
Management of male stress urinary incontinence in high-risk patients: a narrative review
Introduction
The American Urological Association (AUA) and Society of Urodynamics, Female Pelvic Medicine, and Urogenital Reconstruction (SUFU) have published contemporary guidelines for the management of urinary incontinence after prostate therapy (IPT) (1). There is a myriad of options for patients with stress urinary incontinence (SUI), including observation, absorbent wearables, pelvic floor physical therapy, penile clamps, urinary catheters, adjustable balloons, bulking agents, male slings, artificial urinary sphincters (AUS), and urinary diversion. AUS implantation remains the gold standard for patients with moderate to severe SUI, with longstanding evidence of efficacy and durability (2). In patients with a history of pelvic radiotherapy, the AUS remains the preferred management option regardless of incontinence severity; sling placement in these patients has limited efficacy and poor durability (AUA/SUFU IPT guideline statement 24 (1,3-5).
A number of risk factors have been identified to portend higher failure rates with AUS implantation. The risk factors which lead to a “fragile urethra” (or bulbar urethral compromise) have been previously defined as a history of pelvic radiation, a prior failed/eroded AUS, a prior urethroplasty, and urethral atrophy (6). These risk factors all have a common theme: vascular compromise of the bulbar urethra, whether through endarteritis or scar formation through injury or surgery. Similarly, pelvic fracture resulting in traumatic disruption of vascular flow may result in bulbar urethral compromise, resulting in sequelae for cases of incontinence requiring AUS. Herein we assess risk factors that lead to bulbar urethral compromise, bladder pathology that can yield AUS failure, and lower urinary obstruction that can pose a challenge to long-term success. If urethral or bladder pathology is present, it must be carefully considered when determining a management strategy for concomitant SUI. Preoperative cystourethroscopy is recommended in all cases by the AUA/SUFU guidelines, and it remains a critical component of the preoperative evaluation to screen for underlying posterior urethral stenosis, identify bladder pathology, and evaluate the tissue quality of the lower urinary tract for surgical planning (1). Our objective is to provide urological surgeons with a range of strategies for the evaluation, surgical treatment, and follow-up of high-risk patients undergoing first time or repeat implantation of AUS. We present this article in accordance with the Narrative Review reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-727/rc).
Methods
A review of current, English-language literature (see Table 1 for search strategy summary) was performed in PubMed utilizing the search term “artificial urinary sphincter” in conjunction with any of the following additional terms: “radiation”, “urethral stricture”, “posterior urethral stenosis”, “vesicourethral anastomotic stenosis”, “bladder neck contracture”, “pelvic fracture urethral injury”, “penile revascularization”, “inflatable penile prosthesis”, and “erosion”. Articles were screened by JSL to verify their focus on the population of interest (adult men with SUI after prostate surgery or pelvic injury), and findings from relevant articles were synthesized by JSL, AJS, and JCH; guidance is provided based upon expert opinion where existing literature was sparse or nonexistent.
Table 1
| Items | Specification |
|---|---|
| Date of search | September 1–20, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | “artificial urinary sphincter”, “radiation”, “urethral stricture”, “posterior urethral stenosis”, “vesicourethral anastomotic stenosis”, “bladder neck contracture”, “pelvic fracture urethral injury”, “penile revascularization”, “inflatable penile prosthesis”, and “erosion” |
| Timeframe | Jan 1, 1985–Sept 1, 2022 |
| Inclusion criteria | English language, all study types were included for review |
| Selection process | Selection performed by JSL, consensus with AJS and JCH |
| Any additional considerations, if applicable | Additional consideration and search were performed for concomitant bowel use with artificial urinary sphincter placement, pathophysiology of radiotherapy, and urethral fistula |
Results
A total of 920 articles were reviewed: 175 from radiation, 198 from urethroplasty/stricture, 53 from posterior stenosis, 5 from pelvic fracture urethral injury, 54 from inflatable penile prosthesis (IPP), and 387 from AUS erosion. No search results returned for penile revascularization. Twenty-seven articles from radiation, 15 from urethroplasty/stricture, 2 from posterior stenosis, 1 from pelvic fracture urethral injury, 6 from IPP, and 59 from AUS erosions were included. The following is a summary of the relevant findings and our interpretation of existing literature in these disease states. Our knowledge of the pathophysiology of pelvic fracture related injury can be applied to inform care for men with SUI after prostate surgery and/or radiation, another form of injury to the genitourinary organs. In all cases, the blood supply to the urethra may be compromised by prior injuries or treatments and can in turn lead to failure of subsequent interventions in the form of recurrent stricture disease or AUS cuff erosion.
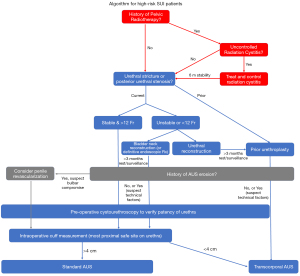
In this manuscript we have divided the discussion into three high-risk disease states: bulbar urethral compromise, bladder pathology, and lower urinary tract complications. We describe the microvascular and macrovascular consequences of AUS erosion, pelvic radiotherapy, pelvic fracture urethral injury (PFUI), urethroplasty, and low testosterone on the bulbar urethra and their consequences on device survival. We highlight important considerations in patients with bladder dysfunction or disease requiring urinary instrumentation. Furthermore, we discuss management of concomitant lower urinary tract complications: anterior urethral stricture, prostatic fossa calcifications, and posterior urethral stenosis. Finally, we synthesize the existing literature and propose an algorithm for management of these high-risk patients in Figure 1 with important considerations for follow-up after AUS implantation.
Discussion/summary
Risk factors
Bulbar urethral compromise
Prior AUS cuff erosion
Prior studies have examined risk factors associated with AUS cuff erosion, with the vast majority being single-center retrospective studies with small cohorts (7-26). Previously described risk factors include diabetes, smoking status, obesity, coronary artery disease, previous urethroplasty, history of radiation, and previous AUS cuff erosion. Pelvic radiotherapy and urethroplasty are risk factors that will be discussed in subsequent sections and other risk factors will be discussed here.
Multiple patient co-morbidities have been identified to increase risk of device failure. In two retrospective studies examining primary AUS placements, diabetes was shown to be independently associated with AUS erosion or infection on multivariable analysis with a hazard ratios ranging from 2.26–2.50. Interestingly, both groups also found increasing body mass index (BMI) to be a protective factor for erosion and infection, in otherwise healthy men (27,28). In the study by Viers et al., this was seen in patients categorized as obese (BMI ≥30.0) with a hazard ratio of 0.39. This finding trended towards significance in overweight patients (BMI between 25–30). They also noted that a greater BMI was associated with a decrease in the proportion of patients with pad use ≤1 pad/day. In the same study, coronary artery disease was found to be an independent risk factor for AUS erosion with a hazard ratio of 1.87 (27). Another study by Ortiz et al. found CAD similarly associated with a risk of erosion, with a hazard ratio of 3.7 (29). Multiple other studies have found this association on univariate analysis, however only trended towards significance in multivariate analysis (30,31). Diabetes and CAD are well known to yield chronic systemic microvascular disease, which may compromise the health of the urethra in a similar manner to that seen after pelvic radiotherapy.
Studies have also found increasing age as a factor for increasing AUS erosion (27,28,30) Ziegelmann et al. found that particularly patients older than 80 years are at a significantly increased risk of erosion, with a hazard ratio of 4.13 on multivariable analysis. Age was not a factor for mechanical failure or urethral atrophy (28). Concerning smoking as a risk facture, despite its known adverse effects on perioperative outcomes and wound healing (32-34). Godwin et al. found that current and previous smoking status did not increase rate of device complications (35).
As with other controllable medical comorbidities, the authors recommend patient optimization prior to AUS placement. Diabetes, coronary artery disease, age/fragility, and smoking affect wound healing and urethral health may be compromised by systemic microvascular disease. These factors may have a compounding effect. Patients have a much higher risk of subsequent AUS removal after a single erosion event, and thus medical optimization is critical with every AUS implant (22,36,37).
If an AUS erosion is identified, prompt device explantation and a delay of 3 to 6 months prior to AUS reimplantation is recommend (AUA/SUFU IPT guideline statement 31) (1). During device explantation, it is recommended that full explant be performed. Various studies have reported lower urinary tract sequelae after explantation, including an incidence of urethral stricture ranging from 12–61.5% (38-42). Management of the urethra varies and multiple studies have compared urethral catheter placement, suture urethrorrhaphy (also referred to as abbreviated urethroplasty or in situ urethroplasty), and excisional urethroplasty (primary urethral anastomosis) (38-40). At this time, there is no definitive or high-level evidence suggesting superior outcomes with a particular technique. However, these studies have uniformly shown that increased severity of erosion results in a greater risk of urethral stricture formation, especially with circumferential erosions. Our group recommends surgical repair of the eroded segment when possible in an effort to mitigate the risk of stricture formation.
AUS replacement after erosion is particularly challenging, with a very high risk of repeat erosion and poor long-term device survival (some groups have reported close to 50% 5-year explant-free survival) (7,11). Thus, it is critical to optimize patient factors prior to reimplantation. Cystourethroscopy around 3–6 months after device removal should be performed to confirm complete and circumferential healing of the urethra and to rule out de novo urethral stricture disease prior to AUS reimplantation.
During AUS reimplantation, TC-AUS cuff placement can be considered. El-Akri et al. have demonstrated a trend towards prolonged explant-free survival compared to bulbar AUS placement in subgroup analysis of patients with previous AUS explantation (2-year explantation-free survival: 61.9% vs. 58.2%; P=0.096) (43). Maurer et al. compared dual-cuff AUS to TC-AUS cuff in the salvage setting and found comparable perioperative outcomes including infection, erosion, mechanical failure, and explantation. Furthermore, they found equivalent objective and social continence outcomes (44).
Prior pelvic radiotherapy
Despite modern improvements in targeting of radiation delivery, such as high-linear accelerators, conformal radiation delivery, and intensity-modulated radiation therapy, bystander effects of radiation to local structures continue to pose significant challenges in the setting of urinary incontinence (45). Through these mechanisms, pelvic radiation therapy may yield microvascular compromise of bulbar urethral perfusion and have consequences on device survival. The reported incidence of AUS cuff erosion ranges from 1–13% in patients without risk factors (7,46). This erosion risk is higher among patients with a history of pelvic radiation, with rates reported as high as 33.3% (range, 3.4–33.3%) (7-15). Numerous studies have examined AUS revision and erosion in irradiated patients, with mixed results (7-23). The vast majority of these studies are single-center and retrospective in nature and often include patients with multiple comorbidities such as history of urethroplasty or previous AUS erosion, which may further increase their risk of AUS complications.
In a multi-institutional retrospective study, Kaufman et al. examined 56 patients who had an idiopathic cuff erosion. Radiated patients were found to have a faster time to erosion. In patients who had an AUS erosion, median erosion-free device survival was 1 year in irradiated patients compared to 3.15 years in non-irradiated patients (23). In another multi-institutional study, Fuller et al. examined device revision and explantation (rather than erosion specifically) in radiated and non-radiated patients. Radiated patients had a shorter median time to explant of their first (26.4 vs. 35.6 months) and second (30.1 vs. 38.7 months) AUS implants compared to non-radiated patients. This difference was not seen with the third AUS explant. Although the group examined any cuff revision or device explantation rather than AUS erosions specifically, they did find that erosion occurred more commonly in radiated patients during the first explant. This difference was not seen for second or third explants. Finally, when adjusted for covariates patients with any urethral risk factor had a compromised revision-free survival; this finding was compounded in those with multiple risk factors. In patients with a 4.0 cm cuff without risk factors, 5- and 10-year revision free survival was 83.1% and 71.9%. For radiated patients, this was reduced to 72.6% and 56.4%, respectively. In those with prior pelvic radiotherapy and urethroplasty, this was reduced further to 46.0% and 24.9% at 5 and 10 years, respectively (22).
Both studies observed that in patients with radiation, device explantation occurred much more rapidly in radiated patients compared to non-radiated patients (22,23). Fuller et al. found that regardless of radiation history, there was no significant difference in etiology (infection, erosion, or device malfunction) for patients undergoing device explantation of their second or third AUS implantation. Furthermore, median time to explant was no longer significantly different for the third AUS explant between radiated and non-radiated patients. This finding seems to show that pelvic radiotherapy plays an influential role on AUS survival with the first device implant, whereas other factors, such as prior erosion, likely grow in relative importance among those requiring revision surgeries. Stated differently, with every device revision or explant, a patient’s risk of subsequent device explantation cumulatively increases and the role pelvic radiotherapy plays as a risk factor for explantation inversely decreases. This is evidenced by the lack of difference in the median-time to explantation between radiated and non-radiated patients after the first AUS is explanted (22).
Prior pelvic fracture urethral injury
As mentioned above, radiotherapy may induce endarteritis that can potentially lead to microvascular compromise of the bulbar urethra. In contrast, traumatic injury to the urethra can lead to macrovascular compromise of the urethra, which may have consequences to device implantation. After posterior urethroplasty for pelvic fracture urethral injury (PFUI), 1.5% to 8% of patients may develop SUI, with identified risk factors including urethral injuries that extended proximally into the prostatic urethra and bladder neck (47,48). PFUI and subsequent posterior urethroplasty compromise the external urethral sphincter, resulting in a reliance on the function of the internal sphincter/bladder neck for continence. Mundy et al. have shown that up to 57% of those with proximal injury extension after urethroplasty have incontinence requiring placement of an AUS (49).
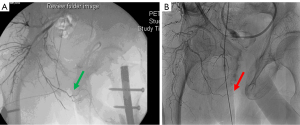
Patients surviving severe pelvic trauma may also have associated injury-related or treatment-related macrovascular compromise involving the internal iliac/internal pudendal arterial system. Cases of bulbar necrosis and early urethroplasty failure are thought to be related to this mechanism of bulbar vascular compromise, and the authors propose that inadequate perfusion in the setting of chronic extrinsic compression with a urinary sphincter cuff underlies a primary mechanism for cuff erosion risk after PFUI. Figure 2 shows angiography of two patients after pelvic fracture with associated PFUI. One demonstrates an angiogram with an intact right internal pudendal artery and the other with a truncation of the right pudendal vessel. Perfusion may also be further compromised by pelvic embolization or surgical vascular ligation when it is required to control pelvic bleeding after trauma. Several studies have shown that pelvic angioembolization is associated with genitourinary end-organ dysfunction (50-54). There is wide variability in injury patterns after pelvic fracture, and it is critical to consider the trauma, subsequent interventions, and their sequelae when evaluating SUI after PFUI.
AUS placement can be difficult after posterior urethroplasty due to scar tissue from the previous dissection, with potential fixation of the previously mobilized urethra yielding additional risk to the periurethral dissection. Previous operative notes and medical records should be carefully reviewed as maneuvers such as corporal splitting, crural rerouting, gracilis flap interposition, and post-operative urine leak can affect the patient’s urethral anatomy and tissue planes. One may consider a bladder neck AUS placement for PFUI patients with complex urethral anatomy. Alternatively, a more distal AUS cuff may be considered, however this may compromise incontinence outcomes. A transcorporal AUS (TC-AUS) cuff can be considered should dorsal dissection prove to be challenging or to avoid placing a 3.5 cm cuff, which may be prone to erosion as seen in patients with history of radiation (55).
Concerning alternative approaches to AUS placement, surgeons should cautiously weigh the potential limitations against plausible benefits in patients with prior pelvic fracture. While post-pelvic fracture erectile dysfunction (ED) is prevalent in up to 42–62% of patients, many can be managed medically and some will ultimately recover independent erectile function; for those with mild ED or normal postinjury function, TC-AUS placement could contribute to worsened or de novo ED due to compromised veno-occlusive function (56). In addition, patients with PFUI are often younger than most other populations undergoing AUS placement, which may prompt greater consideration for device longevity with bladder neck cuff placement if prior pelvic surgeries and associated injuries do not preclude it. Few studies have compared bulbar urethral AUS cuff placement to bladder neck AUS cuff placement, though Khene et al. saw a trend towards longer explant-free survival in patients with bladder neck AUS with median explant-free survival of 18.5 years in bulbar urethral AUS cuffs and 24.5 years in bladder neck cuffs (57).
Prior urethroplasty/urethral transection
Urethroplasty remains an independent risk factor for device erosion and failure. Multiple studies have consistently shown patients with a history of urethroplasty to be at a much higher risk of erosion and device removal. Sayedahmed et al. prospectively evaluated AUS outcomes after urethroplasty, excluding those with radiation and a previous AUS (58). They reviewed a cohort of 105 patients, with 30 having undergone prior urethroplasty; the overall erosion rate was 12.3%, with a 23.3% erosion rate in those with a history of urethroplasty. On univariable logistic regression analysis, previous urethroplasty conferred a higher risk of device explant with an odds ratio (OR) of 4.18; multivariable analysis was not performed due to a small number of events. The group also noted a trend toward a positive correlation between median stricture length and need for AUS explant. Median stricture length was 3.5 centimeters in the explant group compared to 1.4 in those not requiring explant (P=0.056). Mann et al. also found a history of urethroplasty was an independent risk factor for a shorter interval to erosion, with a hazard ratio of 2.12 (24). McGeady et al. found that patients with a history of urethroplasty had a higher rate of failure (device malfunction, infection, or erosion) with a hazard ratio of 8.14 when compared to patients without urethral risk factors (11). McKibben et al. found that compared to ≥4 cm cuff, in patients with 3.5 cm cuff, patients with history of urethroplasty had a higher risk of erosion with hazard ratio of 5.11 (36). Similarly, Fuller et al. found that history of urethroplasty conferred worse all-cause revision-free survival, making it a greater risk factor than radiotherapy. The 5- and 10-year revision free survival was 83.1% and 71.9% for patients without risk factors, 72.6% and 56.4% respectively for patients with a history of radiation, and even lower at 63.9% and 44.9% respectively for patients with prior urethroplasty (22).
While a history of urethral stricture (involving spongiofibrosis of the periurethral vascular sinusoids) and any prior urethroplasty likely represent a risk for AUS failure in all cases, the type of surgical repair may contribute differentially to the risk of AUS failure. Traditional anastomotic repairs disrupt dual antegrade-retrograde urethral perfusion, yielding a distal stump dependent upon retrograde blood flow and a proximal stump still maintained via antegrade perfusion. With AUS cuff placement, a segment of urethra between the prior anastomosis and the cuff site could become ischemic and more prone to breakdown due to cuff compression. Non-transecting anastomotic repairs are thought to maintain dual urethral perfusion by allowing scar excision while preserving healthy spongiosum, and this approach has been shown to yield benefits in the form of preserved sexual function (59-61). Further study is needed to investigate if this technique may decrease the risk of erosion after urethroplasty. The authors acknowledge findings by Sayedahmed et al. suggesting an increased erosion risk specifically after substitution urethroplasty. This study included only a small cohort (19 transecting anastomotic and 11 substitution urethroplasties), and the observed correlation may be a surrogate for the severity of stricture and associated spongiofibrotic vascular compromise. Patients undergoing substitution urethroplasty tend to have more complex or longer segment strictures, and Sayedahmed’s group noted that median stricture length was longer in those requiring AUS explantation (58). Non-transecting techniques and substitution repairs theoretically stand to mitigate cuff erosion risk through greater arterial preservation, although dedicated investigation is needed.
The studies identifying prior urethroplasty as a risk factor, while small in number, are nevertheless consistent in their findings. They highlight the importance of close follow-up and the continued need for strategies to mitigate failure in these high-risk patients. We recommend more frequent follow-up with cystourethroscopy to evaluate urethral mucosal quality at the cuff site at least in the first year after AUS implantation. This serves to evaluate for both urethral stricture recurrence and the health of the urethra.
Low testosterone
Systemic androgens seem to play a role in urethral health and the risk of device erosion. In a study of urethral tissue harvested during urethroplasty, patients with low testosterone (LT) were found to have decreased androgen receptor expression and significantly decreased vessel density (62). In a prospective analysis of 53 consecutive patients undergoing AUS implantation at a single-institution, Hofer et al. found low testosterone to be a significant risk factor for AUS erosion. Of the 53 patients, 20 patients (37.7%) had an AUS erosion with 90% found to have LT. In contrast, of the 33 patients without erosion, 36.4% had LT on serum assay. On multivariable logistic regression, LT remained the sole independent risk factor for AUS erosion with an odds ratio of 15.78 (95% CI: 2.77–89.92) (63). In a retrospective single-center study, Wolfe et al. examined patients with a serum testosterone level within 24 months of AUS placement. When examining patient demographic factors and surgical factors (coronary artery disease, prior AUS, radiation therapy, TC-AUS, and 3.5 cm cuff), again only LT was predictive of AUS cuff erosions on multivariable binary logistic regression analysis (31). It is important to note that these studies did not find a significant difference in the rate of AUS erosion in patients with a history of androgen deprivation therapy (ADT). Bailey et al. compared patients with greater than 6 months use of ADT within 2 years of AUS placement and found no difference in device infection, erosion, mechanical failure, or urethral atrophy (64).
While these studies do highlight low testosterone as a significant and independent risk factor for AUS erosion, it is unclear whether testosterone supplementation may prevent cuff erosion or improve device survival. In a retrospective, single-center study, nearly half of patients with preoperative serum testosterone levels had LT prior to AUS placement (65). No studies have examined testosterone supplementation prior to AUS placement. This remains an important area of future study. However, preoperative serum testosterone assays can be informative and can be important in patient counseling regarding individualized risk and device outcomes.
Bladder pathology
Radiation induced bladder pathology
Patients with radiation cystitis and urinary incontinence after pelvic radiotherapy pose a particularly challenging scenario. Management of the radiation cystitis is recommended prior to placement of an AUS. Cystoscopy with clot evacuation and fulguration, hyperbaric oxygen, and intravesical instillation of astringent agents can be utilized based upon patient needs and available resources (66). If possible, the authors pursue sustained resolution of hemorrhagic cystitis for at least 3–6 months prior to AUS placement. Should hemorrhagic cystitis prove refractory, a patient may be better served with cystectomy and urinary diversion to manage both conditions. Management of acute clot retention from radiation cystitis often entails the use of large-bore urethral catheters and endoscopic treatments, all of which can lead to urethral cuff erosion. In cases of clot retention due to radiation cystitis in the setting of an AUS, placement of a suprapubic tube or an open clot evacuation should be considered to protect the AUS cuff. If urethral access is required for intervention, device uncoupling is recommended for prolonged urethral instrumentation. Section “Need for lower urinary tract instrumentation” below provides recommendations for patients requiring urinary instrumentation in the setting of an AUS.
Urinary adverse effects from radiation damage may also include urgency, frequency, decreased bladder storage volumes, and bleeding complications from radiation cystitis. The incidence of adverse effects varies by radiation modality, however it is noted that improvements in radiation delivery have reduced the prevalence of these toxicities (67-69). Following pelvic radiotherapy, the incidence of Grade 2 or higher adverse effects ranges from 7–41%, grade 3 effects specifically ranging from 5–13%, and grade 4 effects in about 0.1% of patients (70-79). Counseling the patient about bladder function, especially radiation-induced overactive bladder is important for postoperative expectation setting (AUA/SUFU Guideline for non-neurogenic overactive bladder) (80).
If a patient has prolonged and severe urinary incontinence after radiotherapy, surgeons should evaluate the bladder capacity. Temporary use of an external penile clamp can serve as a simple screening tool for underlying poor storage function. The penile clamp mimics the basic function of an AUS cuff, allowing the bladder to cycle and potentially unmasking severe storage symptoms in those with limited capacity. Urodynamic studies (UDS) may be helpful to evaluate storage pressures and capacity before finalizing an incontinence treatment plan. Should bladder capacity be found to be severely limited or if the patient cannot tolerate bladder cycling, it is generally not recommended to pursue AUS placement. Alternatives such as chronic suprapubic cystostomy or urinary diversion should be considered. If a patient desires orthotopic diversion, orthotopic neobladder with AUS placement can be considered as described by Patil et al. (81).
Pelvic fracture related bladder dysfunction:
In patients who sustained a severe pelvic fracture, exclusion of a neurologic cause of incontinence should also be considered as patients with devastating pelvic injuries can have disruption of the pelvic nerves supplying the bladder. Lefaivre et al. prospectively assessed urinary symptoms utilizing the International Consultation Incontinence Questionnaire (ICIQ) at baseline, 6 months, 1, 2, and 5 years after surgical treatment for pelvic fracture. The group found that men had significant worsening and persistent urinary symptoms 5 years after injury. Furthermore, in men, neurologic dysfunction was found to be predictive of worse ICIQ scores (82). In patients with urinary incontinence and history of pelvic fracture, bladder function must be assessed. For example, patients who have suffered from a sacral fracture are at risk for lower motor neuron injury and pressure-flow urodynamics studies should be considered to evaluate for an atonic bladder prior to AUS placement. This is critical for counseling as patients who require clean intermittent catheterization (CIC) may be at risk of erosion.
Need for lower urinary tract instrumentation (bladder cancer, CIC, hemorrhagic cystitis)
The need for CIC or lower urinary tract instrumentation does not necessarily preclude patients from obtaining an AUS. Studies examining CIC have largely been in the pediatric population of mixed genders, with bladder neck AUS cuff implantation and often the creation of catheterizable channels to avoid urethral catheterization (83-85). One study found no erosions in 22 patients requiring CIC, with 50% requiring CIC for >30 months (86). Patients requiring surveillance and treatment for non-muscle invasive bladder cancers represent a similar challenge. Heiner et al. have shown safety of cystoscopic surveillance of 14 patients with AUS and non-muscle invasive bladder cancer. With a median follow-up of 7.2 years, only 1 patient (5.6%) experienced an iatrogenic AUS cuff erosion related to urethral manipulation (87). The need for large-caliber scopes, large-bore catheters, or prolonged urethral catheterization are widely thought to place patients at significant risk for iatrogenic AUS erosions. No modern studies have examined device outcomes in patients requiring instrumentation, however, Otis-Chapados et al. have examined passage of urinary catheters (12 to 22 Fr) and cystoscopes (19 to 26 Fr) through AUS cuffs (3.5 to 6 cm) ex-vivo. They utilized three blind observers to rate the safety of passage, taking into account bulbar urethral thickness and compressibility of urethras (88). These findings can serve as a guide when considering urinary instrumentation and the authors advise caution and careful counseling as these studies have not be studied in the patient setting.
Patients requiring repeat endoscopic resections with large cystoscopes may benefit from temporary cuff uncoupling via a small separate perineal incision to preserve a device in situ and prevent urethral injury and subsequent device erosion. This should be performed prior to cystoscopy to prevent contamination of the surgical field. This incision may be closed temporarily, and a barrier applied to the skin while cystoscopy is performed. Device may be recoupled or the device may be left uncoupled depending on the need for repeat intervention or for prolonged urethral catheterization.
Lower urinary tract complications
Anterior urethral stricture
Management of patients with concomitant urethral pathology poses a challenge to both the patient and surgeon. Any urethral surgery prior to AUS placement may compromise the vascularity of the urethra and may place patients at high risk of AUS complications such as urethral injury during device placement and cuff erosion. Furthermore, multiple studies have found that a history of urethroplasty portends a poor prognosis for device survival, and urethroplasty is often classified as a risk factor for a “fragile urethra” (6).
The authors consider the stability and caliber of the urethra when determining initial management, dividing patients into those with stable asymptomatic, non-flow limiting strictures (≥12 Fr) and those with symptomatic or otherwise clinically apparent (<10–12 Fr) strictures. In those with a prostate in situ, it is also important evaluate the prostate/bladder outlet as contributory factors to any baseline lower urinary tract symptoms.
For patients with asymptomatic moderate caliber strictures (≥12 Fr), we ensure adequate bladder emptying and then repeat in-office cystourethroscopy after an interval of 3–6 months to confirm urethral stricture stability. If patients have worsening of stricture disease with a narrower lumen, development of obstructive symptoms, or new elevated post-void residual measurements, repair of the urethral stricture should be performed prior to AUS placement. AUS placement should be deferred for 3–6 months after urethroplasty, and cystoscopy is important before anti-incontinence surgery to verify success of the urethral reconstruction.
For patients with symptomatic or narrow caliber strictures (<10–12 Fr), urethroplasty should be performed prior to AUS placement. Urethroplasty approach can be determined according to surgeon preference and other patient factors, utilizing the AUA urethral stricture guidelines as a reference (89). A dorsal substitution repair may yield additional challenge with subsequent urethral mobilization, requiring cuff placement at a more proximal/distal site or TC-AUS cuff placement. A non-transecting approach (such as a non-transecting anastomotic urethroplasty or substitution repair) is thought to preserve antegrade urethral perfusion and could limit device erosion risk as noted previously. Non-transecting approaches have been shown to help decrease sexual adverse effects after urethroplasty, such as soft or cold glans, however no studies have extrapolated this for urethral perfusion and its effect on outcomes after AUS placement (90,91).
During AUS placement, difficulty may be encountered after bulbar urethroplasty. Options for alternative cuff location include a more distal bulbar or penobulbar site or a TC-AUS cuff. Transcorporal placement may be the best option after a dorsal substitution graft, as it avoids a second dorsal mobilization and potential graft compromise. If a more distal cuff is placed, some have advocated tandem cuff placement to increase continence; however, this may further contribute to erosion risk in a cohort already prone to such complications due, in part, to their prior urethroplasty (43,44). There are no comparative studies to identify the most appropriate surgical modifications to account for risks from prior urethroplasty. Most studies exploring the aforementioned techniques are retrospective in nature and small in number.
In rare cases, patients may have multiple risk factors and have exhausted their options for stricture repair. For example, a patient with radiation history, prior buccal mucosa graft (BMG) urethroplasty, and previous AUS cuff erosion may develop a new stricture at the area of the previous erosion. Such scenarios illustrate the great importance of patient counseling and understanding the goals of the patient. If continence is the most important goal for them, AUS placement could be cautiously considered with close follow-up, knowing that erosion risk is high. Suprapubic catheter placement perioperatively can establish reliable bladder drainage. This tube can be capped once their AUS is activated and serves as a backup system in case of worsening obstruction due to urethral stricture disease. Some patients might even elect to connect the SP tube to drainage at night (with or without AUS deactivation) to address nocturia or establish a period of cuff site urethral rest when not active. Should such a high-risk patient have an erosion and AUS reimplant deem not an option, permanent urethral ligation as described by VanDyke et al. can restore continence and serve as an alternative to a urinary diversion (92).
Another option for a similar patient may be creation of a continent catheterizable channel to establish an alternative urinary drainage mechanism. Some authors have described placing a bladder neck AUS at the same time of the channel creation, although surgeons may pursue AUS placement separately due to concern about bowel surgery contaminating a sterile device. Studies examining synchronous versus staged bowel surgery and AUS placement have been mixed and largely performed in the pediatric population (93-97). Some studies have demonstrated simultaneous AUS implantation and urinary reconstruction to be safe with good bowel prep, ensuring urine sterility, and separation of surgical fields. It is generally recommended to perform the AUS implantation first, with complete device coverage and incision closure prior to opening the bowel. However, several studies have also found higher erosion and infection risk with concomitant bowel surgery during AUS placement in the pediatric population (94,95). Given high morbidity from device infection or erosion, we recommend staging as two separate procedures whenever possible.
Prostatic fossa calcifications
Patients with recalcitrant prostatic fossa calcification (Figure 3A) or extensive necrosis (Figure 3B) after pelvic radiotherapy should be managed similarly to those with radiation cystitis. Patients should have stable disease without frequent or ongoing need for endoscopic intervention related to cumulative calcification, as repeated treatments could compromise the integrity of the urethra at the AUS cuff. If there is extensive necrosis or calcification, abandonment of the lower urinary tract may need to be considered as endoscopic procedures are likely inadequate to resolve this difficult problem. The dystrophic calcification results from the contact of urine with necrotic tissue. Despite repeated resection of these calcifications, the necrotic tissue remains and will continue to reaccumulate calcifications. These calcifications subject the patient to recurrent urinary tract infections, gross hematuria, and pelvic pain. Figure 3 highlights patients with urethras devastated from prostate radiation. If the patient elects for non-continent urinary diversion, the authors recommend safe resection of bladder tissue and mucosa. In hostile pelvises, as seen in patients with prior radiation and surgery, a partial cystectomy may be performed with fulguration of any remaining bladder mucosa.

Proposed alternatives include resection of this necrotic tissue through salvage prostatectomy or revision of the vesicourethral anastomosis if the patient had a prior prostatectomy. These procedures have a considerable risk of major morbidity, but subsequent AUS can be considered for those who undergo a successful salvage procedure and establish a stable patent lower urinary tract. If a perineal approach is taken for salvage lower urinary tract reconstruction, surgeons can consider placing a “space-saver” AUS cuff; this may facilitate safe subsequent periurethral access, since repeat perineal dissection may be challenging after prior radiation and extensive prior dissection. In one study, a total of 8 patients underwent salvage cystoprostatectomy with orthotopic neobladder for defunctionalized bladder and recalcitrant posterior urethral stenosis. While 5 of 8 patients underwent placement of a “space-saver” AUS cuff at time of lower urinary tract reconstruction, 3 required explantation due to perineal infection. AUS implantation occurred at a mean of 74 days after reconstruction, with 4 patients experiencing AUS erosion (2 patients with “space-saver” AUS cuff and 2 patients who did not have a “space-saver” placed). At a median follow-up of 58 months, there was no recurrence of stenosis. Fifty percent of patients were completely dry and 50% required 1–2 pads per day (81).
Posterior urethral stenosis
After surgical correction of posterior urethral stenosis, it is critical that patients are monitored for urethral patency. A post-void residual should be measured to ensure the bladder can empty prior to AUS. Kahokehr et al. developed an algorithm for treatment of posterior stenoses and recommend stability of >3 months, prior to AUS implantation (98).
If a perineal dissection is to be done for stenosis treatment, one can consider placing a “space-saver” AUS cuff, though a major drawback from this technique is the cost. If SUI is expected after repair, an AUS cuff may be placed at the bulbar urethra during the time of posterior urethroplasty. Then, after adequate urethral rest to allow for neovascularization and to examine for any recurrence of stenosis, AUS implantation may be performed (81). This technique allows surgeons to avoid a challenging dissection of the urethra and avoid urethral injury at the time of perineal dissection for AUS cuff placement. If perineal dissection is not required during stenosis repair, subsequent AUS placement should not be different than in the standard patient.
Surgical considerations for high-risk patients
Patient comorbidities such as diabetes, coronary artery disease, age/fragility, and smoking can play a role in microvascular compromise to urethral health, leading to increased risk of device complications. Similarly, iatrogenic insult such as pelvic radiation can cause microvascular insult to bulbar urethral health. Similarly, pelvic fracture and prior urethral manipulation such as AUS erosion or urethroplasty can result in macrovascular compromise to the urethra. Several surgical techniques have been employed by urologic surgeons to mitigate these risks. Table 2 lists the preoperative and surgical considerations for such high-risk patients.
Table 2
| Patient optimization and surgical techniques for high-risk patients |
| Preoperative optimization of testosterone (cohort series) |
| Penile revascularization (expert opinion) |
| Avoid placement of 3.5 cm AUS cuff (cohort series) |
| Transcorporal AUS cuff +/– corporal wrap (cohort series) |
| Preservation of bulbospangiosus muscle (case report) |
| Relocation of AUS cuff site (expert opinion) |
| “Space-safer” AUS (case series) |
| Lower PRB of 51–60 cmH2O (expert opinion) |
| Intermittent nocturnal deactivation (case series) |
AUS, artificial urinary sphincters; PRB, pressure-regulating balloon.
Cuff size and transcorporal placement
For patients with a history of prior radiation or other bulbar compromise, surgeons should be conservative when sizing the AUS cuff intraoperatively and select a cuff that is up to 0.5 cm larger than the measured urethral circumference, at the expense of some degree of continence. In addition, implanters should avoid using a 3.5 cm AUS cuff, as it has been associated with a high risk of erosion, especially in the high-risk patient. Simhan et al. found that 21% of their radiated patients with a 3.5 cm AUS cuff experienced an erosion, compared to 4% in the non-radiated group. Of the factors examined, history of radiation was the only predictor of erosion with an odds ratio of 6.2 (55). To avoid the placement of a 3.5 cm cuff in these scenarios, the surgeon may need to utilize a TC-AUS placement to increase the circumference of the urethral unit (99,100). This technique can also be utilized to avoid a challenging dorsal urethral dissection, especially in patients with a history of AUS erosion. Several studies have demonstrated TC-AUS to be safe, without additional risk of device erosion (11,25). However, one group compared 3.5 cm standard cuffs to TC-AUS cuffs and found TC-AUS to have a significantly increased risk of erosion on multivariate analysis with a hazard ratio of 6.11 (101). Additionally, Ortiz et al. examined location of cuff erosion and found that TC-AUS placement was not protective of dorsal erosion as previously hypothesized. The group found that most AUS cuff erosions occur ventrally after both standard (79.5%) and TC-AUS (66.7%) cuff placement. Lateral erosions occurred at a rate of 20.5% for standard and 33.3% for TC-AUS cuffs. Dorsal erosions were least common with 5.1% for standard and 20% for TC-AUS cuffs (29). Larger multi-institutional studies combine patients with high risk of erosion, or “fragile urethras”: history of pelvic radiotherapy, history of AUS explanation, and history of urethroplasty (24,43,102). Extrapolating from that data, transcorporal cuffs tended to have longer explant-free survival. Thus, it is our recommendation to preferentially attempt transcoporal cuff placement in this patient population to avoid dorsal dissection, especially in those with dorsal substitution grafts.
In patients with incontinence after lower urinary tract fistula repair or posterior urethroplasty, the surgeon has be to aware that gracilis interposition flaps may have been used (103,104). In our experience, gracilis flaps have not interfered with placement of a bulbar AUS cuff, as the flap is located proximal and away from the standard AUS cuff location.
Another consideration in the patient with a “fragile urethra” is intermittent nocturnal deactivation of the device to allow for urethral rest and unrestricted perfusion of the cuff segment. This is extrapolated from previous published data suggesting an association between urethral atrophy and nocturnal deactivation, where patients treated in a practice that utilized nocturnal deactivation had a 10% rate of urethral atrophy while those in a separate practice not utilizing this technique had a 21% atrophy rate (105). However, further studies would be needed to investigate this adjunctive technique in a rigorous manner.
Urethral protection maneuvers to be considered can include a circumferential urethral wrap or preservation of the bulbospongiosus muscle. Vasan et al. described wrapping the urethra circumferentially with bilateral 2 cm by 2 cm flaps of corpus cavernosal tissue in the Gullwing technique. This is then secured at the ventral urethra with interrupted suture (106). This may mitigate previous concerns about TC-AUS cuffs, however long-term studies are needed to examine the durability of this technique in preventing erosion in high-risk patients. Other groups have also proposed the preservation of the bulbospongiosus muscle and placing the AUS cuff over it in an effort to protect the urethra from erosion (107,108). Prospectively collected data of 82 patients revealed encouraging results with no intraoperative complications. 4.9% required device revision or explant (erosion, infection, or pump/cuff relocation) and device survival at 60 months was 62.6% (108). However, the bulbospongiosus muscle may be atrophic in the case of previous urethral surgery. If examination of the proximal bulbar urethra suggests urethral atrophy of the corpus spongiosum, the surgeon can consider a more distal location for the AUS cuff and potentially placing a TC-AUS to avoid the use of a 3.5 cm cuff.
AUS and penile prosthesis
Patients with vascular compromise of the bulbar urethra may present with ED in addition to urinary incontinence. If the ED does not respond to medical therapy, they have the option of pursuing a penile prothesis placement. Per AUA/SUFU guidelines, an AUS and IPP placement may be staged or synchronous per surgeon and patient discretion (1). Previous studies examining staged versus synchronous implantation have shown variable results (109-113). Many studies have demonstrated its safety, while others have shown increased erosion risk, increased rates of surgical revision, and lower device survival. However, 2 large retrospective studies have shown similar device survival and device revision in metachronous placement compared to synchronous placement (112,113). Patel et al. found that synchronous placement of IPP and AUS did not affect AUS reoperation rate (9.2%) at 3 years when compared to AUS placement alone. However, they did find an increase in IPP reoperation rate at both 1 and 3 years (112). Similarly, Boysen et al. found that in 61 patients with synchronous placement, there was no difference in IPP or AUS device survival on Kaplan-Meier analysis. With a median follow-up of 61 months, AUS survival was 84.5% and 81.7% at 5 years in AUS alone and synchronous IPP and AUS, respectively (113). With synchronous placement of AUS and IPP, complications from one device may affect the other, requiring dual device explantation. One benefit from metachronous placement may be a separation of surgical fields which may isolate one device from the other, thus limiting the effect and spread of device infection or erosion to the other device. If synchronous placement is performed, we recommend separating the surgical fields through separate incisions to prevent contamination. We recommend placement of the IPP first with all incisions closed prior to placement of the AUS. The rationale comes from the increase revision and device complication rate from AUS placement. This may stem from a different level of sterility with urethral manipulation during AUS placement. At our institution, we perform metachronous placement of devices. We generally recommend placement of AUS first. In a patient with concomitant ED and SUI, it would be generally desirable to achieve continence prior to improvement of erectile function. Once the AUS has been successfully placed and activated, the placement of a penile prosthesis can be considered. In patients with mild incontinence, placement of IPP first may be considered as the urethral compression from IPP may result in an acceptable improvement in SUI and deem incontinence surgery such as the AUS unnecessary.
Nevertheless, some patients may have had a penile prosthesis placed for ED prior to becoming incontinent and desire an AUS placement. During placement of an AUS in the setting of a previous penile prosthesis, careful dissection of the dorsal urethra should be done as to avoid entering the corpora cavernosa. Should a transcorporal cuff be needed, we recommend dissection between tunica albuginea of the corpora cavernosa and the IPP pseudo-capsule. If that plane is not easily dissected then the corporotomies can be made onto the cylinders. Some surgeons may leave the corpora cavernosa open, others might use allograft (e.g., Tudoplast) to close the corporotomy window.
Pressure regulating balloon
When choosing the pressure regulating balloon, surgeons should also carefully consider the health of the urethra. Some may select a lower pressure balloon (51–60 cmH2O) instead of the standard 61–70 cmH2O balloon in cases with significant radiation change as this theoretically has less pressure transmission to the urethra. The authors acknowledge that this is extrapolated from data regarding pressure-regulating balloon (PRB) pressures, with 2 prior studies indicating improved continence but a higher rate of erosion and revision (especially in radiated patients) with upregulating PRB pressures (114,115). Moses et al. identified 22 patients undergoing PRB exchange for SUI persistence or recurrence following AUS placement. Patients had an improvement of their SUI based off pads per day, and Incontinence Symptom Index Score and Incontinence Quality of Life. However, 3 patients (14%) with prior radiation experienced cuff erosion and the explantation/revision rate was 45% at 33.5 months and Kaplan-Meier analysis revealed 41% retained their device for 24 months (114). Loh-Doyle et al. similarly identified 55 patients undergoing PRB exchange to 71–80 cmH2O pressure to treat recurrent SUI. At a median follow-up of 26.4 months, 4 (7.3%) patients developed an erosion with 5 patients showing impending erosion requiring revision surgery (115). Based on this, we postulate that a lower PRB may yield lower rate of erosion (at the expense of some degree of continence) in the high-risk patient. Prolonged urethral rest after AUS placement can also be considered, with delay in device activation up to 10–12 weeks, especially in patients with multiple risk factors such as prior pelvic radiotherapy, history of urethroplasty, and history of AUS cuff erosion (116).
Penile revascularization
In the instance that bulbar vascular compromise is suspected, penile revascularization can also be considered in the salvage AUS setting. Penile revascularization has been utilized in patients with devastating pelvic vascular injury resulting in arterial insufficiency and ED (117,118). There is also a rationale for using revascularization prior to urethroplasty for PFUI to prevent recurrence of stricture disease due to poor blood supply. As in the case of ED, revascularization of the corpus spongiosum should be utilized when the mechanism of injury is vascular disruption and an ischemic etiology is confirmed (Figure 2 shows angiogram of intact and injured pudendal artery). This may be the case in PFUI or in cases of multiple insults to the urethra, such as concomitant radiotherapy, urethral/prostate surgery, and AUS erosion. Revascularization can be considered after AUS erosion as a salvage procedure prior to AUS replacement or prior to impending AUS erosion with suspected ischemic etiology. This procedure may be used in highly select cases. The work up consists of penile doppler with medically induced erection and pelvic angiography to evaluate for arterial insufficiency and to map out the vascular anatomy (Figure 2).
A case at our institution for which revascularization was used to salvage an AUS presented as follows: a 45-year-old male with prostate cancer received external beam radiotherapy followed by salvage radical prostatectomy for recurrence in the prostate. The patient’s course was complicated by a vesicourethral anastomotic stenosis requiring posterior urethroplasty, corporal splitting, and inferior pubectomy. The patient had severe SUI and thus underwent AUS placement. He developed an AUS erosion within 9 months. After AUS explantation, penile doppler ultrasound (PDUS) and pelvic angiogram confirmed arterial insufficiency to his dorsal arteries and penile revascularization was performed using the left deep inferior epigastric artery. Following revascularization, the patient was followed with serial PDUS to confirm dorsal artery patency and after appropriate recovery, AUS reimplantation was done at 8 months. A transcorporal 4.0 cm AUS cuff with a 51–60 cmH2O PRB was implanted. He was most recently seen at 7-year follow-up, with a functional device and acceptable social continence. Revascularization should be reserved for these special circumstances and only offered to a carefully selected patient population.
Follow-up for high-risk patients after AUS implantation
Surgeons may consider delay in device activation for up to 8 weeks or longer after implantation in high-risk patients. Furthermore, a more stringent follow-up regimen should be performed as many patients have had multiple insults to the urethra, which compromise urethral health and predispose the patient to device erosion. Mann et al. found most device failures occurred within 2 years of implantation in high-risk patients (24). Though we do not perform routine cystoscopy after AUS implantation in high-risk patients, cystoscopy can be used to inspect the quality of the luminal epithelium, evaluate the urethra to ensure patency of the repair, and exclude gross erosion at about 3–6 months. Patients should be instructed to seek prompt urologic intervention if symptoms of device failure arise, such as hematuria, urinary tract infections, recurrent incontinence, or perineal or penile pain.
During cystourethroscopy, should the urethra underlying the cuff appear thin, a sign of pending erosion, device deactivation with close follow-up can salvage some systems. In this scenario, surgical management with increasing cuff size or placing a lower pressure PRB should be considered after a period of deactivation, and this may prevent erosion while maintaining some level of continence compared to device explanation or complete device deactivation.
Conclusions
A number of patient risk factors are associated with AUS failure and can ultimately lead to device explantation. Each risk factor requires careful consideration and investigation, or intervention as appropriate, prior to device placement. Several surgical strategies to decrease device complications can be considered (Table 2) and we present an algorithm for management of high-risk patients (Figure 1). Optimization of urethral health, confirmation of anatomic and functional stability of the lower urinary tract, and thorough patient counseling are a necessity for these high-risk patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Paul H. Chung and Lindsay Hampson) for the series “Surgical Management of Stress Urinary Incontinence in Men” published in Translational Andrology and Urology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-727/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-727/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-727/coif). The series “Surgical Management of Stress Urinary Incontinence in Men” was commissioned by the editorial office without any funding or sponsorship. JL received travel expenses for “Prosthetic Urology Institute: Fellows Course” from Boston Scientific. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sandhu JS, Breyer B, Comiter C, et al. Incontinence after Prostate Treatment: AUA/SUFU Guideline. J Urol 2019;202:369-78. [Crossref] [PubMed]
- Litwiller SE, Kim KB, Fone PD, et al. Post-prostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for success. J Urol 1996;156:1975-80. [Crossref] [PubMed]
- Bauer RM, Gozzi C, Hübner W, et al. Contemporary management of postprostatectomy incontinence. Eur Urol 2011;59:985-96. [Crossref] [PubMed]
- Cornu JN, Sèbe P, Ciofu C, et al. Mid-term evaluation of the transobturator male sling for post-prostatectomy incontinence: focus on prognostic factors. BJU Int 2011;108:236-40. [Crossref] [PubMed]
- Zuckerman JM, Tisdale B, McCammon K. AdVance male sling in irradiated patients with stress urinary incontinence. Can J Urol 2011;18:6013-7. [PubMed]
- Hoy NY, Rourke KF. Artificial Urinary Sphincter Outcomes in the "Fragile Urethra". Urology. 2015;86:618-24. [Crossref] [PubMed]
- Raj GV, Peterson AC, Toh KL, et al. Outcomes following revisions and secondary implantation of the artificial urinary sphincter. J Urol 2005;173:1242-5. [Crossref] [PubMed]
- Gomha MA, Boone TB. Artificial urinary sphincter for post-prostatectomy incontinence in men who had prior radiotherapy: a risk and outcome analysis. J Urol 2002;167:591-6. [Crossref] [PubMed]
- Walsh IK, Williams SG, Mahendra V, et al. Artificial urinary sphincter implantation in the irradiated patient: safety, efficacy and satisfaction. BJU Int 2002;89:364-8. [Crossref] [PubMed]
- Lai HH, Hsu EI, Teh BS, et al. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol 2007;177:1021-5. [Crossref] [PubMed]
- McGeady JB, McAninch JW, Truesdale MD, et al. Artificial urinary sphincter placement in compromised urethras and survival: a comparison of virgin, radiated and reoperative cases. J Urol 2014;192:1756-61. [Crossref] [PubMed]
- Ravier E, Fassi-Fehri H, Crouzet S, et al. Complications after artificial urinary sphincter implantation in patients with or without prior radiotherapy. BJU Int 2015;115:300-7. [Crossref] [PubMed]
- Hird AE, Radomski SB. Artificial urinary sphincter erosion after radical prostatectomy in patients treated with and without radiation. Can Urol Assoc J 2015;9:E354-8. [Crossref] [PubMed]
- Jhavar S, Swanson G, Deb N, et al. Durability of Artificial Urinary Sphincter With Prior Radiation Therapy. Clin Genitourin Cancer 2017;15:e175-80. [Crossref] [PubMed]
- DeLay KJ, Haney NM, Chiang J, et al. Comparison of Adjuvant Radiation Therapy Before or After Artificial Urinary Sphincter Placement: A Multi-Institutional, Retrospective Analysis. Urology 2018;113:160-5. [Crossref] [PubMed]
- Sathianathen NJ, McGuigan SM, Moon DA. Outcomes of artificial urinary sphincter implantation in the irradiated patient. BJU Int 2014;113:636-41. [Crossref] [PubMed]
- Rivera ME, Linder BJ, Ziegelmann MJ, et al. The Impact of Prior Radiation Therapy on Artificial Urinary Sphincter Device Survival. J Urol 2016;195:1033-7. [Crossref] [PubMed]
- Maurer V, Marks P, Dahlem R, et al. Functional outcomes of artificial urinary sphincter implantation with distal bulbar double cuff in men with and without a history of external beam radiotherapy: an analysis of a prospective database. BJU Int 2019;124:1040-6. [Crossref] [PubMed]
- Mamane J, Sanchez S, Lellouch AG, et al. Impact of radiation therapy on artificial urinary sphincter implantation in male patients: A multicenter study. Neurourol Urodyn 2022;41:332-9. [Crossref] [PubMed]
- Srivastava A, Joice GA, Patel HD, et al. Impact of Adjuvant Radiation on Artificial Urinary Sphincter Durability in Postprostatectomy Patients. Urology 2018;114:212-7. [Crossref] [PubMed]
- Hüsch T, Kretschmer A, Thomsen F, et al. Risk Factors for Failure of Male Slings and Artificial Urinary Sphincters: Results from a Large Middle European Cohort Study. Urol Int 2017;99:14-21. [Crossref] [PubMed]
- Fuller TW, Ballon-Landa E, Gallo K, et al. Outcomes and Risk Factors of Revision and Replacement Artificial Urinary Sphincter Implantation in Radiated and Nonradiated Cases. J Urol 2020;204:110-4. [Crossref] [PubMed]
- Kaufman MR, Milam DF, Johnsen NV, et al. Prior Radiation Therapy Decreases Time to Idiopathic Erosion of Artificial Urinary Sphincter: A Multi-Institutional Analysis. J Urol 2018;199:1037-41. [Crossref] [PubMed]
- Mann RA, Kasabwala K, Buckley JC, et al. The "Fragile" Urethra as a Predictor of Early Artificial Urinary Sphincter Erosion. Urology 2022;169:233-6. [Crossref] [PubMed]
- Brant WO, Erickson BA, Elliott SP, et al. Risk factors for erosion of artificial urinary sphincters: a multicenter prospective study. Urology 2014;84:934-8. [Crossref] [PubMed]
- Moser DC, Kaufman MR, Milam DF, et al. Impact of Radiation and Transcorporeal Artificial Sphincter Placement in Patients with Prior Urethral Cuff Erosion: Results from a Retrospective Multicenter Analysis. J Urol 2018;200:1338-43. [Crossref] [PubMed]
- Viers BR, Linder BJ, Rivera ME, et al. The Impact of Diabetes Mellitus and Obesity on Artificial Urinary Sphincter Outcomes in Men. Urology 2016;98:176-82. [Crossref] [PubMed]
- Ziegelmann MJ, Linder BJ, Rivera ME, et al. Outcomes of artificial urinary sphincter placement in octogenarians. Int J Urol 2016;23:419-23. [Crossref] [PubMed]
- Ortiz NM, Wolfe AR, Baumgarten AS, et al. Artificial Urinary Sphincter Cuff Erosion Heat Map Shows Similar Anatomical Characteristics for Transcorporal and Standard Approach. J Urol 2020;204:1027-32. [Crossref] [PubMed]
- Miller AR, Linder BJ, Rangel LJ, et al. The impact of incontinence etiology on artificial urinary sphincter outcomes. Investig Clin Urol 2017;58:241-6. [Crossref] [PubMed]
- Wolfe AR, Ortiz NM, Baumgarten AS, et al. Most men with artificial urinary sphincter cuff erosion have low serum testosterone levels. Neurourol Urodyn 2021;40:1035-41. [Crossref] [PubMed]
- Myles PS, Iacono GA, Hunt JO, et al. Risk of respiratory complications and wound infection in patients undergoing ambulatory surgery: smokers versus nonsmokers. Anesthesiology 2002;97:842-7. [Crossref] [PubMed]
- Sørensen LT. Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Arch Surg 2012;147:373-83. [Crossref] [PubMed]
- Sørensen LT, Hørby J, Friis E, et al. Smoking as a risk factor for wound healing and infection in breast cancer surgery. Eur J Surg Oncol 2002;28:815-20. [Crossref] [PubMed]
- Godwin CA, Linder BJ, Rivera ME, et al. Effects of Smoking Status on Device Survival Among Individuals Undergoing Artificial Urinary Sphincter Placement. Am J Mens Health 2018;12:1398-402. [Crossref] [PubMed]
- McKibben MJ, Shakir N, Fuchs JS, et al. Erosion rates of 3.5-cm artificial urinary sphincter cuffs are similar to larger cuffs. BJU Int 2019;123:335-41. [Crossref] [PubMed]
- Huang MM, Huffman P, Dani H, et al. Association between Previous Pelvic Radiation and All-Cause and Cause-Specific Failure of Replacement Artificial Urinary Sphincters. J Urol 2022;207:1268-75. [Crossref] [PubMed]
- Rozanski AT, Tausch TJ, Ramirez D, et al. Immediate urethral repair during explantation prevents stricture formation after artificial urinary sphincter cuff erosion. J Urol 2014;192:442-6. [Crossref] [PubMed]
- Siegel JA, Tausch TJ, Morey AF. In situ urethroplasty after artificial urinary sphincter cuff erosion. Transl Androl Urol 2015;4:56-9. [PubMed]
- Chertack N, Chaparala H, Angermeier KW, et al. Foley or Fix: A Comparative Analysis of Reparative Procedures at the Time of Explantation of Artificial Urinary Sphincter for Cuff Erosion. Urology 2016;90:173-8. [Crossref] [PubMed]
- Gross MS, Broghammer JA, Kaufman MR, et al. Urethral Stricture Outcomes After Artificial Urinary Sphincter Cuff Erosion: Results From a Multicenter Retrospective Analysis. Urology 2017;104:198-203. [Crossref] [PubMed]
- Chertack NA, Caldwell KM, Joice GA, et al. Long-term lower urinary tract sequelae following AUS cuff erosion. Neurourol Urodyn 2022;41:229-36. [Crossref] [PubMed]
- El-Akri M, Bentellis I, Tricard T, et al. Transcorporal vs. bulbar artificial urinary sphincter implantation in male patients with fragile urethra. World J Urol 2021;39:4449-57. [Crossref] [PubMed]
- Maurer V, Dahlem R, Rosenbaum CM, et al. Distal Double Cuff Vs Transcorporal Cuff as Salvage Options-A Prospective Analysis of Different Artificial Urinary Sphincter (AMS 800) Implantation Sites. Urology 2019;133:234-9. [Crossref] [PubMed]
- Panganiban RA, Snow AL, Day RM. Mechanisms of radiation toxicity in transformed and non-transformed cells. Int J Mol Sci 2013;14:15931-58. [Crossref] [PubMed]
- Kim SP, Sarmast Z, Daignault S, et al. Long-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective review from the University of Michigan. J Urol 2008;179:1912-6. [Crossref] [PubMed]
- Corriere JN Jr, Rudy DC, Benson GS. Voiding and erectile function after delayed one-stage repair of posterior urethral disruptions in 50 men with a fractured pelvis. J Trauma 1994;37:587-9; discussion 589-90. [Crossref] [PubMed]
- Mundy AR. Pelvic fracture injuries of the posterior urethra. World J Urol 1999;17:90-5. [Crossref] [PubMed]
- Mundy AR, Andrich DE. Pelvic fracture-related injuries of the bladder neck and prostate: their nature, cause and management. BJU Int 2010;105:1302-8. [Crossref] [PubMed]
- Poujade O, Ceccaldi PF, Davitian C, et al. Uterine necrosis following pelvic arterial embolization for post-partum hemorrhage: review of the literature. Eur J Obstet Gynecol Reprod Biol 2013;170:309-14. [Crossref] [PubMed]
- Hundersmarck D, Hietbrink F, Leenen LPH, et al. Pelvic packing and angio-embolization after blunt pelvic trauma: a retrospective 18-year analysis. Injury 2021;52:946-55. [Crossref] [PubMed]
- Luan JY, Li X, Xiang Y, et al. Prognosis of embolization of internal iliac artery during the endovascular repair for abdominal aortic aneurysm. Beijing Da Xue Xue Bao Yi Xue Ban 2014;46:917-9. [PubMed]
- Chun JY, Mailli L, Abbasi MA, et al. Embolization of the internal iliac artery before EVAR: is it effective? Is it safe? Which technique should be used? Cardiovasc Intervent Radiol 2014;37:329-36. [Crossref] [PubMed]
- Matityahu A, Marmor M, Elson JK, et al. Acute complications of patients with pelvic fractures after pelvic angiographic embolization. Clin Orthop Relat Res 2013;471:2906-11. [Crossref] [PubMed]
- Simhan J, Morey AF, Singla N, et al. 3.5 cm artificial urinary sphincter cuff erosion occurs predominantly in irradiated patients. J Urol 2015;193:593-7. [Crossref] [PubMed]
- Johnsen NV, Kaufman MR, Dmochowski RR, et al. Erectile Dysfunction Following Pelvic Fracture Urethral Injury. Sex Med Rev 2018;6:114-23. [Crossref] [PubMed]
- Khene ZE, Paret F, Perrouin-Verbe MA, et al. Artificial Urinary Sphincter in Male Patients with Spina Bifida: Comparison of Perioperative and Functional Outcomes between Bulbar Urethra and Bladder Neck Cuff Placement. J Urol 2018;199:791-7. [Crossref] [PubMed]
- Sayedahmed K, Olianas R, Kaftan B, et al. Impact of previous urethroplasty on the outcome after artificial urinary sphincter implantation: a prospective evaluation. World J Urol 2020;38:183-91. [Crossref] [PubMed]
- Andrich DE, Mundy AR. Non-transecting anastomotic bulbar urethroplasty: a preliminary report. BJU Int 2012;109:1090-4. [Crossref] [PubMed]
- Bugeja S, Andrich DE, Mundy AR. Non-transecting bulbar urethroplasty. Transl Androl Urol 2015;4:41-50. [PubMed]
- Chapman DW, Cotter K, Johnsen NV, et al. Nontransecting Techniques Reduce Sexual Dysfunction after Anastomotic Bulbar Urethroplasty: Results of a Multi-Institutional Comparative Analysis. J Urol 2019;201:364-70. [Crossref] [PubMed]
- Hofer MD, Morey AF. Role of androgens for urethral homeostasis. Transl Androl Urol 2018;7:521-5. [Crossref] [PubMed]
- Hofer MD, Morey AF, Sheth K, et al. Low Serum Testosterone Level Predisposes to Artificial Urinary Sphincter Cuff Erosion. Urology 2016;97:245-9. [Crossref] [PubMed]
- Bailey GC, Linder BJ, Rivera ME, et al. The impact of androgen deprivation on artificial urinary sphincter outcomes. Transl Androl Urol 2016;5:756-61. [Crossref] [PubMed]
- McKibben MJ, Fuentes J, Shakir N, et al. Low Serum Testosterone is Present in Nearly Half of Men Undergoing Artificial Urinary Sphincter Placement. Urology 2018;118:208-12. [Crossref] [PubMed]
- Zwaans BM, Nicolai HG, Chancellor MB, et al. Challenges and Opportunities in Radiation-induced Hemorrhagic Cystitis. Rev Urol 2016;18:57-65. [PubMed]
- Vargas C, Martinez A, Kestin LL, et al. Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys 2005;62:1297-308. [Crossref] [PubMed]
- Valdagni R, Rancati T, Ghilotti M, et al. To bleed or not to bleed. A prediction based on individual gene profiling combined with dose-volume histogram shapes in prostate cancer patients undergoing three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys 2009;74:1431-40. [Crossref] [PubMed]
- Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002;53:1097-105. [Crossref] [PubMed]
- Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 2005;294:1233-9. [Crossref] [PubMed]
- Zelefsky MJ, Wallner KE, Ling CC, et al. Comparison of the 5-year outcome and morbidity of three-dimensional conformal radiotherapy versus transperineal permanent iodine-125 implantation for early-stage prostatic cancer. J Clin Oncol 1999;17:517-22. [Crossref] [PubMed]
- Zelefsky MJ, Cowen D, Fuks Z, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer 1999;85:2460-8. [Crossref] [PubMed]
- Lawton CA, Won M, Pilepich MV, et al. Long-term treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706. Int J Radiat Oncol Biol Phys 1991;21:935-9. [Crossref] [PubMed]
- Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 2006;24:1990-6. [Crossref] [PubMed]
- Peeters ST, Heemsbergen WD, van Putten WL, et al. Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys 2005;61:1019-34. [Crossref] [PubMed]
- Zelefsky MJ, Yamada Y, Cohen GN, et al. Five-year outcome of intraoperative conformal permanent I-125 interstitial implantation for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2007;67:65-70. [Crossref] [PubMed]
- Zelefsky MJ, Hollister T, Raben A, et al. Five-year biochemical outcome and toxicity with transperineal CT-planned permanent I-125 prostate implantation for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys 2000;47:1261-6. [Crossref] [PubMed]
- Chen AB, D'Amico AV, Neville BA, et al. Patient and treatment factors associated with complications after prostate brachytherapy. J Clin Oncol 2006;24:5298-304. [Crossref] [PubMed]
- Keyes M, Miller S, Moravan V, et al. Predictive factors for acute and late urinary toxicity after permanent prostate brachytherapy: long-term outcome in 712 consecutive patients. Int J Radiat Oncol Biol Phys 2009;73:1023-32. [Crossref] [PubMed]
- Lightner DJ, Gomelsky A, Souter L, et al. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment 2019. J Urol 2019;202:558-63. [Crossref] [PubMed]
- Patil MB, Hannoun D, Reyblat P, et al. Total bladder and posterior urethral reconstruction: salvage technique for defunctionalized bladder with recalcitrant posterior urethral stenosis. J Urol 2015;193:1649-54. [Crossref] [PubMed]
- Lefaivre KA, Roffey DM, Guy P, et al. Quantifying Urinary and Sexual Dysfunction After Pelvic Fracture. J Orthop Trauma 2022;36:118-23. [Crossref] [PubMed]
- Herndon CD, Rink RC, Shaw MB, et al. The Indiana experience with artificial urinary sphincters in children and young adults. J Urol 2003;169:650-4; discussion 654. [Crossref] [PubMed]
- Ruiz E, Puigdevall J, Moldes J, et al. 14 years of experience with the artificial urinary sphincter in children and adolescents without spina bifida. J Urol 2006;176:1821-5. [Crossref] [PubMed]
- López Pereira P, Somoza Ariba I, Martínez Urrutia MJ, et al. Artificial urinary sphincter: 11-year experience in adolescents with congenital neuropathic bladder. Eur Urol 2006;50:1096-101; discussion 1101. [Crossref] [PubMed]
- Barrett DM, Furlow WL. Incontinence, intermittent self-catheterization and the artificial genitourinary sphincter. J Urol 1984;132:268-9. [Crossref] [PubMed]
- Heiner SM, Viers BR, Rivera ME, et al. What is the fate of artificial urinary sphincters among men undergoing repetitive bladder cancer treatment? Investig Clin Urol 2018;59:44-8. [Crossref] [PubMed]
- Otis-Chapados S, Kim J, Radomski SB. Artificial urinary sphincter cuffs and safe instrument/catheter passage guidelines. Neurourol Urodyn 2022;41:1764-9. [Crossref] [PubMed]
- Wessells H, Angermeier KW, Elliott S, et al. Male Urethral Stricture: American Urological Association Guideline. J Urol 2017;197:182-90. [Crossref] [PubMed]
- Barbagli G, De Angelis M, Romano G, et al. Long-term followup of bulbar end-to-end anastomosis: a retrospective analysis of 153 patients in a single center experience. J Urol 2007;178:2470-3. [Crossref] [PubMed]
- Palminteri E, Franco G, Berdondini E, et al. Anterior urethroplasty and effects on sexual life: which is the best technique? Minerva Urol Nefrol 2010;62:371-6. [PubMed]
- VanDyke ME, Viers BR, Pagliara TJ, et al. Permanent Bulbar Urethral Ligation: Emerging Treatment Option for Incontinent Men With End-stage Urethra. Urology 2017;105:186-91. [Crossref] [PubMed]
- Gonzalez R, Nguyen DH, Koleilat N, et al. Compatibility of enterocystoplasty and the artificial urinary sphincter. J Urol 1989;142:502-4; discussion 520-1. [Crossref] [PubMed]
- Light JK, Lapin S, Vohra S. Combined use of bowel and the artificial urinary sphincter in reconstruction of the lower urinary tract: infectious complications. J Urol 1995;153:331-3. [Crossref] [PubMed]
- Holmes NM, Kogan BA, Baskin LS. Placement of artificial urinary sphincter in children and simultaneous gastrocystoplasty. J Urol 2001;165:2366-8. [Crossref] [PubMed]
- Mor Y, Leibovitch I, Golomb J, et al. Lower urinary tract reconstruction by augmentation cystoplasty and insertion of artificial urinary sphincter cuff only: long term follow-up. Prog Urol 2004;14:310-4. [PubMed]
- Bar-Yosef Y, Castellan M, Joshi D, et al. Total continence reconstruction using the artificial urinary sphincter and the Malone antegrade continence enema. J Urol 2011;185:1444-7. [Crossref] [PubMed]
- Kahokehr AA, Peterson AC, Lentz AC. Posterior urethral stenosis after prostate cancer treatment: contemporary options for definitive management. Transl Androl Urol 2018;7:580-92. [Crossref] [PubMed]
- Nelson RP. Incorporation of corpora cavernosa in bulbous urethral artificial urinary sphincter. J Urol 1986;136:102-3. [Crossref] [PubMed]
- Guralnick ML, Miller E, Toh KL, et al. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol 2002;167:2075-8; discussion 2079. [Crossref] [PubMed]
- Davenport MT, Akhtar AM, Shakir NA, et al. Comparison of 3.5 cm and transcorporal cuffs in high-risk artificial urinary sphincter populations. Transl Androl Urol 2020;9:62-6. [Crossref] [PubMed]
- Redmond E, Tong S, Zemp L, et al. Improved artificial urinary sphincter outcomes using a transcorporal cuff placement in patients with a "fragile urethra". Can Urol Assoc J 2020;14:E621-4. [Crossref] [PubMed]
- Mundy AR, Andrich DE. Posterior urethral complications of the treatment of prostate cancer. BJU Int 2012;110:304-25. [Crossref] [PubMed]
- Campbell JG, Vanni AJ. Complex Lower Genitourinary Fistula Repair: Rectourethral Fistula and Puboprostatic Fistula. Urol Clin North Am 2022;49:553-65. [Crossref] [PubMed]
- Elliott DS, Barrett DM, Gohma M, et al. Does nocturnal deactivation of the artificial urinary sphincter lessen the risk of urethral atrophy? Urology 2001;57:1051-4. [Crossref] [PubMed]
- Vasan R, Myrga J, Miller D, et al. The Gullwing Technique: A Novel Method of Transcorporal Artificial Urinary Sphincter Placement for the Fragile Urethra. Urology 2022;169:237-40. [Crossref] [PubMed]
- Roth CC, Winters JC. Insertion of artificial urinary sphincter with preservation of bulbospongiosus muscle in patients at risk for sphincter erosion: an assessment of patient satisfaction. Ochsner J 2006;6:54-8. [PubMed]
- Collado Serra A, Domínguez-Escrig J, Gómez-Ferrer Á, et al. Prospective follow-up study of artificial urinary sphincter placement preserving the bulbospongiosus muscle. Neurourol Urodyn 2017;36:1387-94. [Crossref] [PubMed]
- Segal RL, Cabrini MR, Harris ED, et al. Combined inflatable penile prosthesis-artificial urinary sphincter implantation: no increased risk of adverse events compared to single or staged device implantation. J Urol 2013;190:2183-8. [Crossref] [PubMed]
- Sundaram V, Cordon BH, Hofer MD, et al. Is Risk of Artificial Urethral Sphincter Cuff Erosion Higher in Patients with Penile Prosthesis? J Sex Med 2016;13:1432-7. [Crossref] [PubMed]
- Loh-Doyle JC, Ashrafi A, Nazemi A, et al. Dual Prosthetic Implantation After Radical Cystoprostatectomy and Neobladder: Outcomes of the Inflatable Penile Prosthesis and Artificial Urinary Sphincter in Bladder Cancer Survivors. Urology 2019;127:127-32. [Crossref] [PubMed]
- Patel N, Golan R, Halpern JA, et al. A Contemporary Analysis of Dual Inflatable Penile Prosthesis and Artificial Urinary Sphincter Outcomes. J Urol 2019;201:141-6. [Crossref] [PubMed]
- Boysen WR, Cohen AJ, Kuchta K, et al. Combined Placement of Artificial Urinary Sphincter and Inflatable Penile Prosthesis Does Not Increase Risk of Perioperative Complications or Impact Long-term Device Survival. Urology 2019;124:264-70. [Crossref] [PubMed]
- Moses RA, Keihani S, Craig JR, et al. Efficacy of Pressure Regulating Balloon Exchange in Men With Post Artificial Urinary Sphincter Persistent or Recurrent Stress Urinary Incontinence. Urology 2019;123:252-7. [Crossref] [PubMed]
- Loh-Doyle JC, Nazemi A, Ashrafi A, et al. Predictors of Device-related Complications After Exchange of the Pressure-regulating Balloon in Men With an Artificial Urinary Sphincter. Urology 2020;135:154-8. [Crossref] [PubMed]
- Marks JL, Light JK. Management of urinary incontinence after prostatectomy with the artificial urinary sphincter. J Urol 1989;142:302-4. [Crossref] [PubMed]
- Sohn M, Hatzinger M, Goldstein I, et al. Standard operating procedures for vascular surgery in erectile dysfunction: revascularization and venous procedures. J Sex Med 2013;10:172-9. [Crossref] [PubMed]
- Nelson AK, Wessells H, Friedrich JB. Review of microsurgical posterior urethral reconstruction. J Reconstr Microsurg 2011;27:179-86. [Crossref] [PubMed]


