
Prognostic value of galectin-1 and galectin-3 expression in localized urothelial bladder cancer
Highlight box
Key findings
• Gal-1 and Gal-3 expression does not predict for overall survival or progression free survival for patients with urothelial carcinoma.
What is known and what is new?
• Prior studies found that Gal-1 and Gal-3 may serve as prognostic biomarkers in various solid tumors in including bladder cancer.
• We discovered that there is significant intra-tumoral Galectin-1 and Galectin-3 heterogeneity which makes it challenging to use as a potential biomarker in bladder cancer.
• We did not find Gal-1 or Gal-3 to be a prognostic biomarker, as had been shown in prior studies.
What is the implication, and what should change now?
• Due to tumor heterogeneity, additional methods to evaluate Gal-1 and Gal-3 should be considered in future studies, such as serum or urinary galectin levels.
Introduction
Bladder cancer is the sixth most common malignancy of the urinary system, with an incidence of over 80,000 new cases a year and 17,000 deaths per year in the United States (1). Localized bladder cancer may be categorized into two groups: non-muscle invasive bladder cancer (NMIBC, 70–80%) and muscle invasive bladder cancer (MIBC, 20–30%) (2). For patients with NMIBC, 5-year overall survival (OS) is over 90% but for patients with MIBC, five-year survival is only 50% with many patients progressing to metastatic disease despite treatment with chemotherapy in combination with local therapies (surgery or radiation) (3). In the localized setting, there are no validated biomarkers that can help escalate or de-escalate therapy.
Galectins are a class of proteins within the lectin superfamily which bind to β-galactoside sugars within the glycome, deciphering information encoded by glycosylation machinery and translating this information into cellular functions (4). First described in 1975, the galectin pathway has been linked to the tumorigenesis, tissue regeneration, brain development, and cancer pathogenesis (5-7). There are 11 known galectins expressed in humans. Of these, galectin-1 (Gal-1) and galectin-3 (Gal-3) have emerged as two potential biomarkers associated with prognosis in multiple tumor types. For example, in one cohort of patients with pancreatic ductal adenocarcinoma, patients without Gal-1 expression had significantly longer survival than patients with positive Gal-1 expression (8). In thyroid cancer, Gal-1 expression is increased several fold in neoplastic follicular cells compared with benign tissue, and Gal-3 expression is used routinely to differentiate malignant thyroid tumors from benign tumors (9).
For Gal-3, differences in expression between malignant and benign tissue depend on tissue of origin. For tumors of the digestive and urinary system, Gal-3 expression is usually increased but for tumors of the reproductive system, Gal-3 expression is decreased.
In urothelial bladder cancer models, upregulation of Gal-1 and Gal-3 have been associated with increased tumor cell viability in vitro and in xenograft models, with increased proliferation, invasion, and clonogenicity in vitro (10,11). One prior study evaluated Gal-3 protein expression and found that protein expression levels were increased in MIBC compared with NMIBC and elevated Gal-3 protein expression levels were associated with OS (12). However, this study had a limited number of patients, did not describe where Gal-3 expression was measured (benign urothelium vs. muscle invasive disease), and did not control for other clinical variables which may impact OS.
In our study, we sought to determine the relationship between Gal-1 and Gal-3 expression with respect to OS and recurrence-free survival (RFS), in a large cohort of patients who underwent cystectomy for MIBC and NMIBC. We present the following article in accordance with the REMARK reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-494/rc).
Methods
Patient selection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Atrium Health (IRB# LCI-GU-MIUC-GAL-001OB) and individual consent for this retrospective analysis was waived. All patients with MIBC and NMIBC who had a radical cystectomy for UC between January 1, 2000 and May 1, 2010 were identified for the study.
Pathologic data
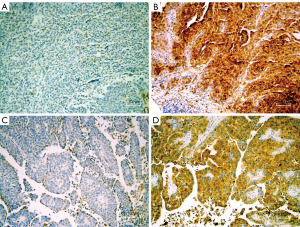
Tissue microarrays (TMA) were generated using formalin-fixed paraffin-embedded tumor blocks from chemotherapy naïve cystectomy specimens which have been previously reported (13,14). For TMA construction, the original hematoxylin and eosin (H&E) slides were reviewed, and diagnostic tissue was marked for biopsy using a manual arrayer (Beecher Instruments, Sun Prairie, WI). One to three tissue cores (each 1.5 mm) of representative areas were used for the array. H&E slides from the TMAs were prepared, and the histopathological diagnosis of tissue samples represented on the TMA were identified for each patient. These tissues included benign urothelium, noninvasive papillary urothelial carcinoma (pTa), urothelial carcinoma in-situ (pTis), invasive urothelial carcinoma (iUC) (pT1-pT4). Immunohistochemistry for Gal-1 (clone C-8, Santa Cruz Biotechnology) and Gal-3 (clone M3/38, Santa Cruz Biotechnology) was performed on unstained TMA slides (15-17). For both antibodies, galectin expression was scored based on the average intensity of staining (0, 1+, 2+, or 3+) and the percentage of tumor cells showing positive staining. All slides were manually scored by a single pathologist with >20 years of experience in surgical pathology and immunohistochemistry (Figure 1). Study pathologist was blinded to all clinical data regarding the patients. The widely accepted quantitative H-score (product of % and intensity) was utilized for analysis, as has been done in prior galectin studies (18,19).
Statistical analysis
Demographic and clinical characteristics were summarized with frequencies and percentages or medians and interquartile ranges (IQR), as appropriate. OS was defined as the time from cystectomy until death. RFS was defined as the time from cystectomy until first recurrence or death. Demographic and clinical characteristics including sex, race, pathologic nodal stage (N stage), pathologic tumor stage (T stage), carcinoma in situ path, gross margins, microscopic margins, preoperative packed cell volume (PCV), preoperative albumin, number of nodes, number of positive nodes, tumor size, American Society of Anesthesiologists class, and Charlson comorbidity index were collected (20-22). Subjects who had pathologic T stage of T2 or higher were classified as muscle invasive, while those with lower than T2 stage were classified as non-muscle invasive.
The variable of interest, H-score, was defined as the product of the percentage of cells staining positive (0–100) and intensity score (0–3) scored by a single specialty trained pathologist (CL), as has been described previously (23). Because subjects had multiple cores, the maximum H-score for the highest T stage core of each subject was used in analyses (i.e., if a patient had cores of normal tissue, Ta, and Tis, the Tis was used).
Survival end points were analyzed using Kaplan-Meier and Cox Proportional Hazards methods. Univariate and multivariable Cox proportional hazards models were fit for each survival endpoint including the variable of interest, Gal-1 or Gal-3 H-scores, and demographic and clinical characteristics. The models were fit separately for Gal-1 and Gal-3, including subjects who had at least one non-missing Gal-1 and Gal-3 score, respectively. All statistical analyses were conducted in SAS 9.4 (SAS Institute, Inc., Cary, NC, USA) with a significance level of 0.05.
Results
A total of 301 patients underwent cystectomy without neoadjuvant chemotherapy. 71% (215/301) of patients had MIBC (pT2 or higher), and 24% (70/297) of patients had pathologic node positive disease. 4 cystectomy specimens did not have data on node status. After a median follow up of 37.6 months, 44% of patients were alive. Clinical and demographic characteristics are summarized in Table 1.
Table 1
| Demographics | Total Number (percentage), N=301 (100%) |
|---|---|
| Sex | |
| Female | 62 (20.6%) |
| Male | 239 (79.4%) |
| Race | |
| White | 279 (92.7%) |
| Black | 17 (5.6%) |
| Other | 3 (1.0%) |
| Unknown | 2 (0.7%) |
| Vital status | |
| Alive | 132 (43.9%) |
| Dead | 169 (56.1%) |
| Recurrence | |
| No | 226 (75.1%) |
| Yes | 75 (24.9%) |
| ASA class | |
| 1 or 2 | 82 (27.2%) |
| 3 | 199 (66.1%) |
| 4 | 20 (6.6%) |
| BMI (kg/m2) | |
| Missing | 65 |
| <18.5 | 4 (1.7%) |
| 18.5–24.9 | 86 (36.4%) |
| 25.0–29.9 | 80 (33.9%) |
| ≥30 | 66 (28.0%) |
| Follow-up time (months) | 37.6 (13.4, 64.0)* |
| PCV Preop (N=299) | 42.0 (38.0, 45.0)* |
| Albumin Preop (N=210) | 4.2 (3.9, 4.5) |
| CCI score age adjusted categorical (N=288) | |
| Missing | 13 |
| 0≤ CCI ≤2 | 108 (37.5%) |
| 2< CCI <5 | 108 (37.5%) |
| CCI ≥5 | 72 (25.0%) |
*, indicates median (25% quartile, 75% quartile). ASA, American Society of Anesthesiologists; BMI, body mass index; PCV, packed cell volume; CCI, Charlson Comorbidity Index.
We analyzed 657 cores from the 301 cystectomy specimens (Figure 2 and Table 2). The median number of cores per cystectomy was 2 (range, 1–7). Cores were taken from adjacent benign urothelium (30.2%), noninvasive papillary urothelial carcinoma (Ta, 4.3%), urothelial carcinoma in situ (Tis, 27.1%), and invasive urothelial carcinoma (pT1-pT4, 38.4%). At the time of sample collection, no distinction was made between pT1 and pT2 or greater disease. One sample could not be classified. Cores may have been taken at the same level (i.e., Patient 852 had 5 cores taken from cystectomy specimen, all benign urothelium cores) or from different levels (i.e., Patient 515 had 5 cores taken: 1 from benign urothelium, 2 from pTa, 1 from TIS, and 1 from invasive tissue).
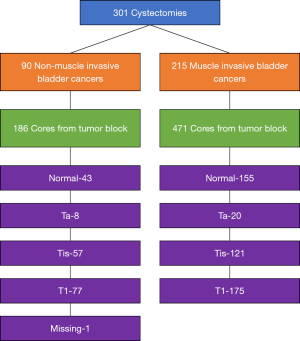
Table 2
| Pathology characteristics | Total (N=301) |
|---|---|
| Pathological tumor stage | |
| Ta | 7 (2.3%) |
| Tis | 38 (12.6%) |
| T1 | 41 (13.6%) |
| T2a | 54 (17.9%) |
| T2b | 44 (14.6%) |
| T3a | 47 (15.6%) |
| T3b | 36 (12.0%) |
| T4a | 32 (10.6%) |
| T4b | 2 (0.7%) |
| Pathological node stage | |
| N0 | 231 (76.7%) |
| N1 | 30 (10.0%) |
| N2 | 40 (13.3%) |
| Presence of Cis | |
| Missing | 4 |
| No | 135 (45.5%) |
| Yes | 162 (54.5%) |
| Gross margins | |
| Missing | 72 |
| Negative | 221 (96.5%) |
| Positive | 8 (3.5%) |
| Microscopic margins | |
| Missing | 6 |
| Negative | 265 (89.8%) |
| Positive | 30 (10.2%) |
| Number of nodes (N=297) | 8.0 (5.0, 14.0)* |
| Tumor size (N=288, centimeter) | 2.5 (1.0, 4.0)* |
*, indicates median (25% quartile, 75% quartile). Ta, noninvasive papillary carcinoma; Tis, carcinoma in situ; T1, tumor invades lamina propria; T2a, tumor invades superficial muscularis propria; T2b, tumor invades deep muscularis propria; T3a, tumor invades perivesical tissue microscopically; T3b, tumor invades perivesical tissue macroscopically; T4a, extravesical tumor invades prostatic stroma, seminal vesicles, uterus, vagina; T4b, extravesical tumor invades pelvic wall, abdominal wall; N0, no lymph node metastasis; N1, single regional lymph node metastasis in the true pelvis; N2, multiple regional lymph node metastasis in the true pelvis; Cis, carcinoma in situ.
T stage, surgical margins, and preoperative packed cell volume (PCV) were associated with OS (Table 3) in the model with the Gal-1 cohort. T stage, surgical margins, and preoperative PCV were associated with OS (Table 4) in the model with the Gal-3 cohort. However, Gal-1 and Gal-3 were not independently associated with OS (Tables 3,4) or RFS (Tables S1,S2).
Table 3
| Variable | Cox univariate, hazard ratio (95% CI) | Cox univariate, P value | Cox multivariate, hazard ratio (95% CI) | Cox multivariate, P value (n=233) |
|---|---|---|---|---|
| Sex | 0.1199 | 0.1190 | ||
| Female | – | – | ||
| Male | 0.72 (0.47, 1.09) | 0.68 (0.43, 1.09) | ||
| Race | 0.7238 | 0.6630 | ||
| Black | – | – | ||
| Other | 1.79 (0.37, 8.62) | 2.25 (0.42, 12.21) | ||
| White | 1.28 (0.60, 2.75) | 1.23 (0.56, 2.70) | ||
| pT | <0.0001 | 0.0004 | ||
| T2, T3, or T4 | 3.02 (1.96, 4.65) | 2.33 (1.43, 3.81) | ||
| Ta, Tis, or T1 | – | – | ||
| pN | <0.0001 | 0.0783 | ||
| N0 | – | – | ||
| N1 or N2 | 2.70 (1.89, 3.87) | 1.58 (0.96, 2.62) | ||
| ASA class | 0.0581 | 0.1845 | ||
| 1 or 2 | – | – | ||
| 3 | 1.44 (0.95, 2.20) | 1.42 (0.90, 2.25) | ||
| 4 | 2.17 (1.10, 4.28) | 1.88 (0.87, 4.03) | ||
| Tis path | 0.2249 | 0.2930 | ||
| No | – | – | ||
| Yes | 0.81 (0.58, 1.14) | 1.24 (0.83, 1.83) | ||
| Surgical margins | <0.0001 | 0.0118 | ||
| Negative | – | – | ||
| Positive | 3.78 (2.39, 6.00) | 2.12 (1.21, 3.71) | ||
| CCI score age adjusted | 0.0004 | 0.2055 | ||
| 0≤ CCI ≤2 | 0.43 (0.28, 0.65) | 0.64 (0.39, 1.05) | ||
| 2< CCI <5 | 0.64 (0.43, 0.97) | 0.74 (0.47, 1.17) | ||
| CCI ≥5 | – | – | ||
| PCV preop | 0.94 (0.91, 0.97) | 0.0004 | 0.958 (0.922, 0.995) | 0.0280 |
| Number of nodes | 0.99 (0.97, 1.02) | 0.6167 | 0.99 (0.96, 1.02) | 0.4645 |
| Positive nodes | 1.18 (1.11, 1.25) | <0.0001 | 1.096 (1.003, 1.199) | 0.0686 |
| Tumor size (cm) | 1.15 (1.09, 1.21) | <0.0001 | 1.05 (0.97, 1.15) | 0.2279 |
| Galectin-1 H-score (for 25 units increase) | 1.00 (0.96, 1.04) | 0.9790 | 1.02 (0.98, 1.06) | 0.4443 |
Ta, noninvasive papillary carcinoma; Tis, carcinoma in situ; T1, tumor invades lamina propria; T2a, tumor invades superficial muscularis propria; T2b, tumor invades deep muscularis propria; T3a, tumor invades perivesical tissue microscopically; T3b, tumor invades perivesical tissue macroscopically; T4a, extravesical tumor invades prostatic stroma, seminal vesicles, uterus, vagina; T4b, extravesical tumor invades pelvic wall, abdominal wall; N0, no lymph node metastasis; N1, single regional lymph node metastasis in the true pelvis; N2, multiple regional lymph node metastasis in the true pelvis; ASA, American Society of Anesthesiologists; PCV, packed cell volume; CCI, Charlson Comorbidity Index.
Table 4
| Variable | Cox univariate, hazard ratio (95% CI) | Cox univariate, P value | Cox multivariate, hazard ratio (95% CI) | Cox multivariate, P value (n=232) |
|---|---|---|---|---|
| Sex | 0.0608 | 0.0565 | ||
| Female | – | – | ||
| Male | 0.67 (0.43, 1.02) | 0.621 (0.388, 0.995) | ||
| Race | 0.8398 | 0.8055 | ||
| Black | – | – | ||
| Other | 1.60 (0.33, 7.69) | 1.77 (0.32, 9.64) | ||
| White | 1.15 (0.54, 2.46) | 1.18 (0.54, 2.61) | ||
| pT | <0.0001 | 0.0011 | ||
| T2, T3, or T4 | 2.84 (1.83, 4.39) | 2.19 (1.34, 3.57) | ||
| Ta, Tis, or T1 | – | – | ||
| pN | <0.0001 | 0.2326 | ||
| N0 | – | – | ||
| N1 or N2 | 2.57 (1.79, 3.69) | 1.39 (0.81, 2.38) | ||
| ASA class | 0.1051 | 0.2411 | ||
| 1 or 2 | – | – | ||
| 3 | 1.41 (0.95, 2.11) | 1.42 (0.91, 2.22) | ||
| 4 | 1.93 (0.97, 3.82) | 1.62 (0.74, 3.54) | ||
| Tis path | 0.1724 | 0.2849 | ||
| No | – | – | ||
| Yes | 0.79 (0.57, 1.11) | 1.24 (0.84, 1.82) | ||
| Micro margins | <0.0001 | 0.0133 | ||
| Negative | – | – | ||
| Positive | 3.58 (2.20, 5.83) | 2.23 (1.22, 4.11) | ||
| CCI score age adjusted | 0.0004 | 0.2090 | ||
| 0≤ CCI ≤2 | 0.43 (0.28, 0.66) | 0.64 (0.39, 1.05) | ||
| 2< CCI <5 | 0.60 (0.40, 0.91) | 0.76 (0.48, 1.21) | ||
| CCI ≥5 | – | – | ||
| PCV preop | 0.94 (0.91, 0.97) | 0.0004 | 0.959 (0.923, 0.996) | 0.0316 |
| Number of nodes | 0.99 (0.96, 1.02) | 0.5250 | 0.985 (0.957, 1.013) | 0.2851 |
| Positive nodes | 1.18 (1.11, 1.26) | <0.0001 | 1.132 (1.008, 1.271) | 0.0605 |
| Tumor size (cm) | 1.15 (1.09, 1.21) | <0.0001 | 1.07 (0.98, 1.17) | 0.1317 |
| Galectin-3 H-score (for 25 units increase) | 1.01 (0.98, 1.05) | 0.4331 | 1.01 (0.97, 1.05) | 0.7167 |
Ta, noninvasive papillary carcinoma; Tis, carcinoma in situ; T1, tumor invades lamina propria; T2a, tumor invades superficial muscularis propria; T2b, tumor invades deep muscularis propria; T3a, tumor invades perivesical tissue microscopically; T3b, tumor invades perivesical tissue macroscopically; T4a, extravesical tumor invades prostatic stroma, seminal vesicles, uterus, vagina; T4b, extravesical tumor invades pelvic wall, abdominal wall; N0, no lymph node metastasis; N1, single regional lymph node metastasis in the true pelvis; N2, multiple regional lymph node metastasis in the true pelvis; ASA, American Society of Anesthesiologists; PCV, packed cell volume; CCI, Charlson Comorbidity Index.
Given that patients with MIBC have worse outcomes than patients with NMIBC, Gal-1 and Gal-3 were analyzed amongst these two different cohorts. For patients with MIBC, Gal-1 and Gal-3 H-scores were not predictive of OS or RFS (Table S3) univariately. For patients with NMIBC, Gal-1 and Gal-3 H-scores were also not predictive of RFS or OS (Table S4).
Galectin H-scores differed on level of invasion for the cystectomy core (Figure 3A,3B) for all patients. Gal-1 H-score was significantly higher in T1 and higher cores than Tis, Ta, and benign tissue, and the inverse relationship was found with Gal-3 (median Gal-1 H-score was 0 across non-invasive tissue types and 200 in invasive, P<0.01 and median Gal-3 score was 270 across non-invasive tissue types and 70 in invasive, P<0.01). Further analysis was done separating patients who had ≥pT2 disease and those who had <pT2 disease and then examining H scores amongst the level of core amongst each cohort. We found no significant differences after separating patients with muscle invasive disease and non-muscle invasive disease. Again, we found that the median Gal-1 H-score was higher in invasive cores than non-invasive cores and the median Gal-3 H scores were higher in non-invasive cores than invasive cores (Tables 5,6).
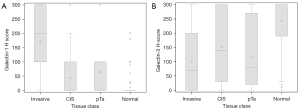
Table 5
| Core biopsy depth | Invasive cohort, median (25% quartile, 75% quartile) | Non-invasive cohort, median (25% quartile, 75% quartile) | P value |
|---|---|---|---|
| Normal (N=68, 14)** | 0 (0, 0) | 0 (0, 45) | 0.74 |
| Ta (N=18, 6)** | 0 (0, 20) | 0 (0, 0) | 0.59 |
| Tis (N=40, 23)** | 0 (0, 20) | 0 (0, 90) | 0.15 |
| Invasive (N=75, 36)** | 150 (50, 200) | 100 (16.67, 200) | 0.73 |
**, Invasive, non-invasive. Ta, noninvasive papillary carcinoma; Tis, carcinoma in situ.
Table 6
| Core biopsy depth | Invasive cohort, median (25% quartile, 75% quartile) | Non-invasive cohort, median (25% quartile, 75% quartile) | P value |
|---|---|---|---|
| Normal (N=69, 14)** | 270 (180, 300) | 260 (180, 300) | 0.44 |
| Ta (N=18, 6)** | 80 (40, 270) | 97.5 (10, 300) | 0.95 |
| Tis (N=41, 20)** | 75 (20, 185) | 130.8 (55, 199.17) | 0.20 |
| Invasive (N=76, 35)** | 35 (0, 122.5) | 60 (6.67, 130) | 0.25 |
**Invasive, non-invasive. Ta, noninvasive papillary carcinoma; Tis, carcinoma in situ.
Intra-tumoral Gal-1 and Gal-3 expression heterogeneity was observed. 69 of the 108 subjects who had 2 or more core specimens at the same level of invasion had discordant Gal-1 H scores. For example, Patient ID 604 had 4 cores from the cystectomy specimen, all T1 or greater with Gal-1 H scores of 100, 200, and 300. Of the 69 patients who had discordant Gal-1 H scores at the same level of invasion, the average difference between the highest and lowest H scores was 155.
Eighty-five of the 108 subjects had 2 or more core specimens at the same level of invasion had discordant Gal-3 H scores. Of the 85 patients who had discordant Gal-3 H scores at the same level of invasion, the average difference between the highest and lowest H scores was 146.
Irrespective of level of invasion, 123 patients had 2 or more core specimens with Gal-1 H scores, where the average difference between the highest and lowest H scores was 95 for Gal-1. Of the 124 patients who had 2 or more core specimens with Gal-3 H scores, the average difference between the highest and lowest H scores irrespective of level of invasion was 109 for Gal-3.
Discussion
In this study, we examined the relationship between Gal-1/Gal-3 expression and RFS/OS amongst 301 patients who underwent cystectomy without neoadjuvant chemotherapy. This represents the largest study to examine the relationship between Gal-1/Gal-3 expression and survival in a clinically annotated bladder cancer dataset. Neither Gal-1 nor Gal-3 tissue expression were associated with OS or RFS in univariable or multivariable analysis for patients with localized bladder cancer. This differs from prior studies which have described a relationship between galectin expression and OS in patients with bladder cancer (12,24). Canesin et al. investigated Gal-3 expression by transcript profiling and protein expression on 105 frozen bladder tumors. They found that Gal-3 transcript levels were highest in invasive (T24, J82, HT1376, HT1197) or metastatic bladder cancer cell lines (TCCSUP), and lower transcript levels were found in papillary or low-grade cell lines. In the tumor specimens, there were increased Gal-3 expression in MIBC compared with NMIBC. We found no significant difference in Gal-1 or Gal-3 expression between patients with MIBC and NMIBC (Tables 5,6), and this was consistent in all levels of the tissue block cores.
Our study differs from their study as they restricted their OS analysis for high grade T1 tumors, while we included survival data for all tumors, Tis to T4. However, even when we restrict our analysis to only patients with pathologic T1, we found no association with Gal-1 or Gal-3 with OS or RFS (Table S5). In our study, we examined Gal-3 expression via tumor cores at multiple levels from individual cystectomy specimens including adjacent normal tissue and utilized the highest galectin expression from the most invasive tissue type. It is unknown from their study where the Gal-3 expression was defined. Lastly, differences between our studies may exist based on our patient populations. Our patients either had muscle invasive urothelial carcinoma, high volume NMIBC, or NMIBC with progression on prior local therapies such as BCG, and thus represent a high-risk population (25,26). In Canesin et al., NMIBC specimens from transurethral resection (who had not undergone cystectomy) were included, and thus may represent a different population of patients.
One prior study described that Gal-3 expression increases from NMIBC to MIBC (27). Gendy et al. examined 35 patients with Ta to T3 disease and found that Gal-3 expression increased as T stage increased. We found that Gal-3 expression was associated with the level of the cystectomy tumor block cores (invasive vs. non-invasive vs. benign). For example, Gal-3 expression was significantly higher in benign tissue cores (Figure 3A,3B), than at the level of pT1, Tis, or T1 or greater cores (Table 4). However, at the same level of tumor core, Gal-3 expression did not differ between patients with muscle invasive disease or non-muscle invasive disease (Table 6).
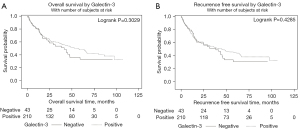
In terms of Gal-1 and urothelial carcinoma, the largest prior study to date evaluated 185 cases of primary localized bladder cancer (24). The highest T stage was used for Gal-1 staining and Gal-1 cytoplasmic immunoreactivity was scored from 1+ to 3+, with positive cases defined as Gal-1 cytoplasmic immunostaining in at least 5% of tumor cells. They found that Gal-1 overexpression in tumor cells was significantly associated with disease specific survival but Gal-1 expression in tumor stromal cells was not an independent prognosticator. This study differs from ours as it set a Gal-1 positivity cutoff at 5%, whereas we treated Gal-1 as a continuous variable. Given that we did not find Gal-1 was significantly associated with OS or RFS as a continuous variable, defining Gal-1 or Gal-3 as a categorical variable was not warranted. However, if we use the same cutoff as Wu et al., we again find no association with Gal-1/Gal-3 expression and RFS or OS (Figures 4,5).
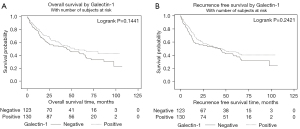
One of the challenges with evaluating Gal-1 and Gal-3 in urothelial carcinoma is the lack of consensus on how to best measure Gal-1 and Gal-3 in urothelial carcinoma, in terms of localization within gross tissue, as well as within cellular compartments. Wu et al. utilized 5% cytoplasmic staining as a cutoff, Su et al. utilized 10%, and Canesin et al. utilized 20% (12,24,28). We treated Gal-1 and Gal-3 as continuous variables rather than having a binary cutoff, as we did not find a relationship between Gal-1/Gal-3 and PFS or OS which would justify a binary cutpoint.
There are several strengths to our study. First, this is the largest study which incorporates both clinical and pathological information in examining the relationship between Gal-1 and Gal-3 expression and OS and RFS in localized bladder cancer. This allowed for multivariable analysis utilizing factors which are known to impact OS and RFS such as the CCI. Second, we are the first to evaluate Gal-1 and Gal-3 expression from multiple tumor cores of the same cystectomy specimen, examining cores from adjacent benign tissue to invasive tumor tissue (Tables 5,6). Lastly, we are the first to demonstrate that there is significant Gal-1 and Gal-3 intratumoral heterogeneity, not only at different levels of tumor invasion, but also within the same level of invasion (Table 7). This is consistent with other biomarker studies describing significant tumor heterogeneity in bladder cancer (29-31).
Table 7
| Galectin | Number of patients with 2 or more core specimens | Average difference between highest and lowest H score | Number of patients with 2 or more cores from same level of core biopsy | Average difference between highest and lowest H score |
|---|---|---|---|---|
| Gal-1 | 123 | 95 | 69 | 155 |
| Gal-3 | 124 | 109 | 85 | 146 |
H score is the product of the percentage of cells staining positive (0–100) and intensity score (0–3).
There are limitations to this study. First, we utilized a tissue microarray for this study. Tissue microarrays may not be representative of the whole tumor specimen due to tissue heterogeneity. A prior study described that binary phenotypes may be reliably investigated on TMAs but complex phenotypes that utilize cutoff values may lead to lower concordance rates, especially if there are a limited number of cores (32). Second, we were not able to differentiate T1 from T2-T4 disease based on our available data. At the time that the TMA was created, the tissues were subdivided only into normal, Tis, Ta, and T1+, and it is unknown whether this distinction may have impacted the analysis. Lastly, we did not have access to patient serum or urine to evaluate Gal-1 and Gal-3. Prior studies have shown that Gal-3 is detectable in urine and serum and is more frequently detected in patients with bladder cancer than controls (12,33). Future studies may consider evaluating urinary levels of Gal-1 or Gal-3 as a potential bladder cancer biomarker for prognosis.
Conclusions
In this study, the highest intra-tumor Gal-1 and Gal-3 H-score per bladder did not independently predict for RFS or OS. This result differs from smaller cohorts which have shown an association between Gal-3 expression and RFS. We discovered significant intra-tumoral Gal-1/Gal-3 heterogeneity which complicates the use of Gal-1 and Gal-3 expression as a prognostic biomarker. Future studies should consider the evaluation of serum and urinary galectins as an approach to mitigate tumor heterogeneity.
Acknowledgments
Funding: This work was supported by the Carolinas Bladder Cancer Fund (No. AH3747).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-494/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-494/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-494/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-494/coif). EFB reports honorarium fees from Exelixis and AstraZeneca, consulting relationship with Johnson and Johnson, and grants from Pfizer and Astellas, and owns stock in Exelixis, Becton Dickinson, Calithera Biosciences, Medtronic, Macrogenics, Arvinas, Autolus. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Atrium Health (IRB# LCI-GU-MIUC-GAL-001OB) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25-41. [Crossref] [PubMed]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005;48:202-5; discussion 205-6. [Crossref] [PubMed]
- Thijssen VL, Heusschen R, Caers J, et al. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim Biophys Acta 2015;1855:235-47. [PubMed]
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer 2005;5:29-41. [Crossref] [PubMed]
- Teichberg VI, Silman I, Beitsch DD, et al. A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc Natl Acad Sci U S A 1975;72:1383-7. [Crossref] [PubMed]
- Ahmed H, AlSadek DM. Galectin-3 as a Potential Target to Prevent Cancer Metastasis. Clin Med Insights Oncol 2015;9:113-21. [Crossref] [PubMed]
- Chen R, Pan S, Ottenhof NA, et al. Stromal galectin-1 expression is associated with long-term survival in resectable pancreatic ductal adenocarcinoma. Cancer Biol Ther 2012;13:899-907. [Crossref] [PubMed]
- Sumana BS, Shashidhar S, Shivarudrappa AS. Galectin-3 Immunohistochemical Expression in Thyroid Neoplasms. J Clin Diagn Res 2015;9:EC07-11. [PubMed]
- Shen KH, Li CF, Chien LH, et al. Role of galectin-1 in urinary bladder urothelial carcinoma cell invasion through the JNK pathway. Cancer Sci 2016;107:1390-8. [Crossref] [PubMed]
- Fang T, Liu DD, Ning HM, et al. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3. Acta Pharmacol Sin 2018;39:1885-93. [Crossref] [PubMed]
- Canesin G, Gonzalez-Peramato P, Palou J, et al. Galectin-3 expression is associated with bladder cancer progression and clinical outcome. Tumour Biol 2010;31:277-85. [Crossref] [PubMed]
- Reddy OL, Cates JM, Gellert LL, et al. Loss of FOXA1 Drives Sexually Dimorphic Changes in Urothelial Differentiation and Is an Independent Predictor of Poor Prognosis in Bladder Cancer. Am J Pathol 2015;185:1385-95. [Crossref] [PubMed]
- Warrick JI, Raman JD, Kaag M, et al. Enhancer of zeste homolog 2 (EZH2) expression in bladder cancer. Urol Oncol 2016;34:258.e1-6. [Crossref] [PubMed]
- Sharma S, Cwiklinski K, Sykes DE, et al. Use of Glycoproteins-Prostate-Specific Membrane Antigen and Galectin-3 as Primary Tumor Markers and Therapeutic Targets in the Management of Metastatic Prostate Cancer. Cancers (Basel) 2022;14:2704. [Crossref] [PubMed]
- Brittoli A, Fallarini S, Zhang H, et al. "In vitro" studies on galectin-3 in human natural killer cells. Immunol Lett 2018;194:4-12. [Crossref] [PubMed]
- Tang D, Yuan Z, Xue X, et al. High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer. Int J Cancer 2012;130:2337-48. [Crossref] [PubMed]
- Abdou AG, Hammam MA, Farargy SE, et al. Diagnostic and prognostic role of galectin 3 expression in cutaneous melanoma. Am J Dermatopathol 2010;32:809-14. [Crossref] [PubMed]
- Jiang J, Jin MS, Kong F, et al. Decreased galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PLoS One 2013;8:e81799. [Crossref] [PubMed]
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Wolters U, Wolf T, Stützer H, et al. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth 1996;77:217-22. [Crossref] [PubMed]
- Kong F, Jin M, Cao D, et al. Galectin-3 not Galectin-9 as a candidate prognosis marker for hepatocellular carcinoma. PeerJ 2020;8:e9949. [Crossref] [PubMed]
- Wu TF, Li CF, Chien LH, et al. Galectin-1 dysregulation independently predicts disease specific survival in bladder urothelial carcinoma. J Urol 2015;193:1002-8. [Crossref] [PubMed]
- Kashif Khan M, Ahmed I, Raza SJ. Factors effecting recurrence and progression of high grade non invasive bladder cancer treated by intravesical BCG. Pak J Med Sci 2014;30:326-30. [PubMed]
- Heney NM, Ahmed S, Flanagan MJ, et al. Superficial bladder cancer: progression and recurrence. J Urol 1983;130:1083-6. [Crossref] [PubMed]
- Gendy HE, Madkour B, Abdelaty S, et al. Diagnostic and Prognostic Significance of Serum and Tissue Galectin 3 Expression in Patients with Carcinoma of the Bladder. Curr Urol 2014;7:185-90. [Crossref] [PubMed]
- Su YL, Luo HL, Huang CC, et al. Galectin-1 Overexpression Activates the FAK/PI3K/AKT/mTOR Pathway and Is Correlated with Upper Urinary Urothelial Carcinoma Progression and Survival. Cells 2020; [Crossref] [PubMed]
- Kang HW, Kim WJ, Choi W, et al. Tumor heterogeneity in muscle-invasive bladder cancer. Transl Androl Urol 2020;9:2866-80. [Crossref] [PubMed]
- Grigg CM, Livasy C, He J, et al. Human epidermal growth factor receptor 2 overexpression is frequently discordant between primary and metastatic urothelial carcinoma and is associated with intratumoral human epidermal growth factor receptor 2 heterogeneity. Hum Pathol 2021;107:96-103. [Crossref] [PubMed]
- Vlachostergios PJ, Faltas BM. The molecular limitations of biomarker research in bladder cancer. World J Urol 2019;37:837-48. [Crossref] [PubMed]
- Hoos A, Cordon-Cardo C. Tissue microarray profiling of cancer specimens and cell lines: opportunities and limitations. Lab Invest 2001;81:1331-8. [Crossref] [PubMed]
- El Gendy H, Madkour B, Abdelaty S, et al. Galectin 3 for the diagnosis of bladder cancer. Arab J Urol 2014;12:178-81. [Crossref] [PubMed]


