
Radial wave therapy does not improve early recovery of erectile function after nerve-sparing radical prostatectomy: a prospective trial
Introduction
Low intensity shockwave therapy (SWT) is an emerging treatment option for men with vasculogenic erectile dysfunction (ED). The efficacy of SWT in this setting has been evaluated in several meta-analyses of randomized trials suggesting that men with vasculogenic ED experience a significant improvement in erectile function after SWT (1-4). The role of SWT in the post-radical prostatectomy (RP) setting for penile rehabilitation (5), however, is less clear, as the original randomized trials of SWT only enrolled men with vasculogenic ED and excluded men who had undergone RP.
The proposed mechanism of action of SWT—microtrauma that stimulates angiogenesis, stem cell proliferation, and nerve regeneration—suggests some potential for clinical efficacy in the post-RP setting (6,7). Furthermore, studies in rat models of cavernosal nerve injury suggest SWT may restore penile blood flow via revascularization and neuronal regeneration (7,8). There have been 5 studies evaluating SWT in the post-RP setting and 1 post-cystoprostatectomy, 3 of which were randomized controlled trials (9-14); these studies support the safety of low-intensity SWT after prostate surgery, but the clinical outcomes from these studies were underwhelming, noting only small increases in international index of erectile function-5 (IIEF-5) score and erectile hardness score (EHS) (11-13).
The pre-clinical data and clinical trials supporting the utility of SWT in ED uniformly used low-intensity focused shockwaves (fSWT). Radial wave therapy (rWT) is an alternative method of creating acoustic waves that differ from fSWT by having lower pressure waves that produce lower peak energy and thus low tissue penetrance (15-17). rWT is commonly utilized in orthopedics, physical therapy, and dermatology (18-21). The data supporting the use of rWT in ED is limited (22); at our institution the results of rWT treatment for men with vasculogenic ED was equivalent to fSWT (23). However, Sandoval-Salinas et al. found no difference between rWT and sham (24). Despite the limited data, rWT is often marketed directly to consumers as evidence-based treatment for ED (25). rWT has not yet been evaluated in the post-RP setting.
Here we report the first trial of rWT for penile rehabilitation after nerve-sparing (NS) RP. We hypothesized that rWT in addition to a phosphodiesterase type 5 inhibitor (PDE5I) would improve early recovery of erectile function following RP compared with a PDE5I alone. We present the following article in accordance with the TREND reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-310/rc).
Methods
Study design and population
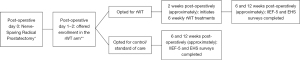
We performed a prospective, non-randomized, open-label trial. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Cleveland Clinic institutional review board (No. 18-919) and informed consent was obtained from all individual participants. All men who underwent either open or robot-assisted RP with any surgeon (8 surgeons were included) at our institution from 2018 to 2020 were considered for inclusion. Men were identified for inclusion if their pre-operative IIEF-5 score was ≥17 (with or without a PDE5I) and they underwent a bilateral NS RP, as dictated on their operative report. Exclusion criteria included pre-operative moderate or severe ED (IIEF-5 score <17), non-NS surgery, adjuvant radiation therapy within the observation period (3 months post-operatively), Grade Group ≥4 prostate cancer (as these patients are at higher risk of requiring adjuvant radiation and we presumed that the NS may not be as thorough), or pre-operative vacuum erectile device or intracavernosal injection use. All eligible men were approached in the early post-operative period (<2 weeks post-operatively) and offered enrollment in the rWT arm. As a referral center with patients traveling from great distances for care, many patients would not be able to travel for the weekly rWT treatments. Those unable to participate in the rWT arm were invited to continue our standard care and allow us to monitor their outcomes in the control arm. A time diagram from day of NS RP through outcome assessments can be seen in Figure 1.
Intervention
At our institution, all post-NS RP patients are offered a PDE5I as part of their baseline penile rehabilitation. Selection of specific PDE5I drug, dosing, and frequency was left to the discretion of the treating surgeon, but most commonly entailed a daily low dose of either sildenafil or tadalafil. Men enrolled in the rWT arm were treated with 6 consecutive weekly sessions beginning approximately 2 weeks post-operatively. The Zimmer enPuls Pro (Zimmer MedizinSysteme GmbH, Neu-Ulm, Germany) rWT device was used to deliver 10,000 “shocks” per treatment at a power of 90 mJ and frequency of 15 Hz. Treatment sites included the distal, mid, and proximal corporal shaft bilaterally as well as the cavernosal neurovascular bundles at the dorsal penopubic junction bilaterally for a total of 8 treatment sites.
Outcome measurements
IIEF-5 scores were collected pre-operatively on all patients undergoing RP in clinic. IIEF-5 and EHS surveys were mailed to participants in both arms to be returned at approximately 6 and 12 weeks post-operatively. The abridged five-item version of the IIEF (also known as the Sexual Health Inventory for Men) is a validated questionnaire that objectively measures erectile function in both clinical and research settings (26,27). The EHS is a validated, single-item 5-point survey to assess erectile hardness; scores 0 through 4 indicate subjective measurement of progressive erection hardness with 3 and 4 indicative of the ability to achieve penetrative intercourse (28). Additionally, patients were asked whether they were taking their PDE5I as prescribed.
The primary endpoint for this study was a comparison of the proportion of men who returned to “near normal” erectile function, defined as IIEF-5 score ≥17 and EHS ≥3, by 3 months post-operatively between the intervention and control arm. Secondary outcomes included comparisons of mean IIEF-5 scores and median EHS between the arms at 6–12 weeks post-operatively.
Statistical analysis
We assumed the baseline recovery of “near normal” erectile function by 12 weeks post-NS RP to be 20% and an absolute benefit of rWT of 25%. Using a power of 0.8 and alpha 0.05, we needed to recruit 54 patients per arm to adequately power our study.
Endpoints with binary outcomes were evaluated using the chi-squared test, while the independent samples t-test was used for continuous outcomes and the Mann-Whitney-Wilcoxon test for ordinal outcomes. P values <0.05 were considered statistically significant. Statistical analyses were performed using Jamovi Version 1.6.23
Results
One hundred and six patients were enrolled (62 in the control arm and 44 in the intervention arm) of whom 73 patients had at least one reported survey response between 6 and 12 weeks post-operatively (30 in the control arm and 43 in the intervention arm); for patients with two survey responses, the latter was used for analysis. Baseline patient characteristics are demonstrated in Table 1. No statistically significant differences were noted between the arms. The mean pre-operative IIEF-5 scores in the control and intervention arms were 22.8 (+/− 2.3) and 22.2 (+/− 2.5), respectively, which were not significantly different.
Table 1
| Characteristic | Control (n=30) | Intervention (n=43) | P value |
|---|---|---|---|
| Mean age (SD), years | 62.5 (7.6) | 59.5 (6.9) | 0.11 |
| Mean pre-operative IIEF-5 (SD) | 22.8 (2.3) | 22.2 (2.5) | 0.34 |
| Diabetes | 1 (3%) | 3 (7%) | 0.49 |
| Hypertension | 10 (32%) | 16 (37%) | 0.48 |
| Coronary artery disease | 0 | 2 (5%) | 0.23 |
| Peripheral artery disease | 0 | 0 | – |
| Any smoking history | 15 (50%) | 16 (37%) | 0.32 |
| Any post-operative PDE5I use | 20 (63%) | 31 (72%) | 0.28 |
| Mean Charlson Comorbidity index (SD) | 2.1 (1.2) | 2.0 (1.1) | 0.60 |
SD, standard deviation; IIEF-5, international index of erectile function-5; PDE5I, phosphodiesterase type 5 inhibitor.
Erectile function outcomes are displayed in Table 2. For our primary outcome, 5 (17%) and 11 (26%) patients recovered early erectile function in the control and intervention arms, respectively, which was not a statistically significant difference (P=0.37). A statistically significant difference was noted in the post-operative median EHS between the control and intervention arms with scores of 1 (interquartile range, 1–2) and 2 (interquartile range, 1–3), respectively (P=0.03). Table 3 lists the categorical post-operative EHS scores for both arms using an EHS of 3 as the cut off; there was no significant difference between the arms (P=0.46). The mean post-operative IIEF-5 scores in the control and intervention arms were 9.4 (+/− 6.6) and 10.9 (+/− 6.7), respectively (P=0.33).
Table 2
| Outcome | Control | Intervention | P value | 95% CI |
|---|---|---|---|---|
| Return to IIEF-5 ≥17 and EHS ≥3 | 5 (17%) | 11 (26%) | 0.37 | – |
| Median EHS [IQR] | 1 [1–2] | 2 [1–3] | 0.03 | −1.03 to −0.06 |
| Mean IIEF-5 score (SD) | 9.4 (6.6) | 10.9 (6.7) | 0.33 | −0.70 to 0.24 |
IIEF-5, international index of erectile function-5; EHS, erectile hardness score; CI, confidence interval; IQR, interquartile range; SD, standard deviation.
Table 3
| Study arm | EHS <3 | EHS >3 | P value |
|---|---|---|---|
| Intervention | 28 | 15 | 0.46 |
| Control | 22 | 8 |
EHS, erectile hardness score.
Of 106 enrolled patients, four adverse events were noted. All four events were related to genital pain during the treatment and required treatment intensity (energy or duration) de-escalation or no treatment changes. One patient un-enrolled from the intervention arm due to concerns of the procedural pain; the plan was to maintain his data in the intervention arm as an intent-to-treat analysis, however, he was subsequently lost to follow-up.
Discussion
In this study, we aimed to determine if rWT had an effect on early recovery of erectile function after NS RP. Though our study was underpowered, we did not find a statistically significant difference in our primary outcome of “near normal” erectile function, defined as a post-operative IIEF-5 score ≥17 and EHS ≥3 within 3 months of NS RP, in the men who selected treatment with rWT compared to non-placebo control. If the true benefit of rWT in our study was 26% recovery in the intervention arm compared to 17% in the control arm, then a trial with 326 subjects per arm would be required. While men in the intervention arm did have significantly higher EHS than did men in the control arm, the reported erectile hardness would not be categorized as suitable for penetrative intercourse.
ED after NS RP is multifactorial and can be a result of neural damage (traction on the cavernous nerves), insufficient arterial inflow (related to ligation of pudendal arterial branches), absence of cavernosal oxygenation and neuropraxia-associated damage to erectile tissue resulting in veno-occlusive dysfunction (5,29). Ischemic hypoxia of the corpus cavernosum can then cause fibrosis, further exacerbating the ED (29). Fibrotic changes of the corpus cavernosum can be present at just 2 months after RP (29). Furthermore, initiation of penile rehabilitation more than 6 months after RP predicts for failure of erection recovery, whereas early penile rehabilitation may improve cavernosal oxygenation and prevent hypoxia-induced fibrosis (5). For this reason, we initiated our intervention soon after Foley catheter removal after RP. Indeed, Porst, in a review of fSWT for treatment of vasculogenic ED, Peyronie’s Disease and post-RP and a description of the author’s own experiences using fSWT, reported treating 12 consecutive post-RP patients with fSWT starting after indwelling catheter removal (at 8–14 days post-RP) (10); ten of these men had return to baseline potency with aid of a PDE5I after fSWT treatment (10). Similarly, Baccaglini et al. performed a randomized controlled trial of post-RP men with the fSWT intervention initiated at 6 weeks post-RP (11). In their study, both arms received daily PDE5I starting at time of indwelling catheter removal with a 2-week PDE5I washout period prior to the 4-month post-op visit; at this time point, there was a significantly improved median IIEF-5 score for the intervention arm compared to control (12.0 versus 10.0, respectively, P=0.006), however, the primary clinical outcome of their study, IIEF-5 difference ≥4 between arms, was not met (11). Furthermore, the percent of men with an IIEF-5 score ≥17 after treatment was not significantly different between groups (11). Jang et al., in a non-sham controlled non-randomized prospective study evaluated fSWT in post-NS RP men initiated on the fourth day after surgery (14). Only post-operative EHS scores were reported, with no statistically significant difference between the treatment and control arms; still, the proportion of men with EHS ≥3 favored the treatment arm. Of note, a pre-operative IIEF-5 score ≥15 was acceptable for inclusion in this study; further, there were no reports of post-operative IIEF-5 scores for either arm (14).
Erectile function after NS RP has been shown to recover in about 60–74% of men at >12 months post-operatively (30,31). Both Frey et al., in a small non-controlled pilot study, and Ladegaard et al., in a randomized placebo-controlled prospective study, performed their trials of fSWT on men with severe ED who were greater than 6 months removed from RP (9,12). Both studies reported statistically significant increases in erectile function outcomes but noted that these improvements were unlikely to have an effect on successful sexual intercourse (9,12). While measuring short term erectile function outcomes could limit the typically expected progressive recovery in function, we sought to determine if a restorative therapy such as rWT could hasten this progression; thus, our primary endpoint was early recovery in erectile function. Furthermore, the strict criteria for our primary endpoint—IIEF-5 score ≥17 and EHS ≥3—was chosen to assess for clinically significant early recovery in erectile function. This timing for our primary outcome was similar to Baccaglini et al.’s outcome measurement (11).
Ours is the first study to evaluate rWT in the post-RP setting. While there is doubt regarding the beneficial effects of rWT as a treatment for ED given the lower intensity acoustic waves and the lack of clinical data, rWT devices are designated as class 1 devices that do not require regulatory approval (15-17). Thus, these devices can be marketed as efficacious ED treatment despite limited supporting data (25). There are two recent studies that reported beneficial effects of rWT on ED (22,23). Wu et al. retrospectively compared fSWT and rWT for treatment of men with vasculogenic ED; at 6 weeks after treatment, there were similar statistically and clinically significant improvements in IIEF-5 scores (23). Yamaçake et al. performed a randomized double-blinded placebo-controlled trial using rWT on renal transplant patients with ED (22). In this trial, with 10 patients per arm, rWT significantly improved IIEF-5 scores at 3 months after treatment compared to sham. Sandoval-Salinas et al., reported a randomized controlled trial of men with mild or moderate ED treated with rWT or sham therapy (40 men per arm) and found no differences in IIEF-5 score and EHS (24). While our primary endpoint also had no difference in IIEF-5 scores between the arms, we did find a significant difference in EHS. IIEF-5 is validated to assess erectile function, but a limitation of this questionnaire is that it focuses on current sexual behavior (32); with our goal of assessing early erectile function recovery, it is likely that study participants were not yet able to achieve an erection sufficient for intercourse. For this reason, we also used the EHS, which simply assesses the hardness of a subject’s erection. While the median EHS after rWT was significantly higher than that of the control arm (2 vs. 1), this may not be clinically significant since an EHS ≥3 correlates with successful penetrative intercourse (33). Thus, we felt that including both assessment scores would be best to evaluate for early return of erectile functions. Another noteworthy outcome of our study is that 42/43 (97.7%) of the patients in the intervention arm completed all 6 treatments with rare limited and no severe adverse effects. Thus, our study supports the safety of rWT in this setting.
Our study has several notable limitations. First, the study is not randomized, blinded or sham-controlled, thus leading to a selection bias of patients who opted for the intervention arm. Given our patients are often from locations that preclude weekly visits to our facility, randomizing only patients who lived within driving distance would have caused prohibitive barriers to enrollment. If the current study design showed a significant signal of erectile improvement with rWT it would have justified the implementation of a larger truly sham controlled study. The lack of a sham control also introduces the potential for a placebo benefit in the treatment group compared to the control arm. Further, we believe that those who chose the intervention arm may self-select for being highly motivated to achieve early erectile function recovery. With the lack of difference between arms in our primary outcome, this selection bias provides further support for the lack of efficacy of rWT on early erectile function recovery after NS RP. Our study is also underpowered based on the pre-study power analysis we performed. This is likely a result of unreturned surveys, as 106 men were enrolled in the study, but only 73 filled out surveys; the vast majority of those who did not return surveys were from the control arm. Last, given that we have multiple surgeons across several hospitals performing RPs at our institution, we were unable to enforce a standard for PDE5I use; still, there was no significant difference in percentage of men who used PDE5I between arms and there is no evidence that one PDE5i regimen for penile rehabilitation is significantly more effective than another.
Conclusions
ED post-NS RP is an important quality of life issue that both patients and providers struggle to manage. In this prospective, non-randomized, open-label trial, rWT did not substantially improve the recovery of early erectile function after NS RP. rWT may contribute to improvement in erectile hardness, but the clinical effect is likely marginal. While rWT is safe, we cannot conclude that it has a positive effect on early recovery of erectile function after post-NS RP. Future studies may consider focusing on longer-term outcomes with a larger sample size.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-310/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-310/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-310/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-310/coif). DAS serves as an unpaid editorial board member of Translational Andrology and Urology from August 2022 to July 2024. DAS is a full-time employee of Exact Sciences whose products are unrelated to this manuscript. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Cleveland Clinic institutional review board (No. 18-919) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clavijo RI, Kohn TP, Kohn JR, et al. Effects of Low-Intensity Extracorporeal Shockwave Therapy on Erectile Dysfunction: A Systematic Review and Meta-Analysis. J Sex Med 2017;14:27-35. [Crossref] [PubMed]
- Lu Z, Lin G, Reed-Maldonado A, et al. Low-intensity Extracorporeal Shock Wave Treatment Improves Erectile Function: A Systematic Review and Meta-analysis. Eur Urol 2017;71:223-33. [Crossref] [PubMed]
- Dong L, Chang D, Zhang X, et al. Effect of Low-Intensity Extracorporeal Shock Wave on the Treatment of Erectile Dysfunction: A Systematic Review and Meta-Analysis. Am J Mens Health 2019;13:1557988319846749. [Crossref] [PubMed]
- Campbell JD, Trock BJ, Oppenheim AR, et al. Meta-analysis of randomized controlled trials that assess the efficacy of low-intensity shockwave therapy for the treatment of erectile dysfunction. Ther Adv Urol 2019;11:1756287219838364. [Crossref] [PubMed]
- Müller A, Parker M, Waters BW, et al. Penile rehabilitation following radical prostatectomy: predicting success. J Sex Med 2009;6:2806-12. [Crossref] [PubMed]
- Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med 2013;10:738-46. [Crossref] [PubMed]
- Li H, Matheu MP, Sun F, et al. Low-energy Shock Wave Therapy Ameliorates Erectile Dysfunction in a Pelvic Neurovascular Injuries Rat Model. J Sex Med 2016;13:22-32. [Crossref] [PubMed]
- Wang HS, Ruan Y, Banie L, et al. Delayed Low-Intensity Extracorporeal Shock Wave Therapy Ameliorates Impaired Penile Hemodynamics in Rats Subjected to Pelvic Neurovascular Injury. J Sex Med 2019;16:17-26. [Crossref] [PubMed]
- Frey A, Sønksen J, Fode M. Low-intensity extracorporeal shockwave therapy in the treatment of postprostatectomy erectile dysfunction: a pilot study. Scand J Urol 2016;50:123-7. [Crossref] [PubMed]
- Porst H. Review of the Current Status of Low Intensity Extracorporeal Shockwave Therapy (Li-ESWT) in Erectile Dysfunction (ED), Peyronie's Disease (PD), and Sexual Rehabilitation After Radical Prostatectomy With Special Focus on Technical Aspects of the Different Marketed ESWT Devices Including Personal Experiences in 350 Patients. Sex Med Rev 2021;9:93-122. [Crossref] [PubMed]
- Baccaglini W, Pazeto CL, Corrêa Barros EA, et al. The Role of the Low-Intensity Extracorporeal Shockwave Therapy on Penile Rehabilitation After Radical Prostatectomy: A Randomized Clinical Trial. J Sex Med 2020;17:688-94. [Crossref] [PubMed]
- Ladegaard PBJ, Mortensen J, Skov-Jeppesen SM, et al. Erectile Dysfunction A Prospective Randomized Placebo-Controlled Study Evaluating the Effect of Low-Intensity Extracorporeal Shockwave Therapy (LI-ESWT) in Men With Erectile Dysfunction Following Radical Prostatectomy. Sex Med 2021;9:100338. [Crossref] [PubMed]
- Zewin TS, El-Assmy A, Harraz AM, et al. Efficacy and safety of low-intensity shock wave therapy in penile rehabilitation post nerve-sparing radical cystoprostatectomy: a randomized controlled trial. Int Urol Nephrol 2018;50:2007-14. [Crossref] [PubMed]
- Jang SW, Lee EH, Chun SY, et al. Comparison of the efficacy of the early LI-SWT plus daily tadalafil with daily tadalafil only as penile rehabilitation for postprostatectomy erectile dysfunction. Int J Impot Res 2022; Epub ahead of print. [Crossref]
- Katz JE, Clavijo RI, Rizk P, et al. The Basic Physics of Waves, Soundwaves, and Shockwaves for Erectile Dysfunction. Sex Med Rev 2020;8:100-5. [Crossref] [PubMed]
- Sandoval-Salinas C, Saffon JP, Corredor HA, et al. Are Radial Pressure Waves Effective in Treating Erectile Dysfunction? A Systematic Review of Preclinical and Clinical Studies. Sex Med 2021;9:100393. [Crossref] [PubMed]
- Liu JL, Chu KY, Gabrielson AT, et al. Restorative Therapies for Erectile Dysfunction: Position Statement From the Sexual Medicine Society of North America (SMSNA). Sex Med 2021;9:100343. [Crossref] [PubMed]
- Speed C. A systematic review of shockwave therapies in soft tissue conditions: focusing on the evidence. Br J Sports Med 2014;48:1538-42. [Crossref] [PubMed]
- van der Worp H, van den Akker-Scheek I, van Schie H, et al. ESWT for tendinopathy: technology and clinical implications. Knee Surg Sports Traumatol Arthrosc 2013;21:1451-8. [Crossref] [PubMed]
- Liao CD, Xie GM, Tsauo JY, et al. Efficacy of extracorporeal shock wave therapy for knee tendinopathies and other soft tissue disorders: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 2018;19:278. [Crossref] [PubMed]
- Adatto MA, Adatto-Neilson RM. Facial treatment with acoustic wave therapy for improvement of facial skin texture, pores and wrinkles. J Cosmet Dermatol 2020;19:845-9. [Crossref] [PubMed]
- Yamaçake KGR, Carneiro F, Cury J, et al. Low-intensity shockwave therapy for erectile dysfunction in kidney transplant recipients. A prospective, randomized, double blinded, sham-controlled study with evaluation by penile Doppler ultrasonography. Int J Impot Res 2019;31:195-203. [Crossref] [PubMed]
- Wu SS, Ericson KJ, Shoskes DA. Retrospective comparison of focused shockwave therapy and radial wave therapy for men with erectile dysfunction. Transl Androl Urol 2020;9:2122-8. [Crossref] [PubMed]
- Sandoval-Salinas C, Saffon JP, Martínez JM, et al. Are Radial Pressure Waves Effective for the Treatment of Moderate or Mild to Moderate Erectile Dysfunction? A Randomized Sham Therapy Controlled Clinical Trial. J Sex Med 2022;19:738-44. [Crossref] [PubMed]
- Goldberg D, Andriessen A, Gold M. Radial shockwave therapy for male erectile rejuvenation in a dermatology and/or medical aesthetic practice. J Cosmet Dermatol 2019;18:1596-600. [Crossref] [PubMed]
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319-26. [Crossref] [PubMed]
- Alwaal A, Awad M, Boggs N, et al. Sexual Health Inventory for Men Questionnaire as a Screening Method for Erectile Dysfunction in a General Urology Clinic. Sex Med 2020;8:660-3. [Crossref] [PubMed]
- Mulhall JP, Goldstein I, Bushmakin AG, et al. Validation of the erection hardness score. J Sex Med 2007;4:1626-34. [Crossref] [PubMed]
- Iacono F, Giannella R, Somma P, et al. Histological alterations in cavernous tissue after radical prostatectomy. J Urol 2005;173:1673-6. [Crossref] [PubMed]
- Tal R, Alphs HH, Krebs P, et al. Erectile function recovery rate after radical prostatectomy: a meta-analysis. J Sex Med 2009;6:2538-46. [Crossref] [PubMed]
- Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol 2012;62:418-30. [Crossref] [PubMed]
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002;14:226-44. [Crossref] [PubMed]
- Mulhall JP, Levine LA, Jünemann KP. Erection hardness: a unifying factor for defining response in the treatment of erectile dysfunction. Urology 2006;68:17-25. [Crossref] [PubMed]


