
State-of-art technologies to detect the DNA damage and repair in sperm and future outlook
Sperm provides half of the DNA required to fertilize an egg. If the DNA in sperm is damaged, it may be unable to fertilize the egg, or if fertilization occurs, the resulting embryo may fail to progress normally or properly implant into the uterine lining. Sperm DNA fragmentation index (DFI) is a measurement used to determine the amount of sperm that have abnormal genetic material and has been becoming widely used in clinic. A DFI above 30% is related to infertility and above 20% is regarded to be suboptimal. Previous study found that in couples with unexplained infertility 26% of men had a DFI of 20% or greater and currently it is estimated that about 5.7% high DFI percentage in the sub-fertility patients. In a recent paper (1), Farkouh et al., have systematically compared the advantage and disadvantage of the current different methods to evaluate the DNA fragmentation in sperm. Furthermore, the authors used strengths-weaknesses-opportunities-threats (SWOT) analysis to provide advice on the DFI in the future clinical usage. However, development on more precise and clinically useful biomarker for sperm DNA damage is still a very challenging task and worth of further investigation.
There are multiple causes leading to a higher DFI, and sperm can have different kinds of oxidative stress from the environment, for example, drugs, varicocele, and smoking which can have significant effects on the oxidative stress levels. Mechanistically, oxidative stress could lead to protein damage, lipid peroxidation, bio-membrane damage, etc. All these adverse damage in cellular level during spermatogenesis can have significant effects on the DNA integrity of sperm (2). The prolonged transition of sperm in epididymis can also cause the oxidative stress. Theoretically, the oxidate stress leads to DNA damage through damage of 8-OHdG in sperm (3). It has been shown that sperm DFI is closely related to sperm function including sperm progressive motility, and other functional parameters. It also has been shown that spermatozoa lack a fully functional DNA repair machinery, as they only have the first DNA repair enzyme, OGG1, yet APE1 and other base-excision pathway is lacking in human and mice sperm while oocytes have abundant APE1 expression to repair sperm damage. Therefore, both DNA damage in sperm and DNA damage repair capability in oocyte is important for the embryo development. Animal studies have shown that treatment with oxidative stress agent led to genomic instability during embryo development defects (4). For example, it has been shown that sperm DNA damage can have significant effect on the embryo (4). Somatic cells have equipped with effective oxidative stress scavenge machinery and has evolved with effective DNA repair mechanism, however sperm cell, as haploid cells, and whether its DNA damage can cause embryo development defects is complex and need more comprehensive evaluation.
A semen analysis does not assess DNA fragmentation. DNA fragmentation is a supplemental test to traditional sperm parameters assessed by a semen analysis. Currently there are several methods to evaluate sperm DFI and each method has the specific advantage and disadvantage. For example, the widely-used flow-cytometry based SCSA method has the advantage of short analysis time. Of particularly noted is that the sperm chromatin dispersion (SCD) method, which is on the other hand based on the staining method. For example, using Comet assay (also known as the single cell gel electrophoresis assay), we have analyzed the sperm samples from the globozoospermia patients, and our result shows that Comet assay can differentiate both the single strand DNA damage and double strand DNA damage (5). Interestingly, we have found that the double-stranded break DFI (DSB-DFI) were significantly higher than that in the fertile controls. Furthermore, we have shown that oxidative stress parameters have positive correlation with DNA damage parameters (5). Therefore, Comet assay hold the promise to have a unique advantage compared with other methods. However, Comet assay is labour intensive, and the operation protocol is relatively complex. Therefore, there is an urgent need to develop more automatic protocol to compensate this short back. In this regard, the new developed micro-fluid method may represent a promising method to increase the efficiency and appliances (6).
It should be noted that several new mechanisms have been proposed on inducing DNA damage. For examples, studies showed that Gunine-rich DNA can fold into highly stable, non-canonical spatial four-stranded DNA structures called G-tetramer (7,8). Failure to properly unhelix these structures will impede DNA replication mechanisms and significantly affect the epigenetics of spermatozoa. In addition, sperm with normal DFI may also carry a large number of abnormal G4 structures. When sperm with normal DFI indicators pass through the egg, sperm carrying G4 structural DNA will largely fail fertilization due to hindered DNA replication. Therefore, providing etiological diagnostic indicators for the detection of G tetramers can effectively predict fertilization success rate and find the real cause for male patients with unexplained infertility. In addition, R-loop within G4, on the other hand, can has significant effect on the DNA replication and transcription. It would be interesting to investigate the detailed relationship between spermatid G4 formation and resulting DNA single-stranded break (SSB) and DSB formation.
With the new development of techniques to measure the DFI index, it has been a trend that high-throughput, automatic detection method would enter the clinic application. In the meantime, the point-of-care testing (POCT) detection is also becoming popular. From the clinic point of view, it needs more robust parameters which can be used to guide the drug intervention based on specific DNA strand mechanism. For example, the flow-cytometry based method, such as pH probe could also be used to aid the detection (9). Furthermore, some infertility genes need to be further examined with the help of fast developed artificial intelligent (AI) methods which certainly would help in this more integrated investigation.
Sperm analysis has been revolutionized in recent year (10). With the fast development of AI system applied in the reproduction felid (8-11), it is predicted that AI could play an important role for the development of innovative detection and screening methods. For example, AI has now been used to train to select the best sperm picture with lowest DFI in testis samples among the large number of pictures of semen samples (12). With the new development in the sperm selection analysis (13), there is urgent need to develop new methods to select the sperm based on the DNA integrity and optimized sperm selection method. For example, the new method using AI based software can select the sperm with least DNA fragmentation and best fertilizing capability.
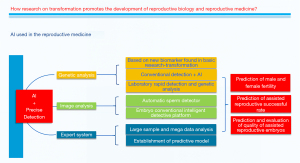
To conclude, we predict with hope that the future integrated methods, especially using AI technology (Figure 1) would provide more intelligent, precise, and reliable knowledge to detect the DNA damage and repair.
Acknowledgments
Funding: This study was funded by the National Science fund of China (No. 82171715).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Andrology and Urology. The article did not undergo external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-870/coif). All authors report that this study was funded by the National Science fund of China (No. 82171715). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Farkouh A, Salvio G, Kuroda S, et al. Sperm DNA integrity and male infertility: a narrative review and guide for the reproductive physicians. Transl Androl Urol 2022;11:1023-44. [Crossref] [PubMed]
- Sheng K, Liang X, Huang S, et al. The role of histone ubiquitination during spermatogenesis. Biomed Res Int 2014;2014:870695. [Crossref] [PubMed]
- Drevet JR, Aitken RJ. Oxidative Damage to Sperm DNA: Attack and Defense. Adv Exp Med Biol 2019;1166:107-17. [Crossref] [PubMed]
- Middelkamp S, van Tol HTA, Spierings DCJ, et al. Sperm DNA damage causes genomic instability in early embryonic development. Sci Adv 2020;6:eaaz7602. [Crossref] [PubMed]
- Huang L, Yao G, Huang G, et al. Association of Zinc deficiency, oxidative stress and increased double-stranded DNA breaks in globozoospermic infertile patients and its implication for the assisted reproductive technique. Transl Androl Urol 2021;10:1088-101. [Crossref] [PubMed]
- Pujol A, García-Peiró A, Ribas-Maynou J, et al. A microfluidic sperm-sorting device reduces the proportion of sperm with double-stranded DNA fragmentation. Zygote 2022;30:200-5. [Crossref] [PubMed]
- Armas P, Calcaterra NB. G-quadruplex in animal development: Contribution to gene expression and genomic heterogeneity. Mech Dev 2018;154:64-72. [Crossref] [PubMed]
- Riegler MA, Stensen MH, Witczak O, et al. Artificial intelligence in the fertility clinic: status, pitfalls and possibilities. Hum Reprod 2021;36:2429-42. [Crossref] [PubMed]
- Li X, Wu S, Yu K, et al. A dual-site controlled pH probe revealing the pH of sperm cytoplasm and screening for healthy spermatozoa. J Mater Chem B 2021;9:3662-5. [Crossref] [PubMed]
- Dai C, Zhang Z, Shan G, et al. Advances in sperm analysis: techniques, discoveries and applications. Nat Rev Urol 2021;18:447-67. [Crossref] [PubMed]
- Zhang Z, Dai C, Shan G, et al. Quantitative selection of single human sperm with high DNA integrity for intracytoplasmic sperm injection. Fertil Steril 2021;116:1308-18. [Crossref] [PubMed]
- Ferrigno A, Ruvolo G, Capra G, et al. Correlation between the DNA fragmentation index (DFI) and sperm morphology of infertile patients. J Assist Reprod Genet 2021;38:979-86. [Crossref] [PubMed]
- Leung ETY, Lee CL, Tian X, et al. Simulating nature in sperm selection for assisted reproduction. Nat Rev Urol 2022;19:16-36. [Crossref] [PubMed]


