
Establishment and validation of a nomogram for predicting the surgical difficulty of lateral retroperitoneal laparoscopic adrenalectomy
Introduction
Laparoscopic adrenalectomy (LA) is considered to be the gold standard surgery for treating most benign and malignant adrenal lesions (1). Compared with open surgery, LA is associated with lower complications, faster operation time and smoother recovery (2). Although several approaches have been reported for LA, transperitoneal and retroperitoneal are the most frequently used surgical approaches for the adrenal glands (3). In contrast to transperitoneal LA (TLA), retroperitoneal LA (RLA) avoids intestinal interference and other associated complications, which is conducive to intestinal recovery (4). However, the retroperitoneal approach has a relatively small operative space, as well as unclear anatomical landmarks, which makes the procedure more technically challenging (5). Therefore, assessing RLA difficulty is vital in terms of reducing operative time, intraoperative blood loss and surgical complications. Although several risk factors have been reported as associated with operative time and blood loss for RLA, at present there is no visual predictive model that can be used to evaluate the surgical difficulty for lateral RLA (LRLA). The most frequently performed retroperitoneal surgical approaches are posterior RLA (PRLA) and LRLA, both which are used in our center. Therefore, we aimed to develop a predictive model of surgical difficulty based on a large series of LRLA cases, which should assist in optimizing the perioperative strategy. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-324/rc).
Methods
Patient selection
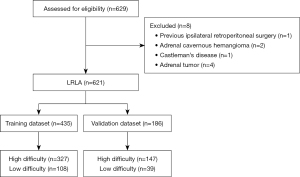
This single-center, retrospective observational study included 621 consecutive LRLA cases from Beijing Anzhen Hospital between January 2012 and December 2021. The inclusion criteria were: (I) patient diagnosed with adrenal lesion, with the histological type including adrenal adenoma, hyperplasia, pheochromocytoma, cyst and myelolipoma; (II) patient underwent LRLA; (III) patient initially underwent LRLA, but converted to open surgery. The exclusion criteria were: (I) patient underwent TLA; (II) patient underwent robot-assisted RLA; (III) incomplete clinical data; (IV) prior ipsilateral retroperitoneal surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Beijing Anzhen Hospital (No. 2022172X). Individual consent for this retrospective analysis was waived.
Data collection
The following clinical data were collected: sex, age, operative time (measured from trocar insertion to abdominal closure), estimated intraoperative blood loss, body mass index (BMI), surgeon’s experience (30 first cases), tumor side (left or right), resection procedure (partial or total adrenalectomy), prior abdominal surgery, tumor diameter (expressed in cm and measured in the major axis), and histological type (adrenal adenoma, hyperplasia, pheochromocytoma, cyst and myelolipoma).
Surgical protocol
All patients underwent LRLA. Briefly, the operating table was flexed to expand the working space between the iliac crest and the 12th rib whiles the patient was in the lateral decubitus position. A 1.5-cm incision was made in the midaxillary line, followed by a 2-cm incision above the iliac crest. The retroperitoneal space was expanded using the surgeon’s index finger. Through a 10-mm trocar, an observation mirror was used through a 30° laparoscope, and the two other trocars were located in the anterior axillary line and posterior axillary line of the subcostal space with a diameter of 5 and 10 mm, respectively. Following the placement of the trocar, the upper pole of the renal was mobilized so the adrenal gland could be exposed. In this procedure, the edge of the adrenal gland was divided using ultrasound, and the central vein in the adrenal gland was ligated through hem-o-lock clips. The specimen was retrieved as a partial or total adrenalectomy.
Outcome definition and predictor selection
The media operation time of these 621 LRLA cases were 105 min, and the operation time above the 75th percentile (>140 min) was chosen as the cut-off. An operation that was >140 min, converted to open surgery or had an intraoperative blood loss >100 mL was considered as a high-difficultly surgery. Otherwise, it was defined as a low-difficulty surgery.
The 621 cases were randomly divided into two groups: the training dataset (n=435) and the validation dataset (n=186). The training dataset was used to develop the predictive model, and the validation dataset was used to evaluate the model’s performance (Figure 1). Secondly, least absolute shrinkage and selection operator (LASSO) regression analysis, which minimizes the impact of factors on feature selection, was used to identify the predictive factors of surgical difficulty in the training cohort. In the LASSO regression model, the optimal λ was selected with a 10-fold cross-validation process and one standard error (1-SE) of the minimum criteria, while retaining the non-zero coefficients.
Statistical analysis
For normally distributed data, continuous variables are presented as mean ± standard deviation, and Student’s t-test was conducted for difference comparison between high- and low-difficulty surgery groups. Continuous variables with a non-normal distribution are presented as median plus interquartile range (IQR), and the Wilcoxon rank sum test was used for comparison. For comparisons between groups among the categorical variables, Chi-square test or Fisher’s exact test were used as appropriate. The “glmnet” package of R was applied for LASSO feature selection, and the “rms” package was used to establish a predictive model and nomogram based on multivariate logistic regression. To assess the accuracy of the predict model, the area under the curve (AUC) of receiver operating characteristic (ROC) and calibration curve plots were created by the “rms” package. An evaluation of the calibration of this nomogram was conducted using calibration plots, along with both Hosmer-Lemeshow and unreliability tests. In addition, decision curve analysis (DCA) was applied to evaluate the clinical value of the nomogram by assessing net benefits at different threshold probabilities in both the training and validation datasets. All the analyses were conducted with R language (version 4.1.0 for Mac) and STATA (version 16.0 for Mac). A P value <0.05 was considered statistically significant.
Results
Patients’ demographics and clinical characteristics
We analyzed the clinical data for 621 LRLA cases comprising 321 men and 300 women; median age was 53 years (range, 17–80 years), median BMI was 25.2 kg/m2 (range, 15.8–43.2 kg/m2), and median lesion diameter was 1.74 cm (range, 0.4–10.2 cm). A summary of the patients’ characteristics in the training and validation cohorts is given in Table 1. In terms of their baseline characteristics, there were no significant differences between the training and validation datasets. In the training dataset, the proportion of cases of high-difficulty surgery was 24.8% versus 21.0% in the validation dataset.
Table 1
| Factor | Training group | Validation group | P value |
|---|---|---|---|
| Sample size (n) | 435 | 186 | NA |
| Age (years), median (IQR) | 53.0 (44.0, 61.0) | 52.0 (41.0, 61.0) | 0.096 |
| Size (cm), median (IQR) | 1.8 (1.3, 2.3) | 1.6 (1.3, 2.3) | 0.41 |
| BMI (kg/m2), median (IQR) | 25.1 (23.2, 27.5) | 25.3 (23.5, 27.6) | 0.58 |
| Operative time (min), median (IQR) | 105.0 (85.0, 140.0) | 100.0 (80.0, 130.0) | 0.27 |
| Blood loss (mL), median (IQR) | 20.0 (10.0, 50.0) | 20.0 (10.0, 50.0) | 0.23 |
| Sex, n (%) | 0.051 | ||
| Male | 236 (54.3%) | 85 (45.7%) | |
| Female | 199 (45.7%) | 101 (54.3%) | |
| Surgeon’s experience, n (%) | 0.89 | ||
| ≤30 | 124 (28.5%) | 52 (28.0%) | |
| >30 | 311 (71.5%) | 134 (72.0%) | |
| Location, n (%) | 0.089 | ||
| Left | 244 (56.1%) | 118 (63.4%) | |
| Right | 191 (43.9%) | 68 (36.6%) | |
| Histological type, n (%) | 0.40 | ||
| Adenoma | 302 (69.4%) | 131 (70.4%) | |
| Hyperplasia | 98 (22.5%) | 38 (20.4%) | |
| Pheochromocytoma | 15 (3.4%) | 7 (3.8%) | |
| Cyst | 15 (3.4%) | 4 (2.2%) | |
| Myelolipoma | 5 (1.1%) | 6 (3.2%) | |
| Prior abdominal surgery, n (%) | 0.82 | ||
| No | 326 (74.9%) | 141 (75.8%) | |
| Yes | 109 (25.1%) | 45 (24.2%) | |
| Resection procedure, n (%) | 0.60 | ||
| Partial | 329 (75.6%) | 137 (73.7%) | |
| Total | 106 (24.4%) | 49 (26.3%) |
Data presented as median (IQR) or number and percentage in parentheses. NA, not applicable; IQR, interquartile range; BMI, body mass index.
Establishment of the nomogram for predicting surgical difficulty in the training cohort
LASSO regression was used first to identify the effective predictors related to surgical difficulty in the training cohort. As shown in Figure 2, if log(λ) increased, most of the coefficients decreased toward zero. Consequently, based on 10-fold cross-validation and the 1-SE criterion, the optimal λ was selected in the LASSO regression model: log(λ)=−3.56, and the remaining non-zero coefficients were finally selected. As a result, surgeon’s experience, tumor diameter, resection procedure, histological type of adrenal hyperplasia/pheochromocytoma, patient’s sex and BMI were identified as predictors of surgical difficulty. From this, a predictive model was constructed, and presented in the form of a nomogram (Figure 3, Table 2).
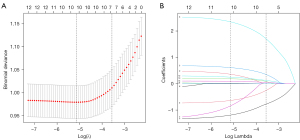
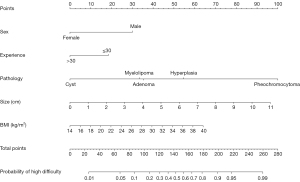
Table 2
| Intercept and variable | Coef. | 95% CI | P value |
|---|---|---|---|
| Female | −1.180 | −1.712, −0649 | <0.001 |
| Experience (>30) | −0.723 | −1.243, −0.204 | 0.006 |
| Histological type | |||
| Hyperplasia | 0.756 | 0.214, 1.297 | 0.006 |
| Pheochromocytoma | 2.532 | 1.106, 3.957 | <0.001 |
| Cyst | −1.387 | −3.347, 0.572 | 0.165 |
| Myelolipoma | −0.081 | −2.232, 2.070 | 0.941 |
| Size (cm) | 0.343 | 0.102, 0.585 | 0.005 |
| BMI (kg/m2) | 0.098 | 0.026, 0.17 | 0.008 |
| Intercept | −3.647 | −5.592, −1.702 | <0.001 |
LRLA, lateral retroperitoneal laparoscopic adrenalectomy; Coef., coefficient; BMI, body mass index.
Evaluation of the nomogram in the training and validation cohorts
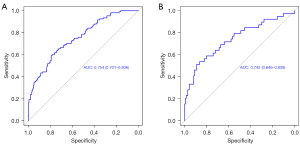
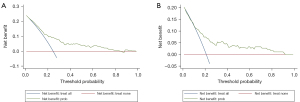
The nomogram was validated in both the training and validation cohorts. As illustrated in Figure 4, the AUC was 0.754 (95% CI: 0.701–0.806), and 0.742 (95% CI: 0.646–0.838) in the training and validation datasets, respectively, which implied favorable discrimination of the model. Furthermore, calibration curves were used to determine predictive accuracy. As illustrated in Figure 5, in the training cohort, the calibration’s unreliability test statistic was –0.005 with a P value of 0.999, whereas the validation cohort was –0.006 with a P value of 0.444. DCA is a novel method for evaluating diagnostic tests, predictive models and molecular markers. It combines the mathematical simplicity of accuracy measures, such as sensitivity and specificity, with the clinical applicability of decision analytic approaches. In this study, DCA was also applied to assess the clinical use of the nomogram. As shown in Figure 6, when the threshold probability increased from 10% to 90%, using the nomogram to predict surgical difficulty was more beneficial than either treating all patients or treating none.

Discussion
We developed and validated a predictive model for the surgical difficulty of LRLA, which was well discriminated and calibrated for individualized prediction. To our knowledge, we are the first to evaluate a surgical difficulty nomogram for LRLA in a large cohort. Similar to other studies (6-8), the operative time, conversion to open surgery and blood loss were used to evaluate surgical difficulty. Our nomogram was based on six predictive factors: patient’s sex and BMI, histological type, tumor diameter, surgeon’s experience, and resection procedure.
Among these predictive factors, pheochromocytoma was the strongest predictor of surgical difficulty. It is a rare neuro-endocrine, catecholamine-secreting tumor that originates in adrenal cortex chromaffin cells (9). In general, LA for pheochromocytoma is considered a high-risk procedure due to the hemodynamic changes that occur during and after the procedure (10). It has been reported that resection of pheochromocytoma correlates with longer operative time, greater blood loss, and longer hospital stay (11,12). Similar results were obtained in our current study, explained by the fact that pheochromocytomas are composed of fragile, necrotic and highly vascularized tissues, making surgery more difficult.
Besides the histological type of lesions, tumor size was another important factor for predicting difficulty of LRLA. Our results indicated that surgery becomes more difficult as the tumor increases in size. In fact, the limitation of tumor size for LRLA is still controversial. It has been reported that retroperitoneal laparoscopy is only appropriate for small to medium-sized lesions (13,14). Nevertheless, with increasing surgical experience and the improvements in laparoscopic instruments, tumor size is no longer an absolute contraindication to LRLA (15). Despite this, the retroperitoneum is a limited operative space, especially if the adrenal mass is large, making the procedure more challenging (16). Additionally, the limited retroperitoneal space can cause interference and collision of surgical instruments, increasing the difficulty of exposing and isolating the adrenal lesion (15). Hence, surgeons prefer the transperitoneal approach for large adrenal masses because there is more working space (17).
Obesity is related to the difficulty of any operation, and there is controversy regarding whether BMI makes laparoscopic adrenal surgery more difficult. Consistent with our study results, a recent study involving 353 patients undergoing RLA suggested that the overweight group with BMI >30 kg/m2 had a prolonged operative time compared with the normal BMI group, but no difference was found in postoperative length of stay, estimated blood loss, or postoperative complications (18). There is more extraperitoneal and suprarenal fat in overweight individuals, which makes it more difficult to detect and isolate the adrenal glands. Interestingly, the effect of BMI on the surgical difficulty of TLA is not consistent with the effect when using the retroperitoneal approach. In a retrospective study, Rodríguez-Hermosa et al. (19) reported that BMI itself does not increase the surgical difficulty of TLA. Furthermore, it was reported that there were no differences in intraoperative blood loss or operative time between obese and non-obese patients undergoing LA via a transabdominal approach (20). Therefore, the transabdominal approach for LA might be a potentially useful surgical option for high BMI and obese patients.
There is currently limited data on the effect of sex on the surgical difficulty of LRLA. Oh et al. (21) summarized their surgical outcomes between the posterior and lateral approaches for RLA. In their results males were significantly associated with ≥90 min operative time for both lateral and posterior RLA (P=0.019). The effect of sex on surgical difficulty might be explained by the difference in fat distribution between males and females at the same BMI (22). In addition, compared with females, males are more muscular, and have greater thickness and density of retroperitoneal fat, thus leading more difficult surgery (23,24).
In addition, our results indicated that prior abdominal surgery did not influence the surgical difficulty of LRLA, which might be attributed to the fact that the transabdominal approach was chosen for LA in patients with a history of ipsilateral retroperitoneal surgery. Therefore, these patients were not enrolled in the present study. The prior abdominal surgery involved in this study was transperitoneal rather than retroperitoneal operation.
However, there are several limitations to the present study. Firstly, it is important to note that this was a retrospective study from one center, which led to some bias. Secondly, the cases of adrenal pheochromocytoma, cyst and myelolipoma in this study were relatively limited, reducing the reliability of the final results to some extent. Moreover, several anatomic factors including perinephric fat tissue, location of adrenal gland and skin-to-adrenal distance were not considered for inclusion in the analysis.
Conclusions
We have presented a surgical difficulty predictive nomogram that incorporates patient’s sex, histological type, tumor diameter, surgeon’s experience, BMI and resection procedure, which may be used to facilitate the preoperative prediction of surgical difficulty in LRLA, contributing to more efficient preoperative management for adrenal gland lesions and providing patients with information during the consent process. If a patient seems to be at high risk for LRLA, a transabdominal approach is a potential option for the surgical treatment of adrenal disease.
Acknowledgments
Funding: This work was supported by the “Undergraduate Scientific Research Innovation” project of Capital Medical University (No. XSKY2022296).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-324/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-324/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-324/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-324/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Beijing Anzhen Hospital (No. 2022172X) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vatansever S, Nordenström E, Raffaelli M, et al. Robot-assisted versus conventional laparoscopic adrenalectomy: Results from the EUROCRINE Surgical Registry. Surgery 2022;171:1224-30. [Crossref] [PubMed]
- Al-Jalabneh T, Al-Shawabkeh O, Al-Gwairy I, et al. Laparoscopic Versus Open Adrenalectomy: a Retrospective Comparative Study. Med Arch 2021;75:41-4. [Crossref] [PubMed]
- Prudhomme T, Roumiguié M, Gas J, et al. Comparison between retroperitoneal and transperitoneal laparoscopic adrenalectomy: Are both equally safe? J Visc Surg 2021;158:204-10. [Crossref] [PubMed]
- Liu Z, Li DW, Yan L, et al. Comparison of lateral transperitoneal and retroperitoneal approaches for homolateral laparoscopic adrenalectomy. BMC Surg 2021;21:432. [Crossref] [PubMed]
- Tuncel A, Langenhuijsen J, Erkan A, et al. Comparison of synchronous bilateral transperitoneal and posterior retroperitoneal laparoscopic adrenalectomy: results of a multicenter study. Surg Endosc 2021;35:1101-7. [Crossref] [PubMed]
- Nassar AHM, Hodson J, Ng HJ, et al. Predicting the difficult laparoscopic cholecystectomy: development and validation of a pre-operative risk score using an objective operative difficulty grading system. Surg Endosc 2020;34:4549-61. [Crossref] [PubMed]
- Duralska M, Dzwonkowski J, Sierdziński J, et al. A Retrospective Study of 881 Lateral Transabdominal Laparoscopic Adrenalectomies Performed Between 1997 and 2017 at a Single Center in Poland to Determine Factors Associated with Surgery Time. Med Sci Monit 2022;28:e936272. [Crossref] [PubMed]
- van Uitert A, van de Wiel ECJ, Ramjith J, et al. Predicting surgical outcome in posterior retroperitoneoscopic adrenalectomy with the aid of a preoperative nomogram. Surg Endosc 2022;36:6507-15. [Crossref] [PubMed]
- Nölting S, Bechmann N, Taieb D, et al. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr Rev 2022;43:199-239. [Crossref] [PubMed]
- Gunseren KO, Cicek MC, Bolat D, et al. Is laparoscopic adrenalectomy for pheochromocytoma safe and effective in geriatric patients? Int J Clin Pract 2021;75:e14427. [Crossref] [PubMed]
- Wang K, Tang G, Peng Y, et al. Adrenal pheochromocytoma: is it all or the tip of the iceberg? Jpn J Radiol 2022;40:120-34. [Crossref] [PubMed]
- Koyama K, Miura N, Watanabe R, et al. Predictors of Hypotension after Adrenalectomy for Pheochromocytoma. Acta Med Okayama 2021;75:345-9. [PubMed]
- Wang J, Yang B, Sun S, et al. Perioperative factors influencing the difficulty of retroperitoneal laparoscopic adrenalectomy: a single-center retrospective study. BMC Urol 2022;22:22. [Crossref] [PubMed]
- Arezzo A, Bullano A, Cochetti G, et al. Transperitoneal versus retroperitoneal laparoscopic adrenalectomy for adrenal tumours in adults. Cochrane Database Syst Rev 2018;12:CD011668. [Crossref] [PubMed]
- Chen W, Liang Y, Lin W, et al. Surgical management of large adrenal tumors: impact of different laparoscopic approaches and resection methods on perioperative and long-term outcomes. BMC Urol 2018;18:31. [Crossref] [PubMed]
- Ji C, Lu Q, Chen W, et al. Retrospective comparison of three minimally invasive approaches for adrenal tumors: perioperative outcomes of transperitoneal laparoscopic, retroperitoneal laparoscopic and robot-assisted laparoscopic adrenalectomy. BMC Urol 2020;20:66. [Crossref] [PubMed]
- Sahbaz NA, Dural AC, Akarsu C, et al. Transperitoneal laparoscopic surgery in large adrenal masses. Wideochir Inne Tech Maloinwazyjne 2020;15:106-11. [Crossref] [PubMed]
- Hu Q, Hang Z, Ho Y, et al. Impact of Obesity on Perioperative Outcomes of Retroperitoneal Laparoscopic Adrenalectomy. Urol Int 2015;95:361-6. [Crossref] [PubMed]
- Rodríguez-Hermosa JI, Planellas-Giné P, Cornejo L, et al. Comparison of Outcomes between Obese and Nonobese Patients in Laparoscopic Adrenalectomy: A Cohort Study. Dig Surg 2021;38:237-46. [Crossref] [PubMed]
- Inaishi T, Kikumori T, Takeuchi D, et al. Obesity does not affect peri- and postoperative outcomes of transabdominal laparoscopic adrenalectomy. Nagoya J Med Sci 2018;80:21-8. [PubMed]
- Oh JY, Chung HS, Yu SH, et al. Comparison of surgical outcomes between lateral and posterior approaches for retroperitoneal laparoscopic adrenalectomy: A single surgeon's experience. Investig Clin Urol 2020;61:180-7. [Crossref] [PubMed]
- Lee SS, Ryu SW, Kim IH, et al. Impact of gender and body mass index on surgical outcomes following gastrectomy: an Asia-Pacific perspective. Chin Med J (Engl) 2012;125:67-71. [PubMed]
- Chai YJ, Yu HW, Song RY, et al. Lateral Transperitoneal Adrenalectomy Versus Posterior Retroperitoneoscopic Adrenalectomy for Benign Adrenal Gland Disease: Randomized Controlled Trial at a Single Tertiary Medical Center. Ann Surg 2019;269:842-8. [Crossref] [PubMed]
- Rah CS, Kim WW, Lee YM, et al. New predictive factors for prolonged operation time of laparoscopic posterior retroperitoneal adrenalectomy; retrospective cohort study. Int J Surg 2021;94:106113. [Crossref] [PubMed]


