
Determination of microbiological characteristics and risk factors associated with bacteriuria and symptomatic urinary tract infection in patients with retained ureteral stents: an observational study
Introduction
Urinary tract infection (UTI) is one of the most common types of infection in the general population. Symptoms related to UTI have a broad clinical spectrum, ranging from asymptomatic bacteriuria to severe sepsis accompanied by acute pyelonephritis or renal abscess (1). Globally, UTIs constitute the largest portion of healthcare-associated infections, which are potentially serious complications in hospitalized patients, leading to increasing costs, morbidity, and mortality rates (2,3). Mostly, asymptomatic bacteriuria does not require antimicrobial treatment, similar to bacteriuria associated with indwelling urethral catheters (4). However, UTI, with clinical symptoms such as dysuria and suprapubic pain, is differentiated from asymptomatic bacteriuria in that it requires the use of antibiotics, especially when the infection is complicated by catheter-associated sepsis (4).
Ureteral stenting is a widely used method for urinary drainage through the ureter into the bladder to relieve ureteral obstruction due to various etiologies and supports postoperative drainage in urology care. However, stenting can induce various minor complications, such as dysuria, suprapubic pain, and hematuria, as well as major complications such as UTI (5).
Among these complications, UTI related to ureteral stenting is frequently encountered in patients with ureteral stents and requires adequate treatment to prevent progression to more severe conditions (6). A previous study has reported a high incidence of bacteriuria in patients with ureteral stents with the highest reported incidence rate up to 100% in patients with permanent stents (7). In addition, extended-spectrum beta-lactamase (ESBL)-producing bacteria and multidrug-resistant microorganisms have been isolated in patients with ureteral stents requiring specific treatment, as seen in other foreign materials used as prosthetic devices (8,9). ESBL-producing bacteria represent a major concern, especially in the hospitalized patient population, and a costly therapeutic challenge requiring broad-spectrum antibiotics with an increased length of hospital stay with economic consequences (10).
To prevent the development of UTI and resistant microorganisms, indwelling ureteral stents must be removed, if possible, in a short time. Regarding this, several studies demonstrated that the duration time of ureteral stents was significantly associated with bacteriuria occurrence (11-13). However, ureteral stents need to be maintained in patients with severe ureteral stricture or ureteral obstruction due to malignancy. In such circumstances, ureteral stents should be replaced periodically to prevent the development of UTI. Although stents should be replaced periodically, patients with maintained ureteral stents in place might be at a higher risk of developing UTI than other groups of patients.
Considering this, knowing the microbiological profile associated with the use of these devices and their resistance pattern is essential to avoid the indiscriminate broad use of antibiotics and prevent occurrence of multidrug-resistant bacteria in patients with maintained ureteral stents in place. Moreover, the identification of predisposing factors for the development of symptomatic UTI associated with ureteral stents is important to prevent sepsis or other complications following UTI and establish an appropriate strategy for using antimicrobial treatment.
Most previous studies have mainly evaluated the pattern of asymptomatic bacteriuria and the colonization rate of ureteral stents in patients with these medical devices (12,14,15). However, the results reported in the literature are extremely varied. Moreover, these studies did not make clear distinctions between bacteriuria and symptomatic UTI. Additionally, only a few studies have assessed the pathophysiology and risk factors for the development of symptomatic UTI in these patients. Recently, two literature reviews have attempted to clarify the epidemiological characteristics and risk factors for UTI in patients with ureteral stents; however, they could not come to clear conclusions due to discrepancies in methodology and a low level of evidence of the included studies (16,17).
Accordingly, this study aimed to identify the microbiological profile of bacteriuria and the incidence of ESBL-producing bacteria in patients who, in particular, required retained ureteral stents and to determine the predisposing factors associated with the bacteriuria and ESBL-producing bacteria in these patients. Additionally, we aimed to determine the incidence rate and risk factors for the development of symptomatic UTI, including factors such as the duration and number of previous replacements of ureteral stents. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-331/rc).
Methods
This study is an observational cross-sectional study. From August 2018 to January 2021, 307 consecutive patients who required indwelling and replaced ureteral stents more than once at Sanggye Paik Hospital were evaluated. Patients with age less than 18 years and those with indwelling ureteral stents placed only once and subsequently removed were excluded from the study. Additionally, patients with ureteral stents inserted through a percutaneous nephrostomy site, bilateral stents, large kidney stones (staghorn stones), ureteral stent migration, or current antibiotic treatment were also excluded from the study because they could have affected the incidence of colonization in urine, leading to inadequate analysis. We excluded patients with urinary stasis developed due to various etiologies, including bladder outlet obstruction and impaired bladder function, because this condition predisposes patients to UTI. We evaluated the voiding pattern and residual urine amount regularly to identify patients with urinary stasis.
Data regarding patient characteristics, including age, sex, body weight, and height, were collected. Underlying systemic diseases of the included patients, such as hypertension, diabetes mellitus (DM), cardiac disease, and the reasons for ureteral stent placement were reviewed. Additionally, laboratory test results, such as estimated glomerular filtration rate (eGFR), glucose level, HbA1c level, lipid profile, and data of patient conditional status, including urethral catheter use and dependent functional capacity, were also collected. We used the Modification of Diet in Renal Disease equation to determine the eGFR according to serum creatinine levels (18). Then, we stratified the patients with chronic kidney disease (CKD) stage by using calculated eGFR. The participants were categorized into the following five stages according to their baseline eGFR: stage 1, eGFR ≥90 mL/min/1.73 m2; stage 2, eGFR of 60–89 mL/min/1.73 m2; stage 3, eGFR of 30–59 mL/min/1.73 m2; stage 4, eGFR 15–29 mL/min/1.73 m2; and stage 5, eGFR <15 mL/min/1.73 m2. We divided the patients stratified by eGFR into 2 groups: group A (preserved urine amount group) including CKD stage 1, 2, and 3, and group B (decreased urine amount group) including CKD stage 4, and 5, due to a small number of patients with CKD stage 5. Moreover, the duration and number of previous ureteral stent replacements were reviewed.
Urine samples from patients systematically collected before ureteral stent replacement were examined using microbiological testing. The acquired urine samples were inoculated on eosin methylene blue (EMB) agar and blood agar for cultural studies. Plates were incubated for 48 hours at 37 ℃. The microorganisms that grew on the agar were evaluated quantitatively [growth of >105 colony-forming units (CFU)/mL was considered significant]. Additionally, all microbial isolates were tested for antibiotic susceptibility. The evaluation and analysis of microbiological characteristics and resistance patterns were conducted according to the definitions and recommendations of the National Committee for Clinical and Laboratory Standards Institute (19,20).
A silicone ureteral stent, double J stent type (Boston Scientific Corp., Natick, MA, USA), was used in all the patients. All stents were inserted through a retrograde route using the endoscopic method under fluoroscopic guidance. After the initial placement of the ureteral stents, they were replaced periodically because the patients required maintenance of these ureteral stents for urinary drainage. However, for different patients, ureteral stents were replaced at different intervals due to the different visiting times and medical conditions of the patients.
The patients with ureteral stents were instructed to visit the hospital immediately in case of developing irritative voiding symptoms, such as dysuria and frequency, fever, flank pain, or pelvic pain during the follow-up period. The patients with clinical symptoms suggestive of UTI, including dysuria, fever, or flank pain who showed the presence of ≥105 CFU/mL of a bacterial species isolated from midstream clean urine were diagnosed with UTI (21,22). Additionally, white blood cell (WBC) count, erythrocyte sedimentation rate, and C-reactive protein values were checked to clarify the disease status.
Asymptomatic bacteriuria was defined as the presence of ≥105 CFU/mL of one bacterial species isolated from two consecutive urine culture samples without any clinical symptoms suggestive of UTI (21). The patients with symptomatic UTI during the study were administered appropriate antibiotics based on microbiological susceptibility results, and the dose and duration of each antibiotic followed clinical practice guidelines (23,24). Subsequently, ureteral stents were removed if the patients’ conditions were stable. Symptomatic UTI was considered to be successfully treated if urinary clinical symptoms disappeared with negative urine culture results.
The primary endpoint was the incidence rate of bacteriuria and ESBL-producing bacteria. The secondary endpoint was the factors predisposing patients with ureteral stents to bacteriuria, ESBL-producing bacteria, and development of symptomatic UTIs.
Statistical analysis
Quantitative data are presented as mean (standard deviation) and categorical data as numbers (n) and percentages. The normal distribution of variables was checked using the Kolmogorov-Smirnov test. Additionally, goodness of fit of logistic regression models was assessed using the Hosmer and Lemeshow test. Univariate and multivariate logistic regression analyses were performed to identify the factors predisposing patients with ureteral stents to bacteriuria, ESBL-producing bacteria, and the development of symptomatic UTI. Results are presented as odds ratios (ORs) and 95% confidence intervals (95% CIs). All tests were two-tailed, and a P value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB) of Sanngye Paik Hospital, Inje University (committee reference No. 2018-11-011), and informed consent was obtained from each patient after careful explanation of the scope of the study.
Results
A total of 307 patients [111 (36.2%) men; 196 (63.8%) women] were included in this study. The mean patient age was 59.4 (±18.1) years. Patient characteristics are presented in Table 1. Overall, 13 (4.2%) had dependent functional capacity, and 23 (7.5%) had indwelling urethral catheters due to persistent bladder distention. The mean duration of placement of ureteral stents was 75.9 (±39.4) days, and the number of previous replacements of ureteral stents was 5.7 (±6.3).
Table 1
| Factors | N (%) or mean ± SD |
|---|---|
| Age (years) | 59.4±18.1 |
| Sex | |
| Male | 111 (36.2) |
| Female | 196 (63.8) |
| Body weight (kg) | 59.0±15.8 |
| Height (cm) | 159.4±10.8 |
| BMI (kg/m2) | 23.6±14.7 |
| Comorbid factors | |
| Diabetes mellitus | 71 (23.1) |
| Hypertension | 122 (39.7) |
| Cardiac disease (CHF, MI) | 21 (6.8) |
| Chronic kidney disease (stratified by CKD stage) | |
| Group A | |
| Stage 1 | 83 (27.0) |
| Stage 2 | 102 (33.2) |
| Stage 3 | 71 (23.1) |
| Group B | |
| Stage 4 | 38 (12.4) |
| Stage 5 | 13 (4.2) |
| Hyperlipidemia | 17 (5.5) |
| Other malignancy | 28 (9.1) |
| Indications for ureteral stenting | |
| Ureteral stricture | 167 (54.4) |
| Hydronephrosis due to ureteral stone | 75 (24.4) |
| Ureteral obstruction due to malignancy | 65 (21.2) |
| Dependent functional capacity | 13 (4.2) |
| Indwelling urethral catheter use | 23 (7.5) |
| Ureteral stent duration period (days) | 75.9±39.4 |
| The number of previous replacements of ureteral stent | 5.7±6.3 |
| Bacteriuria | 187/307 (60.9) |
| ESBL-producing bacteria | 52/187 (27.8) |
| Symptomatic urinary tract infection | 22/187 (11.8) |
CKD stage by using calculated eGFR. Group A including CKD stage 1, 2, and 3. Group B including CKD stage 4, and 5. BMI, body mass index; CKD, chronic kidney disease; CHF, congestive heart failure; ESBL, extended-spectrum beta-lactamase; GFR, glomerular filtration rate; MI, myocardial infarction; SD, standard deviation.
Regarding the results of urine culture, bacteriuria was found in 187 patients (60.9%). Regarding the bacteria identified in urine, Escherichia coli was the most commonly isolated microorganism (60 patients), followed by Enterococcus (44 patients). Additionally, Candida, Staphylococcus, Klebsiella pneumoniae, and Pseudomonas aeruginosa were detected in 25, 17, 13, and 8 patients, respectively (Figure 1). Using the multivariate logistic analysis, the occurrence of bacteriuria was significantly associated with old age, female sex, presence of DM. Additionally, a lower glomerular filtration rate (GFR) and longer duration of ureteral stenting significantly increased the incidence of bacteriuria. Moreover, the patients in group B with CKD stage 4 and 5 showed significantly increased occurrence of bacteriuria compared to those in group A. The number of previous replacements of ureteral stents was not significantly associated with the development of bacteriuria (Table 2).
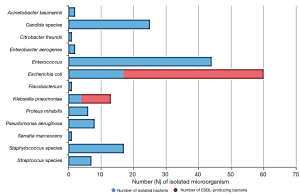
Table 2
| Factors | Non-bacteriuria, n (%) or mean ± SD | Bacteriuria, n (%) or mean ± SD | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||||
| Age (years) | 54.2±18.2 | 62.7±17.3 | 1.03 (1.01–1.04) | <0.001 | 1.02 (1.00–1.03) | 0.030 | |
| Gender | |||||||
| Male | 61 (50.8) | 50 (26.7) | – | – | – | – | |
| Female | 59 (49.2) | 137 (73.3) | 2.83 (1.75–4.59) | <0.001 | 2.92 (1.74–4.90) | <0.001 | |
| Body weight (kg) | 62.2±12.8 | 56.9±13.2 | 0.97 (0.96–0.99) | 0.002 | 1.16 (0.98–1.38) | 0.088 | |
| Height (cm) | 161.4±10.2 | 158.2 ±9.3 | 0.98 (0.96–1.00) | 0.060 | 0.90 (0.79–1.02) | 0.100 | |
| BMI (kg/m2) | 23.8±3.7 | 22.7±4.4 | 0.95 (0.90–1.00) | 0.033 | 0.63 (0.40–1.01) | 0.053 | |
| Comorbid factors | |||||||
| Diabetes mellitus | 17 (14.2) | 54 (29.0) | 2.48 (1.36–4.53) | 0.003 | 2.09 (1.02–4.28) | 0.045 | |
| Hypertension | 37 (30.8) | 85 (45.7) | 1.89 (1.17–3.06) | 0.010 | 0.66 (0.34–1.27) | 0.212 | |
| Cardiac disease (CHF, MI) | 7 (5.8) | 14 (7.5) | 1.31 (0.51–3.36) | 0.568 | – | – | |
| CKD stage (group A vs. group B) | |||||||
| Group A | 109 (90.8) | 147 (78.6) | 2.70 (1.32–5.49) | 0.006 | 2.70 (1.20–6.04) | 0.016 | |
| Group B | 11 (9.2) | 40 (21.4) | |||||
| Hyperlipidemia | 4 (3.3) | 13 (7.0) | 2.17 (0.69–6.81) | 0.186 | 2.92 (0.90–15.2) | 0.102 | |
| Other malignancy | 6 (5.0) | 22 (11.8) | 2.53 (1.00–6.45) | 0.051 | 1.57 (0.81–10.6) | 0.383 | |
| Indications for ureteral stenting | |||||||
| Urethral stricture | 59 (49.2) | 108 (57.8) | 0.94 (0.51–1.71) | 0.831 | – | – | |
| Nephrolithiasis | 39 (32.5) | 36 (19.3) | 0.74 (0.41–1.92) | 0.425 | – | – | |
| Obstruction due to malignancy | 22 (18.3) | 43 (23.3) | – | – | – | – | |
| Dependent functional capacity | 0 (0) | 13 (7.0) | – | 0.003 | – | – | |
| Indwelling urethral catheter use | 2 (1.7) | 21 (11.2) | 7.46 (1.72–32.4) | 0.007 | 3.65 (0.66–20.1) | 0.137 | |
| GFR (mL/min/1.73 m2) | 78.5±38.8 | 65.1±40.5 | 0.99 (0.99–1.00) | 0.007 | 0.99 (0.98–1.00) | 0.002 | |
| Ureteral stent duration (days) | 68.4±43.1 | 80.7±36.1 | 1.01 (1.00–1.01) | 0.008 | 1.01 (1.00–1.02) | 0.010 | |
| The number of previous replacements of ureteral stents | 4.5±5.0 | 6.5±6.9 | 1.07 (1.01–1.12) | 0.011 | 1.05 (0.99–1.11) | 0.090 | |
CKD stage by using calculated eGFR. Group A including CKD stage 1, 2, and 3. Group B including CKD stage 4, and 5. BMI, body mass index; CKD, chronic kidney disease; CHF, congestive heart failure; GFR, glomerular filtration rate; MI, myocardial infarction; OR, odds ratio; CI, confidence interval; SD, standard deviation.
Regarding the resistance of bacteria found in urine, ESBL-producing bacteria were found in 52 isolates (27.8%). Moreover, ESBL-producing bacteria were identified in 43 out of 60 patients (71.7%) with E. coli and 9 out of 13 (69.2%) with K. pneumoniae (Figure 1). The incidence of ESBL-producing bacteria in urine cultures was associated with old age (OR 1.04; 95% CI: 1.02–1.06; P<0.001) and longer duration of ureteral stenting (OR 1.02; 95% CI: 1.00–1.03; P=0.048) using multivariate logistic analysis (Table 3).
Table 3
| Factors | Non-ESBL-producing bacteriuria, n (%) or mean ± SD | ESBL-producing bacteriuria, n (%) or mean ± SD | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||||
| Age (years) | 60.7±17.4 | 67.9±16.3 | 1.04 (1.02–1.06) | <0.001 | 1.04 (1.02–1.06) | <0.001 | |
| Gender | |||||||
| Male | 38 (28.1) | 12 (23.1) | – | – | – | – | |
| Female | 97 (71.9) | 40 (76.9) | 0.766 (0.36–1.62) | 0.484 | – | – | |
| Body weight (kg) | 57.0±14.2 | 56.4±10.1 | 0.99 (0.96–1.01) | 0.221 | – | ||
| Height (cm) | 158.7±9.3 | 156.9±9.3 | 0.98 (0.95–1.01) | 0.106 | 1.04 (0.96–1.12) | 0.316 | |
| BMI (kg/m2) | 22.6±4.5 | 23.0±4.2 | 1.00 (0.96–1.03) | 0.766 | – | ||
| Comorbid factors | |||||||
| Diabetes mellitus | 39 (29.1) | 15 (28.8) | 1.43 (0.73–2.80) | 0.292 | – | – | |
| Hypertension | 59 (44.0) | 26 (50.0) | 1.65 (0.90–3.00) | 0.104 | 0.99 (0.48–2.04) | 0.971 | |
| Cardiac disease (CHF, MI) | 7 (5.2) | 7 (13.5) | 2.82 (0.94–8.50) | 0.065 | 2.17 (0.74–6.39) | 0.159 | |
| CKD stage (group A vs. group B) | |||||||
| Group A | 105 (77.8) | 42 (80.8) | 0.83 (0.37–1.86) | 0.66 | – | – | |
| Group B | 30 (22.2) | 10 (19.2) | |||||
| Hyperlipidemia | 8 (5.9) | 5 (9.6) | 2.15 (0.73–6.40) | 0.167 | 1.92 (0.59–6.28) | 0.281 | |
| Other malignancy | 13 (9.6) | 9 (17.3) | 1.96 (0.78–4.92) | 0.145 | 2.02 (0.81–5.00) | 0.130 | |
| Indications for ureteral stenting | |||||||
| Urethral stricture | 72 (53.8) | 36 (69.2) | 1.51 (0.70–3.26) | 0.29 | – | – | |
| Nephrolithiasis | 30 (22.2) | 6 (11.5) | 0.48 (0.16–1.40) | 0.178 | 0.67 (0.04–11.5) | 0.781 | |
| Obstruction due to malignancy | 33 (24.4) | 10 (19.2) | – | – | – | – | |
| Dependent functional capacity | 11 (8.1) | 2 (3.8) | 0.89 (0.19–4.13) | 0.879 | – | – | |
| Indwelling urethral catheter use | 15 (11.1) | 6 (11.5) | 1.83 (0.68–4.88) | 0.23 | – | – | |
| GFR (mL/min/1.73 m2) | 63.9±41.2 | 68.2±38.8 | 1.00 (0.99–1.01) | 0.694 | – | – | |
| Ureteral stent duration (days) | 78.2±36.6 | 87.1±34.2 | 1.01 (1.00–1.02) | 0.026 | 1.02 (1.00–1.03) | 0.048 | |
| The number of previous replacements of ureteral stents | 6.7±7.7 | 6.2±4.3 | 1.01 (0.96–1.06) | 0.712 | – | – | |
CKD stage by using calculated eGFR. Group A including CKD stage 1, 2, and 3. Group B including CKD stage 4, and 5. BMI, body mass index; CKD, chronic kidney disease; CHF, congestive heart failure; ESBL, extended-spectrum beta-lactamase; GFR, glomerular filtration rate; eGFR, estimated GFR; MI, myocardial infarction; OR, odds ratio; CI, confidence interval; SD, standard deviation.
Additionally, symptomatic UTI developed in 22 patients (7.2%) among all patients. Among patients with symptomatic UTI, the most commonly isolated microorganism was E. coli (five). The following species were identified: Enterococcus (four), Candida species (four), P. aeruginosa (three), and Proteus mirabilis (one). Out of the isolated E. coli, four were ESBL.
When comparing the patients with symptomatic UTI with asymptomatic bacteriuria, asymptomatic bacteriuria was identified in 165 (88.2%), while symptomatic UTI developed in 22 (11.8%) patients with bacteriuria, respectively. No significant differences in bacterial frequency were identified between patients with symptomatic UTI and asymptomatic bacteriuria. However, the proportion of ESBL-producing bacteria among overall E. coli species was higher in patients with symptomatic UTI (80%) than asymptomatic bacteriuria (71%), although it was not significant.
The 22 patients who developed UTI were administered antibiotics according to the urine susceptibility test results. Among them, 15 had their ureteral stent replaced with new stents; subsequently, the UTI symptoms resolved, and bacteriuria was not found in the urine. In the remaining seven patients with symptomatic UTI, ureteral stents were left in place, although they were treated with appropriate antibiotics. Symptoms related to UTI recurred in these patients, and the mean time to symptom recurrence was 10 days. Urine culture obtained in these patients showed positive results. Subsequently, ureteral stents were replaced with new stents after the patients’ condition recovered. After the replacement of ureteral stents, the remaining UTI symptoms were relieved.
Dependent functional capacity and impaired renal function were significantly associated with the development of symptomatic UTI. In particular, group B patients with CKD stage 4 and 5 showed significantly increased development of symptomatic UTI compared to those in group A. Additionally, a longer ureteral stenting duration correlated significantly with the development of symptomatic UTI (Table 4). Age, sex, and the presence of DM were not significantly associated with the development of symptomatic UTI. Similar to bacteriuria, the number of previous replacements of ureteral stents was not significantly associated with the development of symptomatic UTI.
Table 4
| Factors | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (years) | 0.99 (0.98–1.02) | 0.889 | – | – | |
| Gender | |||||
| Male | – | – | – | – | |
| Female | 1.24 (0.51–3.01) | 0.631 | – | – | |
| Body weight (kg) | 0.98 (0.95–1.01) | 0.27 | – | – | |
| Height (cm) | 1.23 (0.98–1.08) | 0.301 | – | – | |
| Comorbid factors | |||||
| Diabetes mellitus | 1.92 (1.06–3.46) | 0.031 | 0.75 (0.23–2.38) | 0.619 | |
| Hypertension | 0.85 (0.35–2.10) | 0.728 | – | – | |
| Cardiac disease (CHF, MI) | 1.40 (0.30–6.42) | 0.669 | – | – | |
| CKD stage (group A vs. group B) | 2.56 (0.99–6.63) | 0.054 | 2.86 (1.06–7.73) | 0.039 | |
| Hyperlipidemia | 1.80 (0.38–8.43) | 0.456 | – | – | |
| Other malignancy | 0.99 (0.22–4.50) | 0.996 | – | – | |
| BMI (kg/m2) | 0.93 (0.86–1.01) | 0.102 | 0.80 (0.34–1.85) | 0.595 | |
| Indications for ureteral stenting | |||||
| Urethral stricture | 0.76 (0.27–2.12) | 0.602 | – | – | |
| Nephrolithiasis | 0.55 (0.15–2.06) | 0.377 | – | – | |
| Obstruction due to malignancy | – | – | – | – | |
| Dependent functional capacity | 6.82 (1.91–24.3) | 0.003 | 17.2 (1.85–159) | 0.012 | |
| Indwelling urethral catheter use | 4.36 (1.44–13.2) | 0.009 | 5.75 (0.89–37.2) | 0.067 | |
| GFR (mL/min/1.73 m2) | 0.99 (0.97–1.00) | 0.055 | 0.98 (0.96–0.99) | 0.008 | |
| Ureteral stent duration (days) | 1.01 (1.00–1.03) | 0.045 | 1.01 (1.00–1.02) | 0.049 | |
| The number of previous replacements of ureteral stents | 0.98 (0.90–1.06) | 0.599 | – | – | |
| ESBL-producing bacteria | 1.10 (0.36–3.39) | 0.872 | – | – | |
CKD stage by using calculated eGFR. Group A including CKD stage 1, 2, and 3. Group B including CKD stage 4, and 5. BMI, body mass index; CKD, chronic kidney disease; CHF, congestive heart failure; GFR, glomerular filtration rate; ESBL, extended-spectrum beta-lactamase; MI, myocardial infarction, OR, odds ratio; CI, confidence interval.
Discussion
In our study, infections related to ureteral stents showed a specific microorganism profile and resistance pattern, in which microorganisms other than E. coli, such as Enterococcus, Staphylococcus species, Klebsiella, and Candida species, were found more frequently when compared to community-acquired UTI. Additionally, we identified the factors associated with bacteriuria and development of symptomatic UTI in patients with retained ureteral stents.
UTI is one of the most frequent complications in patients with indwelling ureteral stents, occurring in as many as 30% of cases leading to increased treatment costs and higher mortality rates (11,25). To prevent these complications, short ureteral stenting duration, if possible, may be an effective method. However, some patients require ureteral stents in place for longer duration due to various circumstances, such as severe ureteral stricture or ureteral obstruction due to malignancy.
Several studies have evaluated the relationship between indwelling urethral catheters and development of UTI. However, few studies have reported on UTI development in patients with ureteral stents (12,14,15). Although the guidelines for bladder catheterization were established by the Infectious Diseases Society of America (26), there have been no guidelines for the management of ureteral stents. Infections related to ureteral stents are expected to exhibit microbiological characteristics different from those found in urethral catheters. Therefore, treatment of UTI in patients with ureteral stents should be specified according to the different microbiological patterns found in these patients.
Ureteral stents are associated with a high incidence rate of UTI, particularly the isolation of ESBL and multidrug-resistant microorganisms (8,9). This is known to be due to colonization of ureteral stents by bacteria, which can induce infectious processes.
The bacteria can invade the bladder at the time of ureteral stent insertion or due to its movement during indwelling, both of which have been associated with catheter-associated infections (27). Moreover, indwelling medical devices, such as ureteral stents, facilitate conditioning film formation on the surface of the biomaterial by deposition of host urinary components, including proteins, electrolytes, and unidentified molecules, which various bacterial species recognize and bind to (28,29). This process by bacterial uropathogens can develop within 24 h after insertion of ureteral stents; up to 90% of indwelling stents when removed from patients contained adherent bacteria (7). The formation of biofilm makes it difficult to achieve significant concentrations of antibiotics. The colonization of the surfaces of materials cannot be prevented, leaving patients at the continued risk of infection or other complications (28). Therefore, once the biofilm is established, eradication of the infection with increased antimicrobial resistance becomes very difficult. Although several studies have been conducted to produce ureteral stents that completely prevent bacterial adhesion and biofilm formation, to date, no such devices exist (30).
Previous studies reported a high incidence of bacteriuria in patients with ureteral stents. Farsi et al. demonstrated that the incidence rate of bacteriuria was 30% in their study (11). In other studies, Paick et al. reported that the incidence rate of bacteriuria was 21% (31), while Kehinde et al. reported that it was 17% (12,32). Similarly, Lifshitz et al. reported a bacteriuria rate of 13% in 65 patients with ureteral stents (25). In addition, Yeniyol et al. reported the presence of bacteriuria in 16% of patients (33) while Akay et al. reported bacteriuria in 24% of patients with ureteral stents at the time of ureteral stent removal (34).
In our study, the incidence of bacteriuria was 60.9%, which is higher than that reported in other studies. This may be because the patients included in this study maintained ureteral stents for longer than in other previous studies, although in this study the stents were periodically replaced. Medical devices retained for longer in the urinary tract were found to be vulnerable to bacterial infection (30). Regarding this, Riedl et al. have reported that bacteriuria was identified in 100% of patients with indwelling permanent stent, compared to 45% in patients with temporary stents (7).
Regarding the microbiological pattern, E. coli was the most commonly isolated microorganism in urine (60 patients), followed by Enterococcus (44 patients). Additionally, Candida, Staphylococcus, K. pneumoniae, and P. aeruginosa were detected in 25, 17, 13, and 8 patients, respectively.
Several previous studies on bacteriuria in patients with ureteral stents have demonstrated similar results, in which bacteria other than E. coli, such as Enterococcus, Staphylococcus species, Klebsiella, and Candida species, were found more frequently compared to those in patients with community-acquired UTI (9,21,34). This may be associated with the longer retention of ureteral stents in patients in the study. Similar to our findings, Akay et al. reported that E. coli was the most commonly found bacterium in urine (34 of 47 patients); the other samples included Enterococcus species (four), Staphylococcus epidermis (two), P. mirabilis (two), and P. aeruginosa (two) (34). In another study, Klis et al. reported that E. coli was the microorganism most frequently isolated from urine, followed by S. epidermidis and S. aureus (13). Altunal et al. reported similar results (35). In addition, previous systematic reviews have reported that Staphylococcus, E. coli, Klebsiella species, P. aeruginosa, Enterococcus, and Candida species were the microorganisms most commonly identified in urine or ureteral stents (16,17).
Lara-Isla et al. reported that in patients with ureteral stents, the most frequently isolated microorganism was E. coli (25.7%), followed by Enterococcus (22.9%). Other microorganisms such as Klebsiella (9%), Pseudomonas (9%), and Candida species (11%) were also frequently isolated, which is in concordance with the results of our study (9). In addition, they demonstrated that regarding the origin of the infection, E. coli was found in 50% of bacteriuria in community-acquired infections, while E. coli and Enterococcus were found in 22.6% of hospital-acquired infections, implying a higher incidence rate of Enterococcus in healthcare-associated infections.
In this study, DM and impaired renal function (in particular, CKD stage 4 and 5) were associated with the occurrence of bacteriuria. Similarly, Kehinde et al. suggested that the incidence of bacteriuria in patients with systemic diseases, including DM, chronic renal failure, and diabetic nephropathy, was significantly higher than that in patients without such diseases (12). Akay et al. reported that the incidence of bacteriuria increased 10-fold in patients with DM or CKD (34).
This finding may have been due to DM, chronic renal failure, diabetic nephropathy, and pregnancy that induce immunosuppression, thereby promoting urinary infection (34). Additionally, these diseases may be responsible for weakening the natural defensive mechanisms of the bladder hence increasing the incidence of bacteriuria (36). DM and CKD predispose individuals to infections occurring in other parts of the body. Therefore, the maintenance of ureteral stents in these patients is expected to lead to an increase in the occurrence of bacteriuria.
Additionally, we found that the female sex was significantly associated with a higher incidence of bacteriuria in patients with ureteral stents than the male sex. In previous studies, several researchers reported similar results in patients with ureteral stents (11,12), while other reports showed that sex was not associated with the incidence of bacteriuria (25,34). The reasons for this difference are not clear. However, a possible explanation could be the anatomically closer location of the urethral orifices with the genital tract in the females.
Moreover, the longer duration of ureteral stenting was significantly associated with bacteriuria occurrence. Several previous studies showed results similar to our study’s (7,11,12). Additionally, Paick et al. (31) reported that bacterial colonization began two weeks after stenting. However, Lifshitz et al. did not find any relationship between stent duration and incidence of bacteriuria (25). They examined 65 patients, a small population compared with our study’s relatively larger population (307 patients). Additionally, recent literature reviews demonstrated that a longer duration of ureteral stenting was an associated risk factor for the occurrence of bacteriuria, although the included studies presented multiple methodological biases (16,17).
Additionally, in our study, the number of previous replacements of ureteral stents did not show a significant association with the development of bacteriuria.
In addition to specific microbiological characteristics, high rates of resistance to antibiotics should be considered when managing UTI in patients with ureteral stents. The antimicrobial resistance of bacteria causing UTI has been recently increasing worldwide, which is thought to be a result of the widespread and indiscriminate use of antibiotics (8). Among them, the increased incidence of ESBL-producing bacteria that present resistance to most antibiotics, except the carbapenem group, is a major concern, with an increased length of stay and economic consequences, as proper antibiotic treatment is challenging (9). Several studies have demonstrated that 27.8% of ESBL-producing bacteria have been identified in patients with healthcare-associated UTIs, including those with ureteral stents (10).
However, few reports have described the resistance patterns and related factors of bacteria isolated from patients with ureteral stents. Kehinde et al. described the susceptibility patterns of isolated bacteria in patients with ureteral stents, which showed that in this population a larger proportion of bacteria was generally more resistant to antibiotics than bacteria cultured from the urine of general hospital patients (12). In another study, Altunal et al. reported that there were 58% ESBL, 67% trimethoprim/sulfamethoxazole, 58% ciprofloxacin, and 58% ceftriaxone resistance among 12 E. coli strains in patients with ureteral stents (35). Lara-Isla et al. reported that Klebsiella showed a higher resistance pattern among isolated bacteria: 66.7% were ESBL-producing Klebsiella in patients with ureteral stents, while 11.1% were ESBL-producing E. coli (9).
Similar to previous studies, in our study, ESBL-producing bacteria were found in 52 (27.8%) of the isolated bacteria. ESBL-producing bacteria were identified in 43 of 60 patients (71.7%) with E. coli and 9 of 13 (69.2%) patients with K. pneumoniae. In addition, the incidence of ESBL-producing bacteria in urine cultures was associated with old age and a longer duration of ureteral stenting using multivariate logistic analysis. The high incidence of ESBL-producing bacteria may be due to ureteral stent insertion being performed in the hospital environment, which is vulnerable to nosocomial infection, with ureteral stent retention in place working as contributing factor resulting in complicated UTIs.
Additionally, we aimed to determine the risk factors for the development of symptomatic UTI, including underlying comorbidities, duration of treatment, and the number of previous ureteral stent replacements.
Only a few studies have been conducted to assess the development of symptomatic UTI and related factors in patients with retained ureteral stents (14,15). Moreover, some previous studies did not differentiate bacterial colonization from true UTI with clinical symptoms. Recently, two literature reviews have tried to clarify the risk factors for UTI in patients with ureteral stents; however, they could not come to clear conclusions due to discrepancies in methodology and a low level of evidence of the included studies (16,17).
Kehinde et al. reported an incidence of symptomatic UTI of 6.4% (32). In their study, patients with symptomatic UTI received appropriate antibiotic treatment. Among the 16 patients with symptomatic UTI, four had no systemic disease, while 12 had systemic diseases, such as chronic renal failure, diabetic nephropathy, and DM. Out of the 16 patients with symptomatic UTI, 7 (43.8%) showed recovery from UTI with antimicrobial therapy. However, nine had septicemia, necessitating removal of ureteral stents. In another study, Altunal et al. reported that 11 (18%) of 60 patients developed symptomatic UTI (35). They demonstrated that a longer duration of ureteral stent placement, presence of DM, and chronic renal failure were significantly associated with the development of symptomatic UTI. Another study reported that about 15% of patients developed symptomatic UTI, which was higher than that reported in a similar study (37).
In our study, the incidence of symptomatic UTI was 22 (7.2%) among all patients. Out of the 22 patients, 15 had their ureteral stents replaced with new ones and were subsequently administered antibiotics based on the susceptibility test results from urine cultures. After using antibiotics and replacement of ureteral stents, the symptoms associated with UTI resolved, and bacteriuria was not found in urine. The remaining seven patients with symptomatic UTI were treated with appropriate antibiotics, with the ureteral stents left in place. However, in seven patients in whom the same ureteral stent was retained, UTI recurred, and the mean time to symptom recurrence was 10 days. After replacement of ureteral stents, the UTI symptoms were relieved.
Similar to our results, Altunal et al. reported that of the 11 patients with symptomatic UTI, six had their ureteral stent replaced and were treated with antibiotics. However, the remaining five were treated with appropriate antibiotics, with ureteral stents left in place. UTI relapsed in all five patients (35).
This finding may have been due to the low effectiveness of antibiotics in bacterial colonization of ureteral stents due to biofilm formation on the surface of the material. Based on this result, we recommend that ureteral stents should be replaced in patients with symptomatic UTI, who require persistent indwelling ureteral stents, after appropriate antibiotic treatment.
In our study, the incidence of symptomatic UTI was associated with dependent functional capacity, impaired renal function, and a longer duration of ureteral stenting. Regarding these findings, it may be thought that dependent functional capacity and longer duration of ureteral stenting were more associated with the development of symptomatic UTI when compared to baseline patient characteristics including age, sex, and presence of DM, although these patient characteristics might also affect the development of UTI to some extent.
Additionally, renal function may be considered as the capacity to produce and excrete urine, in which microorganisms flow into the outer environment. Therefore, severely impaired renal function (in particular CKD state 4 and 5) may result in both concentrated bacteria and subsequent development of symptomatic UTI which is consistent with our results. In previous literature reviews (16,17), CKD appeared to be associated with an increased colonization rate of ureteral stents without any clear evidence of an increase in the development of UTI, although the methodological quality of the included studies was inadequate to derive any clear conclusions. It is known that fewer virulence factors are required for infection in patients with ureteral stents than in those with good function of the urinary tract. Additionally, impaired renal function are known to be potential risk factors for UTI due to the immunosuppression associated with these conditions (36).
A longer duration of ureteral stenting increased the incidence of symptomatic UTI and bacteriuria, which was associated with biofilm formation on the surface of ureteral stents. Our study showed that the number of previous replacements of ureteral stents was not associated with the development of symptomatic UTI, as in bacteriuria. This finding may imply that periodic replacement of ureteral stents in patients who require persistent indwelling ureteral stents would be important to prevent UTI. Additionally, although patients retaining ureteral stents have bacteriuria, not symptomatic UTI, with factors associated with UTI, replacement of ureteral stents with shorter duration should be considered.
Our study was not without limitations and had a potentially limited observational design and small sample size. The study was performed at a single center. However, it included a group of patients commonly managed in urology; therefore, the results may be applied to similar centers. We could not evaluate the incidence of ureteral stent colonization because microbiological tests were not performed for ureteral stents. Colonization of ureteral stents may be a factor associated with bacteriuria. However, colonization of ureteral stents does not necessarily coincide with UTI development. We plan to perform microbiological testing for ureteral stents and assess the incidence of ureteral stent colonization and its association with other factors.
Additionally, it is known that indwelling catheters and female sex are risk factors for bacteriuria and UTI. Therefore, these factors may be potential confounding factors, although analysis of this study was adjusted for variables, including these factors. We have also focused on other factors affecting bacteriuria and symptomatic UTIs, such as longer duration of ureteral stenting. Moreover, since we did not perform microbiological tests for urine samples on a weekly or monthly basis, we could not assess the incidence of bacteriuria and symptomatic UTI with time.
Conclusions
Infections related to ureteral stents showed a specific microorganism profile and resistance pattern compared to community-acquired UTIs. Additionally, we identified the factors associated with the occurrence of bacteriuria, ESBL-producing bacteria, and symptomatic UTI in patients with retained ureteral stents.
In our study, the possible short duration of ureteral stent placement and periodic replacement in patients with prolonged use of ureteral stents prevented the occurrence of bacteriuria, ESBL-producing bacteria, and symptomatic UTI. Considering the microbiological profile and factors associated with bacteriuria and symptomatic UTIs may be associated with better outcomes in patients with retained ureteral stents.
Acknowledgments
The authors thank the entire staff of the Department of Urology, Sanggye Paik Hospital, Inje University College of Medicine. Our Abstract has been published in https://onlinelibrary.wiley.com/doi/10.1111/iju.15019.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-331/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-331/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-331/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-331/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Richter S, Ringel A, Shalev M, et al. The indwelling ureteric stent: a 'friendly' procedure with unfriendly high morbidity. BJU Int 2000;85:408-11. [Crossref] [PubMed]
- Wagenlehner FM, Naber KG. Treatment of bacterial urinary tract infections: presence and future. Eur Urol 2006;49:235-44. [Crossref] [PubMed]
- Haley RW, Culver DH, White JW, et al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol 1985;121:182-205. [Crossref] [PubMed]
- Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients. Arch Intern Med 2000;160:678-82. [PubMed]
- Vallejo Herrador J, Burgos Revilla FJ, Alvarez Alba J, et al. Double J ureteral catheter. Clinical complications. Arch Esp Urol 1998;51:361-73. [PubMed]
- Damiano R, Oliva A, Esposito C, et al. Early and late complications of double pigtail ureteral stent. Urol Int 2002;69:136-40. [Crossref] [PubMed]
- Riedl CR, Plas E, Hübner WA, et al. Bacterial colonization of ureteral stents. Eur Urol 1999;36:53-9. [Crossref] [PubMed]
- Medina-Polo J, Sopeña-Sutil R, Benítez-Sala R, et al. Prospective study analyzing risk factors and characteristics of healthcare-associated infections in a Urology ward. Investig Clin Urol 2017;58:61-9. [Crossref] [PubMed]
- Lara-Isla A, Medina-Polo J, Alonso-Isa M, et al. Urinary Infections in Patients with Catheters in the Upper Urinary Tract: Microbiological Study. Urol Int 2017;98:442-8. [Crossref] [PubMed]
- Medina-Polo J, Arrébola-Pajares A, Pérez-Cadavid S, et al. Extended-Spectrum Beta-Lactamase-Producing Bacteria in a Urology Ward: Epidemiology, Risk Factors and Antimicrobial Susceptibility Patterns. Urol Int 2015;95:288-92. [Crossref] [PubMed]
- Farsi HM, Mosli HA, Al-Zemaity MF, et al. Bacteriuria and colonization of double-pigtail ureteral stents: long-term experience with 237 patients. J Endourol 1995;9:469-72. [Crossref] [PubMed]
- Kehinde EO, Rotimi VO, Al-Hunayan A, et al. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol 2004;18:891-6. [Crossref] [PubMed]
- Klis R, Korczak-Kozakiewicz E, Denys A, et al. Relationship between urinary tract infection and self-retaining Double-J catheter colonization. J Endourol 2009;23:1015-9. [Crossref] [PubMed]
- Lojanapiwat B. Colonization of internal ureteral stent and bacteriuria. World J Urol 2006;24:681-3. [Crossref] [PubMed]
- Ozgur BC, Ekıcı M, Yuceturk CN, et al. Bacterial colonization of double J stents and bacteriuria frequency. Kaohsiung J Med Sci 2013;29:658-61. [Crossref] [PubMed]
- Bey E, Bouiller K, Pimpie R, et al. Recommendations of the AFU Infectious Diseases Committee on the prevention, diagnosis and treatment of infections of endo-ureteral equipment. Prog Urol 2021;31:557-75. [Crossref] [PubMed]
- Vallée M, Bey E, Bouiller K, et al. Epidemiology and risk factors for ureteral stent-associated urinary tract infections in non-transplanted renal patients: a systematic review of the literature. World J Urol 2021;39:3845-60. [Crossref] [PubMed]
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247-54. [Crossref] [PubMed]
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. [Crossref] [PubMed]
- CLSI Standards and Guidelines. CLSI Standards Center, 2021.
- European Association of Urology. Guideline Urological Infections. Arnhem: EAU Guidelines Office; 2020 [last accessed 2021 Feb 17]. Available online: http://uroweb.org/guideline/urologicalinfections/
- Stamm WE, Counts GW, Running KR, et al. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med 1982;307:463-8. [Crossref] [PubMed]
- Eliakim-Raz N, Yahav D, Paul M, et al. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 2013;68:2183-91. [Crossref] [PubMed]
- Sobel JD. Duration of antibiotic treatment for urinary tract infection. Curr Infect Dis Rep 2008;10:483-4. [Crossref] [PubMed]
- Lifshitz DA, Winkler HZ, Gross M, et al. Predictive value of urinary cultures in assessment of microbial colonization of ureteral stents. J Endourol 1999;13:735-8. [Crossref] [PubMed]
- Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50:625-63. [Crossref] [PubMed]
- Reid G, Denstedt JD, Kang YS, et al. Microbial adhesion and biofilm formation on ureteral stents in vitro and in vivo. J Urol 1992;148:1592-4. [Crossref] [PubMed]
- Elwood CN, Lo J, Chou E, et al. Understanding urinary conditioning film components on ureteral stents: profiling protein components and evaluating their role in bacterial colonization. Biofouling 2013;29:1115-22. [Crossref] [PubMed]
- Tieszer C, Reid G, Denstedt J. Conditioning film deposition on ureteral stents after implantation. J Urol 1998;160:876-81. [Crossref] [PubMed]
- Stickler DJ. Bacterial biofilms in patients with indwelling urinary catheters. Nat Clin Pract Urol 2008;5:598-608. [Crossref] [PubMed]
- Paick SH, Park HK, Oh SJ, et al. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology 2003;62:214-7. [Crossref] [PubMed]
- Kehinde EO, Rotimi VO, Al-Awadi KA, et al. Factors predisposing to urinary tract infection after J ureteral stent insertion. J Urol 2002;167:1334-7. [Crossref] [PubMed]
- Yeniyol CO, Tuna A, Yener H, et al. Bacterial colonization of double J stents and bacteriuria frequency. Int Urol Nephrol 2002;34:199-202. [Crossref] [PubMed]
- Akay AF, Aflay U, Gedik A, et al. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double J ureteral stent. Int Urol Nephrol 2007;39:95-8. [Crossref] [PubMed]
- Altunal N, Willke A, Hamzaoğlu O. Ureteral stent infections: a prospective study. Braz J Infect Dis 2017;21:361-4. [Crossref] [PubMed]
- Kunin CM. In defense of the bladder. West J Med 1982;137:237-9. [PubMed]
- Ringel A, Richter S, Shalev M, et al. Late complications of ureteral stents. Eur Urol 2000;38:41-4. [Crossref] [PubMed]


