
A retrospective cohort study of geographic differences in the semen of 1,012 sperm donors in China
Highlight box
Key findings
• We found sperm concentration was significantly higher in sperm donors living in northern China than in southern China.
What is known and what is new?
• Over the years, there have been many debates about whether there are differences in semen quality between different regions.
• This study focused on differences in male semen quality in northern and southern China by comparing different latitudes and different regional divisions.
What is the implication, and what should change now?
• We hypothesize environmental pollution and mental stress due to increased population size may be the main factors underlying the difference in sperm quality between the northern and southern regions of China.
• We suggest future research should focus on the effects of changes in lifestyle habits, environmental pollution, and psychological factors on male reproductive health.
Introduction
Male reproductive health and seminal quality have become a serious concern for public health (1). In a recent study, researchers found the sperm quality of Estonian men was better than that of Finnish men in terms of sperm number and normal sperm count (2), although the limited geographical distribution of the results were not sufficient to explain whether they were related to geographical differences. Furthermore, these results may have been influenced by different factors such as the recruitment of volunteers, semen analysis, and data processing methods (3). A study performed on semen quality among different provinces and municipalities in Spain showed that even in a particular country with uniform processing methods, there were still geographic differences in semen quality (4). This finding supported the hypothesis that there are regional differences in semen quality. Similarly, a study from Qatar also indicated that even within a specific recruitment agency, differences in semen quality between two regions still existed (5).
However, little has been done to investigate variations in semen quality in Asian settings. Preliminary comparisons conducted by reproductive centers across China highlighted some abnormalities in semen parameters and suggested there was a difference between northern and southern geographical regions. Specifically, abnormal sperm parameters were more frequent in the south than the north, possibly indicating a link with regional pollution (6-8), although this may have been be caused by sampling procedures (9).
Therefore, this study focused on differences in male semen quality in northern and southern China by comparing different latitudes and regional divisions. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-578/rc).
Methods
Recruitment
Based on the standard protocol for human sperm banking revised by the Ministry of Health of the People’s Republic of China, eligible donors from 27 provinces and cities whose sperm was kept in a large sperm bank in Beijing from October 2015 to May 2019 were selected. The inclusion criteria were as follows: (I) aged between 22 and 44 years; (II) no noticeable baldness or hair loss; (III) no color weakness and no color blindness; (IV) the donor was in good health, on the basis of both physical examinations and psychological evaluations, including an examination of the reproductive system; (V) following sperm bank regulations, volunteers had abstained from smoking and alcohol for 3 months prior to official sperm donation; (VI) a rigorous medical screening was conducted, and those with a family history of hereditary disease were excluded; (VII) potential donors had undergone laboratory tests to exclude sexually transmitted infections and high-risk genetic diseases, including human immunodeficiency virus 1 and 2, hepatitis B and C, syphilis, gonorrhea, mycoplasma, chlamydia, cytomegalovirus, toxoplasma gondii, rubella virus, herpes simplex virus types 1 and 2, and karyotype analysis; (VIII) as polymorphisms of the human chromosomes have certain clinical effects associated with abortion, stillbirth, and infertility, normal males with chromosome karyotype 46, XY were chosen.
Semen quality was analyzed by trained technicians in accordance with the standardized methods described in the World Health Organization Laboratory Manual (10). Screening criteria for donor semen quality were based on basic human sperm bank standards and technical specifications, and internal quality control was used to ensure there were no significant differences between the research results derived from different technicians. Semen quality needed to meet the following criteria: (I) all semen samples were fresh and drained into a sterile container following masturbation; (II) the liquefaction time was less than 60 minutes and the sperm concentration was greater than or equal to 60×106 mL, the semen volume was more than 2 mL, the proportion of sperm with normal morphology was more than 9%, the proportion of sperm with forward motility was more than 60%, the total motile sperm count was greater than or equal to 12×106, and the freeze-thaw survival rate was more than 60%; and (III) the abstinence period of each sperm donor volunteer was 3 days. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of Peking University Third Hospital (No.2020SZ-002). Individual consent for this retrospective analysis was waived.
Data collection
Trained sperm bank doctors used structured questionnaires to collect demographic and lifestyle information from each donor. The survey covered date of birth, ethnicity, level of education, marriage status, childbearing history, profession, place of birth, year of semen examination, and abstinence period. Each sperm donor was graded by physical examination, including height and weight. According to World Health Organization (WHO) guidelines, body mass index (BMI) was calculated as weight (kg) divided by height squared (m2) (11).
Semen collection and semen analysis
All semen samples were collected into a sterile cup by masturbation in a private room at the sperm bank. Samples were then immediately delivered to the sperm bank laboratory through a delivery window in the semen collection room. Aseptic conditions were guaranteed throughout the process, and samples were analyzed by trained clinical technicians in the same laboratory. Liquefied samples were placed in a water bath at 37 ℃ within an hour of collection. Sperm analysis was performed using a computer-aided sperm analysis (CASA, SuiJia Software, Beijing, China) to determine sperm concentration and progressive motility within 1 hour, in accordance with World Health Organization guidelines (fifth edition) (12).
Several semen quality parameters were assessed, including appearance, volume, viscosity, agglutination, liquefaction time, pH value, sperm concentration, sperm morphology, sperm motility, and the proportion of motile sperm. According to a protocol formulated by the World Health Organization (13), sperm volume was determined by drawing up the entire sample into a wide-mouthed (~2 mm) 5 mL disposable calibrated serological pipette (non-pyrogenic) by means of a mechanical device. To assess sperm concentration and motility, 10 µl of mixed semen was placed in a clean Makler chamber (at 37 ℃) and covered gently with a cover glass (14). The sample was then analyzed at a magnification of ×200, with ten of the 100 squares in the microscopic field randomly scanned and the sperm count recorded by a cytometer. Sperm pH was determined by pH test paper then compared with calibration tape. An ocular grid was used to calculate progressive motility and non-progressive motility, and total motility determined. Finally, the total sperm number as semen volume multiplied by sperm concentration was calculated and the total motile sperm count as total sperm number multiplied by total motility was determined (15).
Each semen sample was analyzed twice. An internal quality control was adopted to ensure there were no significant deviations from the results derived from different clinical technicians.
Statistical analysis
All statistical analyses were performed with SPSS 25.0 (SPSS, Chicago, IL, USA). Median and interquartile range (IQR) was used to describe continuous variables, and frequency and percentage described discrete variables. Pearson’s correlation coefficient was used to evaluate the relationships between different sperm parameters, and for differences between regions or latitudes, the Mann-Whitney U test was used to test for statistical significance (16). For non-normal distribution, median and interquartile range (IQR) and the Kruskal-Wallis H test were used as appropriate (17). The extensive experimental data set obtained from this study was used to build multiple linear regression models so the effect of confounding variables that might affect semen parameters could be excluded. All analyses involved two-sided tests and P<0.05 denoted statistical significance.
Results
A total of 1,012 participants were enrolled between 2015 and 2019. The spatial distribution of participants from 27 provinces in China is shown in Figure 1, which shows the provinces with more than 100 participants were Beijing Municipality [177], Hebei [146], and Shandong [104].
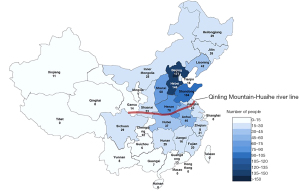
The “Qinling Mountain–Huaihe River” line is an essential geological boundary dividing northern and southern regions of China, and this was used to divide participants according to their location (Table 1). The mean (IQR) age of all participants was 26 years (23–31 years). Of the 1,012 participants, 752 were from northern China with a median (IQR) age of 27 years (23–31 years) and 360 were from southern China with a median (IQR) age of 24 years (22–29 years). In total, 94% of participants were from the Han ethnic group.
Table 1
| Characteristic | Group | Number of subjects | P | ||
|---|---|---|---|---|---|
| China | Northern China | Southern China | |||
| Age (year), n (%) | 22–24 | 126 (12.5) | 85 (11.3) | 41 (15.8) | <0.001 |
| 25–29 | 403 (39.8) | 270 (35.9) | 133 (51.2) | ||
| 30–39 | 417 (41.2) | 345 (45.9) | 72 (27.7) | ||
| 40–44 | 66 (6.5) | 52 (6.9) | 14 (5.3) | ||
| Median (IQR) | 26 (23.0–31.0) | 27 (23.0–31.0) | 24 (22.0–29.0) | ||
| Ethnicity, n (%) | Han | 955 (94.4) | 703 (93.5) | 252 (96.9) | 0.038 |
| Others | 57 (5.6) | 49 (6.5) | 8 (3.1) | ||
| Education, n (%) | Junior college and lower | 167 (16.5) | 114 (15.2) | 25 (9.6) | <0.001 |
| Undergraduate | 612 (60.5) | 456 (60.6) | 156 (60) | ||
| Postgraduate and higher | 233 (23.0) | 182 (24.2) | 79 (30.4) | ||
| Marriage, n (%) | Unmarried | 769 (76.0) | 546 (72.6) | 223 (85.8) | <0.001 |
| Married | 243 (24.0) | 206 (27.4) | 37(14.2) | ||
| Volunteer status, n (%) | Students | 399 (39.4) | 253 (33.6) | 146 (56.2) | <0.001 |
| Others | 613 (60.6) | 499 (66.4) | 114 (43.8) | ||
| BMI, n (%) | <18.5 kg/m2 | 8 (0.8) | 5 (0.7) | 3 (1.2) | 0.009 |
| 18.5–24.9 kg/m2 | 812 (80.2) | 588 (78.2) | 224 (86.2) | ||
| 25–29.9 kg/m2 | 192 (19.0) | 159 (21.1) | 33 (12.6) | ||
IQR, inter quartile range; BMI, body mass index.
As shown in Table 2, progressive motility was significantly and negatively associated with volunteer status and positively associated with BMI, while semen volume was significantly and positively associated with marital status. Statistical analysis showed no significant difference in semen quality between the two regions (Table 3, P>0.05). Although most parameters did not differ between the two regions, some showed a strong correlation with each other, and Table 4 shows the spearman correlation coefficients for these relationships. Semen volume exhibited a convincing negative correlation with sperm concentration (r=−0.239, P<0.001), whereas progressive motility exhibited a convincing positive correlation with sperm concentration (r=0.169, P<0.001).
Table 2
| Characteristic | Semen parameters (β) | |||||
|---|---|---|---|---|---|---|
| SV | SC | PM | FSC | FPM | PSR | |
| Age | −0.017 (−0.227, 0.146) | 0.034 (−4.778, 12.148) | −0.022 (−1.420, 0.791) | 0.064 (−0.453, 4.681) | −0.024 (−2.719, 1.437) | 0.039 (−0.007, 0.022) |
| Marital status | 0.078b (0.010, 0.460) | −0.067 (−19.290, 1.077) | −0.053 (−2.274, 0.386) | −0.040 (−4.722, 1.457) | 0.006 (−2.299, 2.704) | 0.027 (−0.011, 0.024) |
| Volunteer status | 0.008 (−0.179, 0.220) | 0.004 (−8.632, 9.478) | −0.106a (−2.833, −0.472) | 0.017 (−2.135, 3.349) | 0.032 (−1.294, 3.146) | 0.073 (−0.001, 0.030) |
| BMI | 0.013 (−0.156, 0.239) | −0.005 (−9.726, 8.208) | 0.086a (0.440, 2.778) | 0.001 (−2.661, 2.769) | 0.021 (−1.482, 2.915) | −0.019 (−0.020, 0.011) |
| Ethnicity | 0.035 (−0.150, 0.535) | 0.028 (−8.467, 22.555) | −0.018 (−2.608, 1.445) | 0.021 (−3.138, 6.276) | −0.002 (−3.918, 3.704) | 0.034 (−0.012, 0.041) |
| Education | 0.019 (−0.097, 0.175) | 0.051 (−1.466, 10.816) | 0.032 (−0.419, 1.185) | 0.051 (−0.442, 3.285) | 0.013 (−1.206, 1.812) | −0.012 (−0.014, 0.007) |
a and b indicate that the analysis of variance was significant at the P value <0.01 and <0.05 level, respectively. SV, semen volume, mL; SC, sperm concentration, 106/mL; PM, progressive motility, %; FSC, frozen-thawed sperm concentration, 106/mL; FPM, frozen-thawed progressive motility, %; PSR, post-thaw survival rate, %; BMI, body mass index.
Table 3
| Characteristic | Northern China (N=752) | Southern China (N=260) | P |
|---|---|---|---|
| Semen volume, mL | 3.0 (2.4–4.0) | 3.2 (2.4–4.4) | 0.142 |
| Sperm concentration, 106/mL | 134.0 (106.0–168.0) | 128.2 (102.3–163.0) | 0.155 |
| Progressive motility, % | 65.0 (60.0–71.0) | 65.0 (60.0–71.0) | 0.964 |
| Frozen-thawed sperm concentration, 106/mL | 71.9 (63.0–84.9) | 70.0 (62.0–82.0) | 0.379 |
| Frozen-thawed progressive motility, % | 53.0 (48.0–58.0) | 52.0 (48.0–58.0) | 0.223 |
| Post-thaw survival rate, % | 0.8 (0.8–0.9) | 0.8 (0.7–0.9) | 0.256 |
Data are shown as median (IQR). IQR, inter quartile range.
Table 4
| SV | SC | PM | FSC | FPM | PSR | |
|---|---|---|---|---|---|---|
| SV | ||||||
| Spearman Corr. | 1 | −0.239* | 0.019 | −0.214* | −0.055 | −0.079* |
| P value | − | <0.001 | 0.556 | <0.001 | 0.081 | 0.012 |
| SC | ||||||
| Spearman Corr. | −0.239* | 1 | 0.169* | 0.861* | 0.078 | −0.056 |
| P value | <0.001 | − | <0.001 | <0.001 | 0.013 | 0.073 |
| PM | ||||||
| Spearman Corr. | 0.019 | 0.169* | 1 | 0.094* | 0.515* | −0.367* |
| P value | 0.556 | <0.001 | − | 0.003 | <0.001 | <0.001 |
| FSC | ||||||
| Spearman Corr. | −0.214* | 0.861* | 0.094* | 1 | 0.067* | 0.005 |
| P value | <0.001 | <0.001 | 0.003 | − | 0.033 | 0.881 |
| FPM | ||||||
| Spearman Corr. | −0.055* | 0.078* | 0.515* | 0.067* | 1 | 0.555* |
| P value | 0.081 | 0.013 | <0.001 | 0.033 | − | <0.001 |
| PSR | ||||||
| Spearman Corr. | −0.079* | −0.056 | −0.367* | 0.005 | 0.555* | 1 |
| P value | 0.012 | 0.073 | <0.001 | 0.881 | <0.001 | − |
* P value <0.05. Spearman Corr., Spearman correlation coefficient; SV, semen volume, mL; SC, sperm concentration, 106/mL; PM, progressive motility, %; FSC, frozen-thawed sperm concentration, 106/mL; FPM, frozen-thawed progressive motility, %; PSR, post-thaw survival rate, %.
To further illustrate the regional difference in semen quality, we refined the north and south by excluding remote areas while setting provinces in south-eastern China (including Jiangsu, Shanghai, Zhejiang, Fujian, Jiangxi, and Anhui) and southern China (including Guangdong, Guangxi, Hainan, Hongkong, Macao, and Taiwan) as the south, and provinces in northern China (including Beijing, Tianjin, Hebei, Shanxi, and Inner Mongolia) and northeast (including Heilongjiang, Jilin, and Liaoning) China as the north. This reduced the number of samples to 667 and led to a significant difference in sperm concentration between the two regions (Table 5, P=0.015). No significant differences were observed between the two regions in terms of semen volume, progressive motility, post-thaw survival rate, frozen-thawed sperm concentration, and frozen-thawed progressive motility (P>0.05). To further verify this result, we then divided China into four regions by latitude, including regions between 18° to 27° north latitude, 27° to 36° north latitude, 36° to 45° north latitude, and 45° to 54° north latitude, and performed statistical analysis. As shown in Table 6, there were statistically significant differences in sperm concentration among men from different latitudes (P=0.04), but no statistically significant differences in semen volume, progressive motility, frozen-thawed sperm concentration, frozen-thawed progressive motility, and post-thaw survival rate. The sperm concentrations of those from 18° to 27° north latitude were significantly lower than those from 36° to 45° and 45° to 54° [median 131, 134 and 146, respectively, P=0.021 (18° to 27° vs. 36° to 45°) and P=0.01 (18° to 27° vs. 45° to 54°)].
Table 5
| Characteristic | Northern China (N=521) | Southern China (N=146) | P |
|---|---|---|---|
| Semen volume, mL | 3.0 (2.2–4.0) | 3.1 (2.4–4.2) | 0.189 |
| Sperm concentration, 106/mL | 134.0 (106.7–170.0) | 125.0 (99.5–156.3) | 0.015 |
| Progressive motility, % | 65.0 (60.0–70.0) | 64.0 (60.0–70.0) | 0.620 |
| Frozen-thawed sperm concentration, 106/mL | 71.7 (63.0–86.8) | 69.7 (62.0–79.3) | 0.090 |
| Frozen-thawed progressive motility, % | 53.0 (48.0–58.0) | 52.0 (47.8–58.0) | 0.140 |
| Post-thaw survival rate, % | 0.8 (0.8–0.9) | 0.8 (0.8–0.9) | 0.248 |
Data are shown as median (IQR). IQR, inter quartile range.
Table 6
| Characteristic | Latitude (°N) | P | |||
|---|---|---|---|---|---|
| 45–54 (N=29) | 36–45 (N=622) | 27–36 (N=310) | 18–27 (N=51) | ||
| Semen volume, mL | 2.6 (2.2–3.6) | 3.0 (2.2–4.0) | 3.2 (2.4–4.2) | 3.4 (2.4–4.6) | 0.084 |
| Sperm concentration, 106/mL | 146.9 (121.5–177.0)b | 134.0 (105.7–170.0)b | 131.0 (104.5–163.2) | 120.0 (95.1–158.0) | 0.040 |
| Progressive motility, % | 64.0 (60.0–73.5) | 65.0 (60.0–70.0) | 65.0 (61.0–71.0) | 65.0 (59.0–71.0) | 0.758 |
| Frozen-thawed sperm concentration, 106/mL | 73.0 (61.7–84.8) | 72.0 (63.0–86.1) | 71.6 (62.0–82.0) | 65.0 (60.0–75.3) | 0.108 |
| Frozen-thawed progressive motility, % | 50.0 (48.0–56.5) | 53.0 (48.0–58.3) | 52.0 (48.0–58.3) | 53.0 (47.0–58.0) | 0.464 |
| Post-thaw survival rate, % | 0.8 (0.7–0.9) | 0.8 (0.8–0.9) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.080 |
Data are shown as median (IQR). b, Compared with 18-27 °N, P value <0.05. IQR, inter quartile range.
Discussion
Male reproductive health is an area of increasing concern, and whether differences in semen quality exist between different regions remains unclear. In this study, we performed a quantitative correlation analysis of semen quality in 667 sperm donors between northern and southern China. Our data revealed donors living in northern China had significantly higher sperm concentrations than those in southern China. Similarly, there were statistically significant differences in sperm concentration among men from different latitudes. Sperm concentration is an important parameter of semen and is considered an indicator of fertility. Our results are the first to comprehensively describe differences in semen quality between the north and south of China, and these regional differences may have biological significance.
A previous study with a population of healthy Chinese men showed age-related changes were not evident in terms of sperm concentration among different age groups (18). These findings were consistent with a previous meta-analysis performed with a stronger methodology, which also found no relationship between male age and sperm concentration, although other important parameters for male fertility, including motility and morphology, were affected by age (19). Our present analysis concurred with these earlier findings in that we found no significant differences between male age and sperm concentration. According to relevant statistics, the distribution of the Chinese population has undergone tremendous changes. In recent years, the population of the north has been on a decline, whereas the south shows an opposite trend (20). This is believed to be related to the migration caused by different distributions of the regional economy (21) and has led to a heavier level of pollution. Consistent with previous studies demonstrating the negative impact of pollution on semen quality (22), we observed a higher concentration of sperm in the semen samples from donors living in the north, which is in accordance with the milder levels of pollution in that region. This may also explain the better semen quality in another study of volunteers who lived in rural areas (23), where population density is low. In addition, people tend to suffer from greater psychological burdens in more densely populated areas. The association between mental stress and reduced semen quality is supported by a study that demonstrated biological plausibility (24). Under mental stress, the male endocrine system is interrupted, which manifests as low levels of testosterone and luteinizing hormone, interfering with sperm production and reducing fertility (25). Therefore, mental stress may also account for the regional differences in sperm concentration between northern and southern China. The influence of environmental factors and lifestyle on semen quality may be explained by the changes in fertility patterns brought about by migration (26).
In addition to the factors mentioned above, others such as seasons, ethnicity, dietary patterns, and obesity may also affect semen quality. A previous assessment of male infertility data in the United States showed that compared with Asian men, white males had a higher total sperm number but lower sperm concentration (27). In the present study, only 5.6% of our sperm donors were from ethnic minorities, with most from the Han ethnic group. Thus, due to limited data, we were unable to determine whether the difference in semen quality between northern and southern China was related to ethnicity.
In terms of diet, a cross-sectional study in Spain previously assessed the relationship between dietary patterns and fertility and suggested adherence to a healthy diet was positively correlated with sperm concentration and sperm motility (28). A Chinese study also suggested healthy dietary patterns have beneficial effects on sperm concentration, total sperm count, and progressive sperm motility in males (29). In contrast to the western diet, the traditional Chinese diet is based on grains, and due to differences in latitude and climate, people in southern China tend to eat rice while those in the north eat flour. At present, there is no literature relating to the effect of rice and flour on semen quality. However, a previous study used reliable blood glucose index (GI) data sources and showed sperm concentration was negatively correlated with the frequency and quantity of grain consumption (30), because grains are rich in starch. When dietary intake leads to an increase in blood sugar, hyperglycemia will increase the risk of inflammation, leading to a decline in semen quality (31). Data relating to the relationship between diet and semen quality or factors related to male infertility, remains limited, and additional prospective studies, including those exploring specific biological mechanisms, are required (32). Therefore, further investigation is needed to identify how differences in the diet structure of southern and northern Chinese men can influence semen quality.
With regard to seasonality, a previous report found the sperm concentration and total sperm count of outdoor workers in summer were significantly lower than from the same workers collected in winter (33). Previous study has also shown spring is associated with higher sperm motility and morphologically normal sperm in comparison to other seasons (34). In our present study, we did not record the season in which donors provided samples and will incorporate this data in future studies.
With regards to obesity, the reduction of fertility in obese men is thought to be linked to changes in the levels of hormones that help form sperm (35). Since obese sperm donors were excluded from the current study, our results are independent of the influence of obesity. A high BMI is known to be associated with negative effects on sperm quality (36), and a previous study indicated differences in the distribution of BMI between adolescents in northern and southern China. Adolescents in the northern regions of China generally have a bulky figure, a phenomenon that is independent of the level of socio-economic development (37). Previous study suggested adaptation to the surrounding environment may cause regional differences in human growth and development (38). Moreover, sufficient sunshine, low annual average temperature, and the seasonal variability in temperature in northern China are all beneficial to the accumulation of body fat (39,40), which may be the reason for the higher BMI of sperm donors in that region.
This was a preliminary study relating to regional differences in the semen quality of sperm donors in northern and southern China. Future investigations are required to validate the geographic variations in larger regions with a more accurate study design to address the issues listed above. More participants from southern China should also be included in future analyses to verify our conclusions and validate the generalizability of our findings. Due to technical limitations, we currently do not have enough data to show the distribution of air pollution on a map.
Although the reasons for regional differences in semen quality remain unclear, lifestyle and environmental factors have been implicated in the decline of sperm quality (41-45). We hypothesize that environmental pollution and mental stress due to an increased population size may be the main factors underlying the difference in sperm quality in men living in northern and southern China.
Conclusions
We identified differences in sperm quality between men living in northern and southern China. Our data revealed sperm donors living in the north of China have significantly higher sperm concentrations than those in the south.
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Funding: This study was supported by the Beijing Natural Science Foundation (Grant No. 7182177 and No. 7194333) and the National Natural Science Foundation of China (Grant No. 81871204 and No.81901535).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-578/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-578/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-578/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tomaiuolo R, Veneruso I, Cariati F, et al. Microbiota and Human Reproduction: The Case of Male Infertility. High Throughput 2020;9:10. [Crossref] [PubMed]
- Jørgensen N, Carlsen E, Nermoen I, et al. East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod 2002;17:2199-208. [Crossref] [PubMed]
- Auger J, Jouannet P. Evidence for regional differences of semen quality among fertile French men. Fédération Francaise des Centres d'Etude et de Conservation des Oeufs et du Sperme humains. Hum Reprod 1997;12:740-5. [Crossref] [PubMed]
- López-Teijón M, Elbaile M, Alvarez JG. Geographical differences in semen quality in a population of young healthy volunteers from the different regions of Spain. Andrologia 2008;40:318-28. [Crossref] [PubMed]
- Elbardisi H, Majzoub A, Al Said S, et al. Geographical differences in semen characteristics of 13 892 infertile men. Arab J Urol 2018;16:3-9. [Crossref] [PubMed]
- Li Y, Yang X, Guo X, et al. Analysis of semen detection results of 5648 infertile male cases in Guangzhou China. Chinese Journal of Health Laboratory Technology 2014;24:1125-7.
- Wei Y, Chen W, Li J, et al. Analysis of semen quality of 1626 infertile men in western Guangxi China. Chinese Journal of Human Sexuality 2017;26:116-8.
- Ma B, Gao K, Chen S, et al. Semen quality analysis of 6325 infertile men in Gansu China. Chinese Journal of Andrology 2019;33:36-9.
- Fisch H, Goluboff ET. Geographic variations in sperm counts: a potential cause of bias in studies of semen quality. Fertil Steril 1996;65:1044-6. [Crossref] [PubMed]
- World Health Organization. WHO laboratory manual for the examination and processing of human semen, 5th ed. World Health Organization 2010.
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i-xii, 1-253. [PubMed]
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231-45. [Crossref] [PubMed]
- Cole M, Lindeque P, Halsband C, et al. Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 2011;62:2588-97. [Crossref] [PubMed]
- Makler A. The improved ten-micrometer chamber for rapid sperm count and motility evaluation. Fertil Steril 1980;33:337-8. [Crossref] [PubMed]
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the Covid 19 pandemic: What advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci 2020;22:339-42. [Crossref] [PubMed]
- Bürkner PC, Doebler P, Holling H. Optimal design of the Wilcoxon-Mann-Whitney‐test. Biom J 2017;59:25-40. [Crossref] [PubMed]
- Chan Y, Walmsley RP. Learning and understanding the Kruskal-Wallis one-way analysis-of-variance-by-ranks test for differences among three or more independent groups. Phys Ther 1997;77:1755-62. [Crossref] [PubMed]
- Zhu QX, Meads C, Lu ML, et al. Turning point of age for semen quality: a population-based study in Chinese men. Fertil Steril 2011;96:572-6. [Crossref] [PubMed]
- Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril 2001;75:237-48. [Crossref] [PubMed]
- Deng Q. Population Mobility and Urban Future. Think Tank of Science & Technology 2017;1:34-9.
- Lin D. A comparison of social adaptation among rural-to-urban migrants with different careers. Chinese Mental Health Journal 2007;21:400-3.
- Zhang HT, Zhang Z, Cao J, et al. Ambient ozone pollution is associated with decreased semen quality: longitudinal analysis of 8945 semen samples from 2015 to 2018 and during pollution-control period in Beijing, China. Asian J Androl 2019;21:501-7. [Crossref] [PubMed]
- Wang N, Gu H, Gao Y, et al. Study on Influencing Factors of Semen Quality in Fertile Men. Front Physiol 2022;13:813591. [Crossref] [PubMed]
- Ilacqua A, Izzo G, Emerenziani GP, et al. Lifestyle and fertility: the influence of stress and quality of life on male fertility. Reprod Biol Endocrinol 2018;16:115. [Crossref] [PubMed]
- King JA, Rosal MC, Ma Y, et al. Association of stress, hostility and plasma testosterone levels. Neuro Endocrinol Lett 2005;26:355-60. [PubMed]
- Zou Z, Hu H, Song M, et al. Semen quality analysis of military personnel from six geographical areas of the People's Republic of China. Fertil Steril 2011;95:2018-23, 2023.e1-3.
- Khandwala YS, Zhang CA, Li S, et al. Racial Variation in Semen Quality at Fertility Evaluation. Urology 2017;106:96-102. [Crossref] [PubMed]
- Salas-Huetos A, James ER, Aston KI, et al. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod Biol 2019;19:219-24. [Crossref] [PubMed]
- Cao LL, Chang JJ, Wang SJ, et al. The effect of healthy dietary patterns on male semen quality: a systematic review and meta-analysis. Asian J Androl 2022;24:549-57. [Crossref] [PubMed]
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281-3. [Crossref] [PubMed]
- Kristo AS, Matthan NR, Lichtenstein AH. Effect of diets differing in glycemic index and glycemic load on cardiovascular risk factors: review of randomized controlled-feeding trials. Nutrients 2013;5:1071-80. [Crossref] [PubMed]
- Liu CY, Chou YC, Chao JC, et al. The Association between Dietary Patterns and Semen Quality in a General Asian Population of 7282 Males. PLoS One 2015;10:e0134224. [Crossref] [PubMed]
- Levine RJ, Mathew RM, Chenault CB, et al. Differences in the quality of semen in outdoor workers during summer and winter. N Engl J Med 1990;323:12-6. [Crossref] [PubMed]
- Chen Z, Godfrey-Bailey L, Schiff I, et al. Impact of seasonal variation, age and smoking status on human semen parameters: The Massachusetts General Hospital experience. J Exp Clin Assist Reprod 2004;1:2. [Crossref] [PubMed]
- Škurla M, Rybář R. Obesity and reduced fertility of men. Ceska Gynekol 2018;83:212-7. [PubMed]
- Wang EY, Huang Y, Du QY, et al. Body mass index effects sperm quality: a retrospective study in Northern China. Asian J Androl 2017;19:234-7. [Crossref] [PubMed]
- Ji CY, Sun JL. Geographic and population difference of BMI in Chinese school-age youth. Chinese Journal of Pediatrics 2004;42:328-32. [PubMed]
- Takahashi E. Geographic Distribution of Human Stature and Environmental Factors-An Ecologic Study. Journal of the Anthropological Society of Nippon 1971;79:259-86. [Crossref]
- Li Q, Dong K, Xu L, et al. The distribution of three candidate cold-resistant SNPs in six minorities in North China. BMC Genomics 2018;19:134. [Crossref] [PubMed]
- Ji C, Yuan J, Wen D. Environmental Factors Contributing to Regional Differences in the Growth of China’s Rural Youth. Sports Science 1992;5:38-42+6+95.
- Sharpe RM. Lifestyle and environmental contribution to male infertility. Br Med Bull 2000;56:630-42. [Crossref] [PubMed]
- Blay RM, Pinamang AD, Sagoe AE, et al. Influence of Lifestyle and Environmental Factors on Semen Quality in Ghanaian Men. Int J Reprod Med 2020;2020:6908458. [Crossref] [PubMed]
- Choe SA, Kim S, Im C, et al. Nighttime environmental noise and semen quality: A single fertility center cohort study. PLoS One 2020;15:e0240689. [Crossref] [PubMed]
- Skakkebæk NE, Lindahl-Jacobsen R, Levine H, et al. Environmental factors in declining human fertility. Nat Rev Endocrinol 2022;18:139-57. [Crossref] [PubMed]
- Aitken RJ. The changing tide of human fertility. Hum Reprod 2022;37:629-38. [Crossref] [PubMed]


