device malfunction is the most common cause of recurrent incontinence post AUS insertion. Whilst in the past fluid leak from the system was the most common cause of device failure, the advent of kink-resistant tubing and a fluoro-silicone layer around the cuff leaflet has led to decreased rates of system leak (43). In modern cohorts, material fatigue of the pressure regulating balloon has become a topic of interest and further research (44-48). Bergeson et al. evaluated PRB fatigue and identified that during revision surgery, 66% of PRBs did not maintain manufacturer pressure ratings for appropriate fluid volumes, which may subsequently lead to decreased urethral coaptation (48). In a small observational study of 50 patients, Bugeja et al. identified an 85.7% continence rate following PRB exchange alone (47).
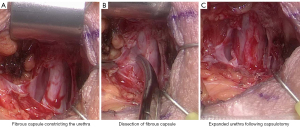
Urethral atrophy has been a well recognised cause of recurrent incontinence following AUS insertion (41,42,49), however some authors in more recent studies have challenged this theory (46-48). Bugeja et al. performed the first described capsulectomies of fibrous tissue that had formed under the urethral cuff (47). Following capsulectomy, the urethra expanded toward its original circumference. This approach has also been evaluated by Pearlman et al. (50) with a modified capsulotomy technique with similar results (see Figure 4). In the past, circumferential narrowing of the urethral tissue under the AUS cuff was thought to occur secondary to ischaemic change with subsequent fibrosis of urethral tissue, however these newer studies, whilst of small case volume, may change our understanding of the processes that underpin recurrent incontinence following AUS insertion.
Urethral erosion can cause recurrent incontinence, but may also present with haematuria, irritative voiding, or urinary retention, as well as device infection or cellulitis (41,43,51,52). Risk factors include pelvic radiotherapy, revision surgery, a smaller cuff, and placement of in-dwelling catheters or endourology procedures, particularly if the cuff had not been adequately deactivated prior to urethral instrumentation (43,52). Once diagnosis is confirmed, antibiotic therapy and explantation of the device should be performed, with urethral repair and insertion of IDC for urethral healing. Reinsertion of a new AUS should be staged for at least 3 months after explanation; with cystoscopic examination performed prior to ensure no urethral stricture development at the site of the previous cuff erosion and repair.
Revision surgery for incontinence post artificial urinary sphincter
Surgical approaches for recurrent incontinence post AUS include: urethral cuff resizing (43,49), urethral cuff repositioning (46,53), tandem urethral cuff (54-57), transcorporal urethral cuff (57-59), PRB exchange (47,60-62), and the seldom reported urethral wrap approach (49,63). There are no systematic reviews that assess best surgical technique for this scenario.
Urethral cuff downsizing is a commonly performed treatment strategy for recurrent incontinence. Development of the 3.5 cm urethral cuff was followed by decreased rates of incontinence (42,64), but also increased risk of cuff erosion and mechanical failure over time (65,66). Following the discovery of Bugeja et al. of fibrous urethral capsules (47), urethral cuff downsizing may become less common with further experience and adoption of the newer capsulotomy technique. Both cuff downsizing and the capsulotomy technique avert the need to mobilise a further segment of urethra. Repositioning the cuff requires mobilisation of a further segment of the urethra, and if performed is more favourably completed proximal to the original cuff site if possible, otherwise more distal, noting that more distally the corpus spongiosum is thinner.
O’Connor et al. (67) described the tandem urethral cuff technique, which involves an additional cuff being placed at time of AUS insertion or revision surgery. Whilst when first introduced the tandem cuff technique demonstrated comparative or favourable results compared with other approaches (53,67), more contemporary works report higher rates of complications compared to single cuff techniques (54-56). The tandem cuff requires two circumferential dissections around the urethra, and therefore can decrease corpus spongiosum vascularity in the intervening segment.
In our practice, for patients whereby urethral vascularity may be compromised and there is high risk of urethral erosion, such as in in patients that have had prior urethral reconstruction, significantly poor corpus spongiosum tissue quality in the context of pelvic irradiation and previous urethral erosion, the insertion of a transcorporally placed cuff is favoured. The transcorporal technique uses the segment of tunica albuginea immediately dorsal to the corpus spongiosum as a tissue bolster in a region in which the urethra is at its thinnest (see Figure 5). Whilst there is support within the literature from observational studies, high quality evidence is lacking and the approach carries of risk of decreased sexual function (56-58). Additionally there may be increased risk of urethral erosion at the ventral aspect of the corpus spongiosum rather than the dorsal aspect.
Replacement of the PRB can be performed as a revision method (60-62). There is no reported benefit of overfilling the PRB above manufacturer recommended standards at time of initial AUS insertion (47). PRB replacement for revision surgery has demonstrated improved post-operative continence as the initial PRB may have suffered material fatigue (47,60-62). Pearlman et al. in a small review including only seven patients that underwent capsulotomy, identified that four of six PRBs assessed during revision had pressures below the recommended manufacturer rating (50). Bergeson et al. also identified a 34.3% PRB failure rate in 177 AUS revision cases, with two-thirds of PRBs having decreased pressure (48). During AUS revision surgery for the treatment of recurrent urinary incontinence, we advocate the concomitant replacement of PRB due to the risk that material fatigue may have occurred regardless of SUI aetiology; as well as replacement of other device components. Single component exchange for devices older than 3 years is not recommended due to the risk of mechanical failure of older device components (46).
Revision due to urethral erosion
Once urethral erosion has been diagnosed, expeditious explantation of the device is important to minimise infectious sequelae. If the urethral defect is small, we proceed with primary urethral closure, which has been demonstrated to reduce risk of urethral stricture, using a 4–0 Vicryl suture in transverse orientation (to reduce stricture risk) followed by a 3-week period of urethral rest with an in-dwelling catheter. However if tissue loss from the erosion is large or circumferential, urethroplasty with substitution graft (such as buccal mucosa graft) may be performed. Following a minimum of 3 months, and exclusion of possible urethral stricture as a complication of urethral trauma, AUS re-insertion can be performed. There is no consensus within the literature as to whether standard placement or transcorporal cuff placement provides better efficacy and safety profile.
Surgical treatment options for a devastated urethra
For a devastated urethra, where urethral surgery is not feasible, urinary diversion is an option to consider to provide improved quality of life in severe urinary incontinence. This may be achieved by offering SPC urinary diversion or ileal conduit urinary diversion techniques.
Limitations and future research
Studies about management of persistent and recurrent urinary incontinence after previous surgery for IPT have largely been observational in nature with no RCT data. Future research directions may include higher case volume studies comparing surgical techniques, and the role of adjunct investigations and treatments addressing corpus spongiosum vascularity, such as the role of doppler ultrasound evaluation, or treatments such as hyperbaric oxygen therapy (HBOT) pre or post AUS revision surgery. The use of medications such as tadalafil to improve corpus spongiosum vascularity is also a topic of potential research. AUS devices with dynamic cuff pressure is an interesting area of surgical device innovation that may see decreased rates of revision surgery required in the future.
Conclusions
IPT is a bothersome condition that adversely impacts patient quality of life. Surgical management of IPT leads to marked improvement in quality of life, however persistent or recurrent urinary incontinence can occur leading to patient frustration and functional morbidity. Studies reporting surgical treatment revision strategies for persistent and recurrent incontinence lack large volume cohort numbers and randomised quality design. Whilst this makes providing an evidence based consensus treatment algorithm difficult, the authors have provided a considered appropriate approach to management. Further high quality, and higher volume studies using comparative data can improve our current understanding of surgical treatments for persistent and recurrent urinary incontinence.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Paul H Chung and Lindsay Hampson) for the series “Surgical Management of Stress Urinary Incontinence in Men” published in Translational Andrology and Urology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-759/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-759/coif). The series “Surgical Management of Stress Urinary Incontinence in Men” was commissioned by the editorial office without any funding or sponsorship. JK serves as the President of North Shore Urology Research Group. AD serves as an unpaid Senior Research Advisor of North Shore Urology Research Group. AC is a proctor for Medtronic and Boston Scientific. AC received honoraria for presentation from Medtronic and Coloplast. AC participates on Advisory Board for Coloplast and is a Member of Board of Directors for Mary’s Meals. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liss MA, Osann K, Canvasser N, et al. Continence definition after radical prostatectomy using urinary quality of life: evaluation of patient reported validated questionnaires. J Urol 2010;183:1464-8. [Crossref] [PubMed]
- Sacco E, Prayer-Galetti T, Pinto F, et al. Urinary incontinence after radical prostatectomy: incidence by definition, risk factors and temporal trend in a large series with a long-term follow-up. BJU Int 2006;97:1234-41. [Crossref] [PubMed]
- Ali M, Hutchison DD, Ortiz NM, et al. A narrative review of pelvic floor muscle training in the management of incontinence following prostate treatment. Transl Androl Urol 2022;11:1200-9. [Crossref] [PubMed]
- Silva LA, Andriolo RB, Atallah ÁN, et al. Surgery for stress urinary incontinence due to presumed sphincter deficiency after prostate surgery. Cochrane Database Syst Rev 2014;2014:CD008306. [Crossref] [PubMed]
- Sandhu JS, Breyer B, Comiter C, et al. Incontinence after Prostate Treatment: AUA/SUFU Guideline. J Urol 2019;202:369-78. [Crossref] [PubMed]
- Donovan JL, Hamdy FC, Lane JA, et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med 2016;375:1425-37. [Crossref] [PubMed]
- Abrams P, Cardozo L, Wagg A, et al. Incontinence - 6th international consultation on incontinence. Bristol: International Continence Society, 2016.
- Burkhard FC, Bosch JLHR, Cruz F, et al. EAU Guidelines on urinary incontinence in adults. Arnhem: European Association of Urology; 2020
- Nam RK, Herschorn S, Loblaw DA, et al. Population based study of long-term rates of surgery for urinary incontinence after radical prostatectomy for prostate cancer. J Urol 2012;188:502-6. [Crossref] [PubMed]
- Averbeck MA, Woodhouse C, Comiter C, et al. Surgical treatment of post-prostatectomy stress urinary incontinence in adult men: Report from the 6th International Consultation on Incontinence. Neurourol Urodyn 2019;38:398-406. [Crossref] [PubMed]
- Carson CC. Artificial urinary sphincter: current status and future directions. Asian J Androl 2020;22:154-7. [Crossref] [PubMed]
- Abrams P, Constable LD, Cooper D, et al. Outcomes of a Noninferiority Randomised Controlled Trial of Surgery for Men with Urodynamic Stress Incontinence After Prostate Surgery (MASTER). Eur Urol 2021;79:812-23. [Crossref] [PubMed]
- Heesakkers J, Farag F, Bauer RM, et al. Pathophysiology and Contributing Factors in Postprostatectomy Incontinence: A Review. Eur Urol 2017;71:936-44. [Crossref] [PubMed]
- Groutz A, Blaivas JG, Chaikin DC, et al. The pathophysiology of post-radical prostatectomy incontinence: a clinical and video urodynamic study. J Urol 2000;163:1767-70. [Crossref] [PubMed]
- Kojima Y, Takahashi N, Haga N, et al. Urinary incontinence after robot-assisted radical prostatectomy: pathophysiology and intraoperative techniques to improve surgical outcome. Int J Urol 2013;20:1052-63. [Crossref] [PubMed]
- Habashy D, Losco G, Tse V, et al. Mid-term outcomes of a male retro-urethral, transobturator synthetic sling for treatment of post-prostatectomy incontinence: Impact of radiotherapy and storage dysfunction. Neurourol Urodyn 2017;36:1147-50. [Crossref] [PubMed]
- Chung ASJ, Suarez OA, McCammon KA. AdVance male sling. Transl Androl Urol 2017;6:674-81. [Crossref] [PubMed]
- Rehder P, Staudacher NM, Schachtner J, et al. Hypothesis That Urethral Bulb (Corpus Spongiosum) Plays an Active Role in Male Urinary Continence. Adv Urol 2016;2016:6054730. [Crossref] [PubMed]
- Bole R, Hebert KJ, Gottlich HC, et al. Narrative review of male urethral sling for post-prostatectomy stress incontinence: sling type, patient selection, and clinical applications. Transl Androl Urol 2021;10:2682-94. [Crossref] [PubMed]
- Collado Serra A, Resel Folkersma L, Domínguez-Escrig JL, et al. AdVance/AdVance XP transobturator male slings: preoperative degree of incontinence as predictor of surgical outcome. Urology 2013;81:1034-9. [Crossref] [PubMed]
- Grabbert M, Mumm JN, Klehr B, et al. Extended follow-up of the AdVance XP male sling in the treatment of male urinary stress incontinence after 48 months: Results of a prospective and multicenter study. Neurourol Urodyn 2019;38:1973-8. [Crossref] [PubMed]
- Lima JP, Pompeo AC, Bezerra CA. Argus T® versus Advance® Sling for postprostatectomy urinary incontinence: A randomized clinical trial. Int Braz J Urol 2016;42:531-9.
- Esquinas C, Angulo JC. Effectiveness of Adjustable Transobturator Male System (ATOMS) to Treat Male Stress Incontinence: A Systematic Review and Meta-Analysis. Adv Ther 2019;36:426-41. [Crossref] [PubMed]
- Choiniere R, Richard PO, Morin M, et al. Evaluation of benefits and harms of surgical treatments for post-radical prostatectomy urinary incontinence: a systematic review and meta-analysis protocol. F1000Res 2019;8:1155. [Crossref] [PubMed]
- Meisterhofer K, Herzog S, Strini KA, et al. Male Slings for Postprostatectomy Incontinence: A Systematic Review and Meta-analysis. Eur Urol Focus 2020;6:575-92. [Crossref] [PubMed]
- Ratan H, Simmerton D, Wilson S, et al. Development and current status of the AMS 800 artificial urinary sphincter. EAU-EBU Update Series 2006;4:117-28. [Crossref]
- Kretschmer A, Nitti V. Surgical Treatment of Male Postprostatectomy Incontinence: Current Concepts. Eur Urol Focus 2017;3:364-76. [Crossref] [PubMed]
- Hüsch T, Kretschmer A, Thomsen F, et al. Antibiotic Coating of the Artificial Urinary Sphincter (AMS 800): Is it Worthwhile? Urology 2017;103:179-84. [Crossref] [PubMed]
- Chen YC, Lin PH, Jou YY, et al. Surgical treatment for urinary incontinence after prostatectomy: A meta-analysis and systematic review. PLoS One 2017;12:e0130867. [Crossref] [PubMed]
- Ostrowski I, Golabek T, Ciechan J, et al. Preliminary outcomes of the European multicentre experience with the ZSI 375 artificial urinary sphincter for treatment of stress urinary incontinence in men. Cent European J Urol 2019;72:263-9. [PubMed]
- Gormley EA, Lightner DJ, Faraday M, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol 2015;193:1572-80. [Crossref] [PubMed]
- Soljanik I, Becker AJ, Stief CG, et al. Repeat retrourethral transobturator sling in the management of recurrent postprostatectomy stress urinary incontinence after failed first male sling. Eur Urol 2010;58:767-72. [Crossref] [PubMed]
- Baron MG, Delcourt C, Nouhaud FX, et al. Sequential treatment with ProACT™ device implantation after male sling failure for male urinary incontinence. Prog Urol 2017;27:1098-103. [Crossref] [PubMed]
- Yiou R, Butow Z, Baron T, et al. Adjustable continence therapy (ProACT™) after male sling failure for patients with post-radical prostatectomy urinary incontinence: a prospective study with one-year follow-up. World J Urol 2015;33:1331-6. [Crossref] [PubMed]
- Munier P, Nicolas M, Tricard T, et al. What if artificial urinary sphincter is not possible? Feasibility and effectiveness of ProACT for patients with persistent stress urinary incontinence after radical prostatectomy treated by sling. Neurourol Urodyn 2020;39:1417-22. [Crossref] [PubMed]
- Ajay D, Zhang H, Gupta S, et al. The Artificial Urinary Sphincter is Superior to a Secondary Transobturator Male Sling in Cases of a Primary Sling Failure. J Urol 2015;194:1038-42. [Crossref] [PubMed]
- Martinez EJ, Zuckerman JM, Henderson K, et al. Evaluation of salvage male transobturator sling placement following recurrent stress urinary incontinence after failed transobturator sling. Urology 2015;85:478-82. [Crossref] [PubMed]
- Angulo JC, Schönburg S, Giammò A, et al. Artificial urinary sphincter or a second adjustable transobturator male system offer equivalent outcomes in patients whom required revision on the initial ATOMS device: An international multi-institutional experience. Neurourol Urodyn 2021;40:897-909. [Crossref] [PubMed]
- Angulo JC, Virseda-Chamorro M, Arance I, et al. Long-term outcome of adjustable transobturator male system for stress urinary incontinence in the Iberian multicentre study. Neurourol Urodyn 2020;39:1737-45. [Crossref] [PubMed]
- Grabbert M, Hüsch T, Kretschmer A, et al. Secondary Sling Implantation after Failure of Primary Surgical Treatment for Male Stress Urinary Incontinence: A Retrospective Study. Urol Int 2020;104:625-30. [Crossref] [PubMed]
- Chung E, Cartmill R. Diagnostic challenges in the evaluation of persistent or recurrent urinary incontinence after artificial urinary sphincter (AUS) implantation in patients after prostatectomy. BJU Int 2013;112:32-5. [Crossref] [PubMed]
- Simhan J, Morey AF, Zhao LC, et al. Decreasing need for artificial urinary sphincter revision surgery by precise cuff sizing in men with spongiosal atrophy. J Urol 2014;192:798-803. [Crossref] [PubMed]
- Dobberfuhl AD, Comiter CV. A Systematic Approach to the Evaluation and Management of the Failed Artificial Urinary Sphincter. Curr Urol Rep 2017;18:18. [Crossref] [PubMed]
- Ziegelmann MJ, Linder BJ, Avant RA, et al. Bacterial Cultures at the Time of Artificial Urinary Sphincter Revision Surgery in Clinically Uninfected Devices: A Contemporary Series. J Urol 2019;201:1152-7. [Crossref] [PubMed]
- Srivastava A, Joice GA, Patel HD, et al. Causes of Artificial Urinary Sphincter Failure and Strategies for Surgical Revision: Implications of Device Component Survival. Eur Urol Focus 2019;5:887-93. [Crossref] [PubMed]
- Terlecki RP, Wilson SK. A new paradigm for surgical revision of the artificial urinary sphincter for recurrent stress urinary incontinence: Wilson's Workshop 11. Int J Impot Res 2022;34:37-43. [Crossref] [PubMed]
- Bugeja S, Ivaz SL, Frost A, et al. Urethral atrophy after implantation of an artificial urinary sphincter: fact or fiction? BJU Int 2016;117:669-76. [Crossref] [PubMed]
- Bergeson RL, Yi YA, Baker RC, et al. Urethral atrophy is now a rare cause for artificial urinary sphincter revision surgery in the contemporary 3.5 cm cuff era. Transl Androl Urol 2020;9:50-5. [Crossref] [PubMed]
- Linder BJ, Viers BR, Ziegelmann MJ, et al. Artificial Urinary Sphincter Mechanical Failures-Is it Better to Replace the Entire Device or Just the Malfunctioning Component? J Urol 2016;195:1523-8. [Crossref] [PubMed]
- Pearlman AM, Rasper AM, Terlecki RP. Proof of concept: Exposing the myth of urethral atrophy after artificial urinary sphincter via assessment of circumferential recovery after capsulotomy and intraoperative pressure profiling of the pressure regulating balloon. Investig Clin Urol 2018;59:275-9. [Crossref] [PubMed]
- Bryan DE, Mulcahy JJ, Simmons GR. Salvage procedure for infected noneroded artificial urinary sphincters. J Urol 2002;168:2464-6. [Crossref] [PubMed]
- Wang R, McGuire EJ, He C, et al. Long-term outcomes after primary failures of artificial urinary sphincter implantation. Urology 2012;79:922-8. [Crossref] [PubMed]
- Eswara JR, Chan R, Vetter JM, et al. Revision Techniques After Artificial Urinary Sphincter Failure in Men: Results From a Multicenter Study. Urology 2015;86:176-80. [Crossref] [PubMed]
- Chertack N, Chaparala H, Angermeier KW, et al. Foley or Fix: A Comparative Analysis of Reparative Procedures at the Time of Explantation of Artificial Urinary Sphincter for Cuff Erosion. Urology 2016;90:173-8. [Crossref] [PubMed]
- Manka MG, Wright EJ. Does Use of a Second Cuff Improve Artificial Urinary Sphincter Effectiveness? Evaluation Using a Comparative Cadaver Model. J Urol 2015;194:1688-91. [Crossref] [PubMed]
- Ahyai SA, Ludwig TA, Dahlem R, et al. Outcomes of single- vs double-cuff artificial urinary sphincter insertion in low- and high-risk profile male patients with severe stress urinary incontinence. BJU Int 2016;118:625-32. [Crossref] [PubMed]
- Maurer V, Dahlem R, Rosenbaum CM, et al. Distal Double Cuff Vs Transcorporal Cuff as Salvage Options-A Prospective Analysis of Different Artificial Urinary Sphincter (AMS 800) Implantation Sites. Urology 2019;133:234-9. [Crossref] [PubMed]
- Vasan R, Myrga J, Miller D, et al. The Gullwing Technique: A Novel Method of Transcorporal Artificial Urinary Sphincter Placement for the Fragile Urethra. Urology 2022;169:237-40. [Crossref] [PubMed]
- Mock S, Dmochowski RR, Brown ET, et al. The Impact of Urethral Risk Factors on Transcorporeal Artificial Urinary Sphincter Erosion Rates and Device Survival. J Urol 2015;194:1692-6. [Crossref] [PubMed]
- Moses RA, Keihani S, Craig JR, et al. Efficacy of Pressure Regulating Balloon Exchange in Men With Post Artificial Urinary Sphincter Persistent or Recurrent Stress Urinary Incontinence. Urology 2019;123:252-7. [Crossref] [PubMed]
- Maximilien B, Aublea A, Gillibert A, et al. Urethral pressure controlled balloon refilling or balloon change for artificial sphincter secondary procedure? Prog Urol 2018;28:209-14. [Crossref] [PubMed]
- Loh-Doyle JC, Nazemi A, Ashrafi A, et al. Predictors of Device-related Complications After Exchange of the Pressure-regulating Balloon in Men With an Artificial Urinary Sphincter. Urology 2020;135:154-8. [Crossref] [PubMed]
- Trost L, Elliott D. Small intestinal submucosa urethral wrap at the time of artificial urinary sphincter placement as a salvage treatment option for patients with persistent/recurrent incontinence following multiple prior sphincter failures and erosions. Urology 2012;79:933-8. [Crossref] [PubMed]
- Kretschmer A, Buchner A, Grabbert M, et al. Risk factors for artificial urinary sphincter failure. World J Urol 2016;34:595-602. [Crossref] [PubMed]
- McKibben MJ, Shakir N, Fuchs JS, et al. Erosion rates of 3.5-cm artificial urinary sphincter cuffs are similar to larger cuffs. BJU Int 2019;123:335-41. [Crossref] [PubMed]
- Loh-Doyle JC, Hartman N, Nazemi A, et al. Mechanical failure rates of artificial urinary sphincter components: Is the 3.5-cm urethral cuff at higher risk? Neurourol Urodyn 2019;38:187-92. [Crossref] [PubMed]
- O'Connor RC, Gerber GS, Avila D, et al. Comparison of outcomes after single or DOUBLE-CUFF artificial urinary sphincter insertion. Urology 2003;62:723-6. [Crossref] [PubMed]





