The office management of ejaculatory disorders
Introduction
Ejaculation, defined as the expulsion of seminal fluid from the urethra, comprises three stages: emission, ejection, and orgasm (1,2). Emission is the deposition of seminal fluid into the posterior urethra through the contractions of the vas deferens, seminal vesicles, and prostate; it is controlled by the sympathetic nerves T10–L2. Ejection involves bladder neck closure to prevent retrograde flow, and seminal fluid expulsion from the urethra, mediated by the parasympathetic nerves S2–S4. Orgasm is a centrally controlled sensory and emotional experience associated with the preceding events.
The ejaculatory reflex is predominantly controlled through the neurotransmission of serotonin [or 5-hydroxytryptamine (5-HT)] and dopamine, with secondary involvement of other neurotransmitters, such as namely norepinephrine, adrenaline, acetylcholine, oxytocin, gamma-aminobutyric acid and nitric oxide (NO) (1,3,4). Serotonin has an inhibitory role in ejaculation, and it stimulates 5-HT1B and 5-HT2C receptors to perpetrate this inhibition (5). By contrast, dopamine activates 5-HT1A receptor to show an opposite effect (6). Furthermore, testosterone, thyrotropin, and prolactin have independent roles in the control of ejaculatory function (7). Therefore, normal antegrade ejaculation involves coordinated neurological, psychological, tissue-related, and endocrinological events, and disruption at any point in this cascade of events may result in an ejaculatory disorder (EjD) (1).
EjDs, erectile dysfunction (ED), and hypogonadism are three major categories of male sexual dysfunctions (8). EjDs comprise a heterogeneous group of dysfunctions that involve altered time and control [premature ejaculation (PE) and delayed ejaculation (DE)], presence [anejaculation (AE)], direction [retrograde ejaculation (RE)], volume [perceived ejaculate volume reduction (PEVR)], or force [decreased force of ejaculation (DFE)] of ejaculation (1).
PE is the most common male sexual dysfunction, with a global prevalence of 20–30% (9-11). PE can be lifelong PE (present since the onset of sexual life) and acquired (developing after a period of normal ejaculatory function) (12). Although the definitions of PE vary, such definitions generally involve three core components (13,14): short ejaculatory latency, lack of control over ejaculation, and negative personal consequences.
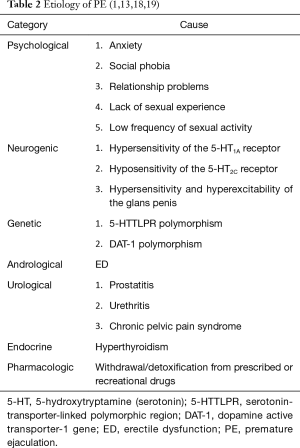
Short ejaculatory latency is typically measured using the intravaginal ejaculatory latency time (IELT), defined as the time from vaginal intromission to intravaginal ejaculation (15). Table 1 provides the latest, commonly used definitions of PE proposed by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) in 2013 (16) and the International Society for Sexual Medicine (ISSM) in 2014 (17). According to the ISSM evidence-based definition of PE, lifelong PE is characterized by an IELT of ≤1 minute since the first intercourse, whereas an IELT of 3 minutes is set as a valid IELT cut-off for the diagnosis of acquired PE (17). The etiology of PE involves multiple biological and psychological factors (Table 2) (1,13,18,19). Lifelong PE may be associated with biological factors (1) such as serotonin dysregulation (20), genetic predisposition (21,22), and glans penis hypersensitivity and hyperexcitability (23). Acquired PE commonly results from ED (24) or psychological problems (25,26) and occasionally urological dysfunctions (27), hyperthyroidism (28), or withdrawal or detoxification from prescribed or recreational drugs (29,30).
EjDs other than PE are less common in the general male population compared with PE. In an Argentinian survey of 2,456 men reporting their ejaculatory function, the absence of ejaculation, DE, and PE was noted in 6.8%, 14.1%, and 28.3% of the participants, respectively (31). In a community-based cross-sectional study of 1,181 Danish men, the prevalence rates of DE and PE were 4.6% and 24.0%, respectively (32). Recently, in an observational analysis of 988 community-dwelling men with at least one EjD other than PE (DE, AE, PEVR, or DFE), 88% of the participants experienced more than one EjDs, and 79% of the participants were diagnosed with ED (33); furthermore, PEVR (88%) was the most prevalent EjD, followed by DFE (81%), DE (62%), and AE (37%).
Despite being extremely common among men, EjDs remain poorly understood, underdiagnosed, and undertreated. Therefore, this article presents the current state-of-the-art knowledge for EjDs management and perspectives on EjD diagnosis and treatment optimization strategies.
Role of primary care physicians in sexual dysfunction treatment
In European surveys of e-mail and telephone helplines for sexual problems, ED (42.9–72.0%) and PE (15.8–36.0%) were the major male sexual concerns, whereas EjDs other than PE accounted for only 3.3–3.4% of the complaints (34-36). Similar to men with ED, men with PE often suffer from anxiety, depression, distress, low self-esteem, low sexual confidence, sexual dissatisfaction, and interpersonal difficulty (37,38). An observational analysis of 988 patients with EjDs other than PE indicated that the majority of patients (68%) considered their symptoms to be bothersome (33). DE is associated with anxiety, depression, and relationship dissatisfaction (39,40). Furthermore, sexual dysfunctions may contribute to infertility or subfertility.
Despite the significant negative effects of sexual dysfunctions on numerous aspects of a man’s life, extremely few men seek treatment, probably because they are embarrassed to describe their condition to a physician. Large multinational surveys have revealed that only 18% of men with sexual problems and 9% of men with PE had attempted to seek medical help from a physician (10,41). The type and duration of sexual dysfunction, age, coexisting health problems, and marital status are factors significantly associated with a man’s willingness to seek medical help (34). Regardless of the high prevalence of PE in community samples, 73% and 44% of urologists from the United States and South Korean treat less than one PE patient and less than two PE patients per week, respectively (42,43).
Most medical problems observed in the primary care practice, such as diabetes mellitus (DM) and cardiovascular and urological diseases, are associated with male sexual dysfunctions, and effective oral treatments are available for ED and PE. Therefore, men generally prefer primary care physicians for seeking treatment for their sexual problems (44-46). However, most primary care physicians do not incorporate questions regarding sexual health into their routine practice because they fail to recognize the high prevalence of sexual dysfunctions among the general male population and the negative effects of such dysfunctions on men’s quality of life. Furthermore, primary care physicians as well as their patients may feel embarrassed to discuss sexual-health-related topics. In the Global Study of Sexual Attitudes and Behaviors survey, only 9% of the participants had discussed their sexual health with a physician during routine visits in the past three years (41). Among 50 primary care physicians in Lisbon, only 15.5% actively inquired patients regarding their sexual health, and this is mainly because the patients had DM, were prescribed medication with adverse effects on sexual function, and were undergoing family planning (47). Therefore, because of the difficulty associated with initiating discussions regarding sexual concerns between affected men and primary care physicians, sexual dysfunctions remain underdiagnosed and undertreated. In addition, although most men experiencing sexual dysfunctions are eager to discuss their sexual problems with their primary care physicians, they generally wait to be asked about them (44,46). Unless physicians proactively start these discussions, diagnosis and treatment are delayed. Hence, primary care physicians should consider this issue and prepare to treat such patients to optimize their sexual health and quality of life.
“ALLOW”, a 5-step proactive management plan, was developed by Sadovsky to facilitate primary care physicians in discussing sexual dysfunctions with their patients (48):
- Step 1: “ask” the patients about their sexual activity
- Step 2: “legitimize” the patients’ sexual problems and recognize them as critical concerns affecting the patients’ quality of life
- Step 3: “limitations” lead the physician to evaluate his or her own ability to work with the patients. The patients can be referred to an appropriate subspecialist for further investigation and treatment when necessary
- Step 4: “open” up the sexual concerns for further discussion
- Step 5: “work” with the patient to develop a suitable treatment plan and set an appropriate goal.
Clinical diagnosis of ejaculatory disorders (EjDs)
Because physical and psychological factors may be involved in the onset of EjDs, accurate and multidimensional diagnosis is essential for effective EjD treatment. Detailed medical and sexual history-taking, along with appropriate physical examinations, laboratory tests, and questionnaires is necessary for identifying EjDs and their potential causes. In addition, the possibility of other coexisting sexual problems, particularly ED, should be considered.
History-taking
Obtaining detailed medical history, including acute or chronic diseases and injuries, urogenital infections or abnormalities, surgeries, medications, and drug or alcohol abuse, is the critical first step in determining the etiology of a patient's sexual dysfunction (1,49,50). A sexual history should involve crucial information such as the date and mode of EjD onset, self-estimated IELT, perceived control over ejaculation, personal distress and interpersonal difficulty associated with ejaculatory problems, intercourse frequency, and presence of other sexual dysfunctions (1,14).
Physical examination
Performing the examination of the genitourinary, endocrine and neurological systems may facilitate determining the anatomical abnormalities associated with EjDs (1,14).
Laboratory tests
Laboratory tests can provide additional information when an organic problem is suspected during history-taking or physical examination.
- Post ejaculatory urinalysis
This analysis can be used to identify patients with RE (49). - Blood examination:
The blood levels of glucose, hormones involved in the control of ejaculatory function (testosterone, thyrotropin, and prolactin), and prostate-specific antigen in blood can be screened for the presence of DM, hormonal disorders and prostatic cancer, respectively (7,51). - Microbiological examination:
Culturing of prostatic secretion, urine, and semen can facilitate the diagnosis of prostatic infection (49,52).
Patient-reported outcomes
Defining PE on the basis of only the IELT does not accurately capture all aspects of PE; therefore, several multidimensional self-reported questionnaires have been designed to assess PE objectively. The following are the tools used commonly for evaluating PE (Table 3):
- Premature ejaculation diagnostic tool (PEDT):
The PEDT is a five-item questionnaire encompassing the essence of the DSM-IV-TR definition of PE control, frequency, minimal sexual stimulation, distress, and interpersonal difficulty (53). Scores of ≥11, 9–10, and ≤8 indicate PE, probable PE, and no PE, respectively. - Premature ejaculation profile (PEP):
The PEP comprises four items assessing perceived control over ejaculation, personal distress associated with ejaculation, satisfaction with sexual intercourse, and interpersonal difficulty associated with ejaculation (54). - Index of premature ejaculation (IPE):
The 10-item IPE provides reliable and valid assessments of control over ejaculation, satisfaction with sex life, and distress in men with PE (55). - Male Sexual Health Questionnaire-Ejaculatory Dysfunction-Short Form (MSHQ-EjD-SF):
The MSHQ-EjD-SF contains three ejaculatory function items (frequency, strength and volume of ejaculation) and one ejaculation bother item for assessing EjDs (56).
Why do patients with premature ejaculation (PE) require active treatment with oral agents?
Various treatment options are currently available for patients with PE, including psychological behavioral therapies, on-demand oral medications, regular oral medications, on-demand topical anesthetic agents, and surgery (13,14,43). The evolution of PE therapy has been influenced by the advances in the new understanding of physiology and etiology (4). Historically, PE was considered a psychosomatic problem; therefore, psychological behavioral therapies were the mainstay for PE management before the emergence of pharmacological therapies (57,58). Psychological behavioral therapies, such as the squeeze technique and the start-and-stop technique, help men in developing sexual skills to control or delay ejaculation, improve self-confidence, reduce performance anxiety, resolve psychological and interpersonal concerns, and promote communication between them and their partners (59,60). However, these approaches are time consuming and costly, and require partners’ cooperation and well-trained sex therapists (61-64). Furthermore, robust evidence regarding the efficacy of these psychological-behavioral therapies is lacking (60,65).
Patients may suffer from PE for a long time before seeking treatment; hence, they require a more aggressive treatment to rapidly restore their sexual satisfaction. The ideal treatment for PE should be on-demand oral administration of medication for a rapid well-tolerable action, effective from the first dose (61).
Dapoxetine, a short-acting, on-demand selective serotonin reuptake inhibitor (SSRI), is the first and only drug licensed for the treatment of PE in men aged 18–64 years. Its mechanism of action may increase serotonin action at pre- and post-synaptic receptors by blocking serotonin reuptake, resulting in DE (66). After oral administration, dapoxetine (30 or 60 mg) is rapidly absorbed, with peak plasma concentrations occurring approximately 1 hour after administration, and its clearance rate is 95% by 24 hours (67). These unique pharmacokinetic characteristics render dapoxetine suitable for on-demand use; thus, compared with drugs requiring daily dosing regimens, dapoxetine is more convenient, flexible, and inexpensive, in addition to causing fewer treatment-emergent adverse events (TEAEs). An integrated analysis of data from five phase III trials of dapoxetine including 6,081 patients with PE worldwide demonstrated that dapoxetine (30 or 60 mg) taken 1–3 hours before intercourse was effective from the first dose (68). At 12 weeks of treatment, the average IELT increased from a baseline of 0.9 minutes to 3.1 minutes with dapoxetine 30 mg and to 3.6 minutes with dapoxetine 60 mg, both compared with an increase to 1.9 minutes with placebo (all P<0.001). In addition to the IELT, both doses of dapoxetine significantly improved control over ejaculation, satisfaction with sexual intercourse, and other patient-reported outcomes. The efficacy of dapoxetine was similar between patients with lifelong PE and those with acquired PE (69). Dapoxetine was generally well-tolerated, and the most commonly reported TEAEs were nausea, dizziness and headache (68,70). Furthermore, dapoxetine had no effect on patient mood, and it was not associated with anxiety, akathisia, suicidality, or discontinuation syndrome (68).
According to a study of practice patterns among 527 South Korean urologists in the management of PE, dapoxetine was the most commonly used medication for PE: it was prescribed by 87.3% of the participants (43); nevertheless, behavioral therapy and other therapies were used to manage PE patients by 47.6% and less than 54.3% of the urologists, respectively. Therefore, dapoxetine can likely fulfill the treatment requirements of most patients with PE.
Optimizing dapoxetine treatment for premature ejaculation (PE)
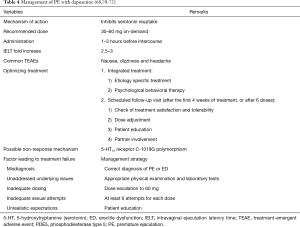
Because of the complexity and variability of PE, optimizing the use of dapoxetine in the management of PE represents a great challenge for physicians (Table 4). Because PE has a multifactorial etiology (1) and a substantial proportion of PE patients also have comorbidity (69,73), the management of patients with PE may be complex and may require a multidisciplinary approach. Optimal treatment outcomes can be achieved through a holistic approach by using appropriate etiology-specific treatments combined with dapoxetine treatments (2). A phosphodiesterase type 5 (PDE5) inhibitor can be used with dapoxetine in the treatment of ED and coexisting PE (73,74). When psychogenic or relationship factors are present, psychological behavioral therapy can be combined with dapoxetine treatment in an integrated treatment program to manage both the emotional and physical aspects of PE (60,62). A systematic review demonstrated that pharmacotherapy combined with psychological behavioral therapy is significantly superior to monotherapy alone in improving the IELT, ejaculation control, sexual satisfaction, and anxiety (60).
In clinical practice, scheduled follow-up visits are an essential aspect of overall management of patients with PE, particularly when comorbidities are present (71). Follow-up visits offer communication opportunities for patients with PE and their physicians to discuss treatment results, identify patient expectations, and provide patient education regarding efficacy optimization. Arranging a follow-up visit is recommended for patients treated with dapoxetine after the first 4 weeks of treatment or after six doses to measure their treatment satisfaction and tolerability and determine the next action (71). Treatment outcomes can be assessed through objective measurements of the IELT and by using simple questions or questionnaires on ejaculation control and sexual satisfaction such as the Clinical Global Impression of Change and PEP (68). Up titrating the dose of dapoxetine from 30 to 60 mg can facilitate achieving optimal therapeutic benefit for patients exhibiting limited response to dapoxetine 30 mg (71). Furthermore, partner involvement in follow-up sessions is suggested so that physicians can fully understand the effect of dapoxetine treatment on PE.
Management of initial non-response to dapoxetine treatment in premature ejaculation (PE) patients
The method for managing PE in patients showing an unsatisfactory response to dapoxetine treatment is a common major concern in clinical practice. Before patient is declared as a true non-responder to dapoxetine, several factors potentially causing treatment failure should be assessed, such as misdiagnosis, unaddressed underlying concerns, inappropriate dosing, inadequate sexual attempts, and unrealistic expectations, should be assessed (Table 4). Patients may confuse the syndromes of PE with those of ED; therefore, ED may be misdiagnosed as PE if diagnostic evaluation is incomplete. The underlying diseases and comorbidity associated with PE treatment failure should be diagnosed and treated simultaneously or before dapoxetine treatment to maximize treatment outcomes.
The number of sexual attempts and dapoxetine dosages are critical factors associated with the extent of ejaculation delaying (68,71,73). The aforementioned integrated analysis of data from five phase III trials of dapoxetine revealed that the effects of dapoxetine could be observed after the first dose, with the most favorable outcome being obtained after 12 weeks of treatment (68). Furthermore, immature sexual technique and low frequency of sexual activity are associated with PE (1). Increasing the frequency of sexual activity may enhance sexual technique, self-confidence, and satisfaction thereby improving dapoxetine treatment outcomes. Before dose escalation, patients should be encouraged to perform at least six sexual attempts with dapoxetine 30 mg (58,71).
The recommended starting dose of dapoxetine for all patients with PE is 30 mg, and the maximum dose of 60 mg is recommended only for those with unsatisfactory response to 30 mg dapoxetine and without intolerable adverse events (71). In the PAUSE study, 17.7% of patients with PE in the dapoxetine treatment group required a higher dose of 60 mg at visits 2–4 (70). Jiann and Huang demonstrated that 42.9% of patients with unsatisfactory response to dapoxetine (30 mg) could benefit from a 60-mg dose (73). However, no clinical study has demonstrated whether dapoxetine (90 or 120 mg) can salvage dapoxetine (60 mg) non-responders.
According to the integrated analysis of data from the five phase III trials (68), the average IELT significantly increased with dapoxetine (30 or 60 mg) to 3.1–3.9 minutes, which was in the adequate IELT range (3–7 minutes) (75). However, a study evaluating patient satisfaction with dapoxetine treatment indicated that the satisfaction rate (45.0%) was much lower than the response rate (74.6%) (73), probably because an unmet need or unrealistic expectations patients with PE, such as a longer than normal intercourse (76). Unsatisfactory results are the main reason for dapoxetine treatment discontinuation in clinical practice (73,76). Moreover, some patients might feel disappointed because PE is not curable and dapoxetine should be taken before each intercourse (77). This highlights the importance of asking patients about their expectations and providing adequate patient education before starting dapoxetine treatment.
On-demand dapoxetine treatment is the only approved pharmacological therapy for PE. In addition to the licensed dapoxetine, several off-label PE therapies are recommended in the ISSM and European Association of Urology (EAU) guidelines on PE treatment, and some of such therapies are outlined as follows: the SSRIs paroxetine sertraline, citalopram and fluoxetine; the tricyclic antidepressant, clomipramine, for treating lifelong and acquired PE; and the topical anesthetic cream, lidocaine or prilocaine, for treating lifelong PE (Table 5) (13,14). These off-label medications can be used in the management of PE after dapoxetine treatment failure. However, SSRIs and clomipramine are not amenable to on-demand dosing (14). Regarding the secondary management choices for PE in the practice patterns of urologists from the United States, 42% of participants preferred changing the SSRI regimen, and a substantial portion of the participants (14%) resorted to topical anesthetics (42). Patients with PE nonresponsive to any SSRI treatment are rare, and 5-HT1A receptor C-1019G polymorphism may have a substantial role in the therapeutic response (72). Daily treatment with long-acting SSRIs increases medication accumulation and consequently increases sexual side effects, such as ED and hypoactive sexual desire (78), as well as the risk of suicidal ideation (79) and SSRI discontinuation syndrome (80). The most common adverse reactions associated with local anesthetics include ED, penile numbness, and vulvovaginal burning sensation in the female partner (81).
Erectile dysfunction (ED) versus premature ejaculation (PE)
ED is a highly common male sexual disorder in daily clinical practice; the affected worldwide population is predicted to increase from 152 million in 1995 to 322 million in 2025 (82). ED is defined as the persistent or recurrent inability for a man to have satisfactory penile rigidity for sexual intercourse (83). Penile erection is mainly modulated by the NO/cyclic guanosine monophosphate system (84), which is different from the mechanisms involved in ejaculation control. However, large-scale studies have indicated that a considerable proportion of men with PE (15–45%) also report ED (10,85,86). According to our 2013 population-based Internet survey on PE, PE coexisted in 30.7% of Taiwanese men complaining of ED (unpublished data), possibly because of a vicious circle between PE and ED: a man with PE attempts to control ejaculation by reducing the level of excitation, which can lead to ED; by contrast, a man with ED attempts to achieve an erection by increasing the level of excitation, which can lead to PE (24). The syndromes of PE and ED are often confused: The main difference between PE and ED is that a man with PE can maintain an erection sufficient for the completion of intercourse, whereas a man with ED cannot maintain an erection before ejaculation (87).
Because PE frequently coexists with ED and these sexual dysfunctions must be treated with different medications, we recommend screening patients with PE for ED, and vice versa before the initiation of treatment. Otherwise, a missing diagnosis of ED may result in PE treatment failure because the penis cannot maintain an erection until ejaculation. The Sexual Health Inventory for Men (SHIM), also known as the five-item International Index for Erectile Function, is generally considered as the gold standard for ED diagnosis (indicated by an SHIM score of ≤21) (88); however, its reliability in patients with PE is limited (73,89,90) as it has a high false-positive rate (33.3%) (89). Jiann and Huang reported that the main problem is caused by question 5 in the SHIM: “When you attempted sexual intercourse, how often was it satisfactory for you?” (73). Similar to patients with ED, those with PE are often dissatisfied with their sexual performance (37). Therefore, some PE patients with normal erectile function respond to the question as “almost never or never” or “a few times”, and are incorrectly diagnosed with ED. To accurately screen for ED in patients with PE, we recommend using a global assessment question “Did your erection last long enough for you to have successful intercourse?”.
Because coexisting ED may be associated with more severe PE symptoms, the treatment for patients with both PE and ED is challenging for physicians (69). Although the current ISSM and EAU guidelines on PE treatment recommend treating ED first (13,14), the use of PDE5 inhibitors indicated for the treatment of ED in PE treatment remains controversial (91). No clinically significant pharmacokinetic interactions between dapoxetine and PDE5 inhibitors have been reported (92). The efficacy, including a significant increase in the IELT and improvement in patient-reported outcomes, and safety of combining dapoxetine with a PDE5 inhibitor for patients with coexisting PE and ED have been demonstrated in the phase III study of McMahon et al. (74) and the real-world practice study of Jiann and Huang (73). Nevertheless, dapoxetine should be prescribed with caution in patients using a PDE5 inhibitor, particularly those at a risk of orthostatic reaction, because this combination treatment may increase the risk of prodromal symptoms that may progress to syncope, compared with monotherapy of dapoxetine or a PDE5 inhibitor (14,74).
Role of female partners in the evaluation and management of premature ejaculation (PE)
Factors that may influence the IELT include female sexual function physiology and response time-course (57). Therefore, concerns regarding sexual partners may also contribute PE development. The burden of PE frequently extends to sexual partners. Several large-scale surveys have revealed significantly lower sexual satisfaction and higher personal distress and interpersonal difficulty among the female partners of men with PE than among those of men without PE (93-95). A recent Internet survey focusing on female partners’ perceptions of PE indicated that 78.6% of women with self-reported sexual problems, such as low libido and sexual dissatisfaction, experienced these problems while being in a relationship with men with PE (95); hence, PE should be considered a couple’s problem.
Integrating partners into diagnosis, treatment discussions, and response evaluation of PE is extremely crucial. Female partners may facilitate clinicians in gaining insight into the sexual-, psychological-, and relationship-related problems associated with PE. Moreover, female partners’ sexual dysfunctions should be diagnosed and treated to prevent considerably reductions in the PE treatment efficacy (42). Treatment preference is ideally based on the specific needs of patients with PE and their partners. Because PE affects both men and their partners and because each individual has different criteria for defining successful outcomes, the treatment satisfaction of both patients and their partners should be evaluated. The integrated analysis of data from the five phase III trials demonstrated significant improvements in satisfaction with sexual intercourse, ejaculation-related personal distress and interpersonal difficulty in the female partners of men treated with dapoxetine versus those treated with placebo (68). Although the benefits of PSD502 (a topical anesthetic) for female partners have been demonstrated using the PEP questionnaire (96), topical anesthetics may cause vulvovaginal burning sensation, discomfort or numbness in partners (81,96,97). These potential drawbacks may limit the acceptability of topical anesthetics for the treatment of PE in patients with PE and their partners (98). Psychological behavioral therapies for improving sexual skills and communication between couples require active participation of both partners (60,99). A cooperative partner also can enhance man’s self-confidence and provide positive feedback on intercourse performance, thereby increasing long-term treatment success.
However, most patients with PE consult their physician in the absence of their partners. According to a PE management survey of 207 urologists from the United States, 51% of the urologists reported that they inquire patients about their partners, but only 8%, 7%, and 4% of them evaluate, refer, or treat partners, respectively (42). Therefore, physicians should pay more attention to the importance of partner involvement in the PE treatment process and encourage patients to bring their partners to their visits to ensure the most favorable treatment outcomes.
General considerations for ejaculatory disorders (EjDs) other than premature ejaculation (PE)
Delayed ejaculation (DE)
DE is also called retarded ejaculation or inhibited ejaculation. According to the DSM-5, the major feature of DE is “a marked delay in or inability to achieve ejaculation, despite the presence of adequate sexual stimulation and the desire to ejaculate” (16). Approximately 25% of patients have lifelong DE, whereas the majority (75%) acquired it after a period of normal ejaculatory function (100). Most DE cases are caused by spinal cord injury (SCI; 68.9%); other common causes include retroperitoneal lymph node dissection (RPLND; 20.7%), idiopathy (7.1%), and DM (2.1%) (50). Furthermore, neurological diseases and serotonergic drugs are associated with severe DE (51).
Thus far, no evidence-based or specific criteria for accurately diagnosing a man with DE have been reported. According to a median IELT of 5.4 min (15), DE can be diagnosed in a man with an IELT more than 25–30 minutes (approximately two standard deviations above the median) and distress associated with this condition (101).
No specific medication has been approved for the treatment of lifelong or acquired DE. However, if DE is suspected to be a side effect of a medication, reducing the dosage or resorting to another medication with fewer side effects can facilitate to reversing DE (102). Amantadine, buproprion, buspirone, cyproheptadine, and yohimbine are recommended by the British Association of Sexual Health and HIV (BASHH) special interest group in sexual dysfunction for managing SSRI-induced DE (50). These drugs facilitate ejaculation through either a central dopaminergic or anti-serotonergic mechanism of action, or a peripheral adrenergic mechanism of action (1,50). When organic or pharmacological causes are excluded, patients with DE can benefit from psychological therapy. Numerous psychotherapeutic strategies, such as cognitive behavioral therapy, couples sex therapy, masturbatory retraining, and sexual tipping point, have been suggested for managing DE to reduce anxiety, enhance penile stimulation, or maximize arousal (2,102).
Anejaculation (AE)
AE involves the complete absence of antegrade or RE because of the failure of emission (49). SCI is responsible for most of AE cases (85.8%), followed by RPLND (6.0%) and idiopathy (5.8%) (103). The evaluation of AE includes detailed history-taking of medical, sexual, and recent medication use as well as physical examination. The absence of sperm in post-ejaculatory urinalysis, which can differentiate between AE and RE, supports AE diagnosis (1).
In the 2015 EAU Guidelines on Male Infertility, drug treatment for AE caused by lymphadenectomy and neuropathy is not recommended because of its low efficacy (49). Vibrostimulation is the first-line therapy for AE caused by SCI, lymphadenectomy and neuropathy (49). An intact lumbosacral spinal cord segment is required for vibrostimulation procedure (49). Vibrostimulation, which involves application of a vibrator to the penile surface or to the lower surface of the glans penis, recruits the ejaculatory reflex to induce ejaculation (103). Kamischke and Nieshlag compared the treatment efficacies of vibrostimulation in various patient subpopulations (103). A significantly higher proportion of patients with idiopathic AE had antegrade ejaculation after vibrostimulation compared with patients with SCI-induced AE. Among patients with SCI-induced AE, patients with lesions on T11 and above (71.6%) had a significantly higher response rate to vibrostimulation than did those with lesions below T11 (35.5%). The side effects of vibrostimulation include paroxysmal hypertension, headache, and signs of autonomic dysreflexia (103). Pretreatment with nifeipine or prazosin in AE patients with a SCI above T6 can facilitate preventing autonomic dysreflexia (103). Therefore, vibrostimulation is a simple, efficient, and safe treatment for AE and can be performed by a patient himself at home (104,105).
Electroejaculation can be used in cases of vibrostimulation failure (49). Electroejaculation is an electric stimulation of the periprostatic nerves responsible for ejaculation through a probe inserted into the rectum (103,104). General anesthesia is required except in cases of complete SCI. In a systematic review of electroejaculation therapy for AE (103), ejaculation could be induced by electroejaculation in approximately 80% of AE patients. No significant differences were observed between the underlying causes of AE and the response to electroejaculation. The side effects of electroejaculation include rectal mucosa injury, transitory erythema, autonomic dysreflexia, paroxysmal hypertension, and vomiting (103). Pretreatment with (10–30 mg) nifeipine can reduce the incidence of autonomic dysreflexia (103).
Retrograde ejaculation (RE)
RE involves ejaculation in an abnormal direction into the bladder; it is engendered by a loss of normal bladder neck closure during ejaculation. The underlying causes of RE can be divided into five main categories: anatomic, neurogenic, toxic, pharmacological, and idiopathic (103). According to an analysis of the underlying causes of RE in 342 patients reported by Kamischke and Nieshlag, RPLND (29.2%) was the leading cause of RE, followed by idiopathic cause (19.9%), DM (13.5%), bladder neck surgery (10.2%), and transurethral resection of the prostate (TURP, 8.5%) (103).
RE can be diagnosed through accurate patient history-taking with emphasis on coexisting medical conditions, medication, and previous surgical procedures. Risks factors for RE (such as DM, SCI, and RPLND; prostate gland or bladder neck surgery; and alpha blockers or psychotropic medication use) must be identified. Patients with RE frequently notice cloudy post-orgasmic urine. The diagnosis of RE can be confirmed through the presence of spermatozoa and fructose in a post-ejaculatory urinalysis (49,101).
RE treatment depends on the underlying cause. In the absence of SCI, urethral anomalies, or drug consumption, oral medications are considered the first-line treatment for RE. Because bladder neck closure is under sympathetic control, medical treatment for RE is based on increasing sympathetic activity or on diminishing parasympathetic activity (103). The adrenergic agonists, ephedrine and midodrine, the antihistamine, brompheniramine, and the tricyclic antidepressants, desipramine and imipramine, are recommended in the 2015 EAU Guidelines on Male Infertility for the treatment of RE (49). A systematic review of 36 studies of medical treatments for RE reported that imipramine showed a significantly higher reversal rate than ephedrine did in patients with RE (106). However, no significant differences were observed between the underlying causes of RE and the response to medical treatment. The side effects of drugs for RE treatment include dizziness, sleep disturbances, weakness, restlessness, dry mouth, nausea, and sweating (103).
If medical management is unsuccessful, Young-Dees type of bladder neck reconstruction (107) or bladder neck collagen injection (108,109) can enable achieving antegrade ejaculation. Pharmacologically induced RE can be reversed through the intermittent use of a medication (which relaxes the bladder neck) or the use of a lower dose (110,111). Furthermore, ejaculation with a full bladder, which increases bladder neck closure, is a simple method for facilitating the restoration of normal ejaculation (112).
Conclusions
Because of their multifactorial etiology, accurate identification of EjDs and their underlying causes is critical for effective treatment. Management approaches should be individualized on the basis of patients’ symptoms, expectations, and underlying etiologies. Dapoxetine treatment can be combined with psychological behavioral therapies, if required, for the overall management of patients with PE. Scheduled follow-up visits are essential for improving patient satisfaction with dapoxetine treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Lue T. editor. Atlas of Male Sexual Dysfunction. Philadelphia: Current Medicine, Inc., 2004.
- Rowland D, McMahon CG, Abdo C, et al. Disorders of orgasm and ejaculation in men. J Sex Med 2010;7:1668-86. [Crossref] [PubMed]
- Donatucci CF. Etiology of ejaculation and pathophysiology of premature ejaculation. J Sex Med 2006;3 Suppl 4:303-8. [Crossref] [PubMed]
- Bettocchi C, Verze P, Palumbo F, et al. Ejaculatory disorders: pathophysiology and management. Nat Clin Pract Urol 2008;5:93-103. [Crossref] [PubMed]
- Giuliano F. 5-Hydroxytryptamine in premature ejaculation: opportunities for therapeutic intervention. Trends Neurosci 2007;30:79-84. [Crossref] [PubMed]
- Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiol Behav 2004;83:291-307. [Crossref] [PubMed]
- Corona G, Jannini EA, Lotti F, et al. Premature and delayed ejaculation: two ends of a single continuum influenced by hormonal milieu. Int J Androl 2011;34:41-8. [Crossref] [PubMed]
- Wolters JP, Hellstrom WJ. Current concepts in ejaculatory dysfunction. Rev Urol 2006;8 Suppl 4:S18-25. [PubMed]
- Laumann EO, Nicolosi A, Glasser DB, et al. Sexual problems among women and men aged 40-80 y: prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res 2005;17:39-57. [Crossref] [PubMed]
- Porst H, Montorsi F, Rosen RC, et al. The Premature Ejaculation Prevalence and Attitudes (PEPA) survey: prevalence, comorbidities, and professional help-seeking. Eur Urol 2007;51:816-23; discussion 824. [Crossref] [PubMed]
- McMahon CG, Lee G, Park JK, et al. Premature ejaculation and erectile dysfunction prevalence and attitudes in the Asia-Pacific region. J Sex Med 2012;9:454-65. [Crossref] [PubMed]
- Godpodinoff ML. Premature ejaculation: clinical subgroups and etiology. J Sex Marital Ther 1989;15:130-4. [Crossref] [PubMed]
- Althof SE, McMahon CG, Waldinger MD, et al. An update of the International Society of Sexual Medicine's guidelines for the diagnosis and treatment of premature ejaculation (PE). J Sex Med 2014;11:1392-422. [Crossref] [PubMed]
- Hatzimouratidis K, Eardley I, Giuliano F, et al. Guidelines on Male Sexual Dysfunction: Erectile dysfunction and premature ejaculation, the 2015 version. Available online: http://uroweb.org/wp-content/uploads/EAU-Guidelines-Male-Sexual-Dysfunction-2015-v2.pdf
- Waldinger MD, Quinn P, Dilleen M, et al. A multinational population survey of intravaginal ejaculation latency time. J Sex Med 2005;2:492-7. [Crossref] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, DSM-5. Washington, DC: American Psychiatric Association, 2013.
- Serefoglu EC, McMahon CG, Waldinger MD, et al. An evidence-based unified definition of lifelong and acquired premature ejaculation: report of the second international society for sexual medicine ad hoc committee for the definition of premature ejaculation. J Sex Med 2014;11:1423-41. [Crossref] [PubMed]
- Jannini EA, Lenzi A. Ejaculatory disorders: epidemiology and current approaches to definition, classification and subtyping. World J Urol 2005;23:68-75. [Crossref] [PubMed]
- Jannini E, McMahon CG, Waldinger MD. editors. Premature Ejaculation: From Etiology to Diagnosis and Treatment. Milan: Springer Science & Business Media, 2012.
- Waldinger MD. The neurobiological approach to premature ejaculation. J Urol 2002;168:2359-67. [Crossref] [PubMed]
- Jern P, Santtila P, Witting K, et al. Premature and delayed ejaculation: genetic and environmental effects in a population-based sample of Finnish twins. J Sex Med 2007;4:1739-49. [Crossref] [PubMed]
- Waldinger MD. Toward evidence-based genetic research on lifelong premature ejaculation: a critical evaluation of methodology. Korean J Urol 2011;52:1-8. [Crossref] [PubMed]
- Xin ZC, Choi YD, Rha KH, et al. Somatosensory evoked potentials in patients with primary premature ejaculation. J Urol 1997;158:451-5. [Crossref] [PubMed]
- Jannini EA, Lombardo F, Lenzi A. Correlation between ejaculatory and erectile dysfunction. Int J Androl 2005;28 Suppl 2:40-5. [Crossref] [PubMed]
- Hartmann U, Schedlowski M, Krüger TH. Cognitive and partner-related factors in rapid ejaculation: differences between dysfunctional and functional men. World J Urol 2005;23:93-101. [Crossref] [PubMed]
- McCabe MP, Connaughton C. Psychosocial factors associated with male sexual difficulties. J Sex Res 2014;51:31-42. [Crossref] [PubMed]
- Lee JH, Lee SW. Relationship between premature ejaculation and chronic prostatitis/chronic pelvic pain syndrome. J Sex Med 2015;12:697-704. [Crossref] [PubMed]
- Carani C, Isidori AM, Granata A, et al. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab 2005;90:6472-9. [Crossref] [PubMed]
- Adson DE, Kotlyar M. Premature ejaculation associated with citalopram withdrawal. Ann Pharmacother 2003;37:1804-6. [Crossref] [PubMed]
- Peugh J, Belenko S. Alcohol, drugs and sexual function: a review. J Psychoactive Drugs 2001;33:223-32. [Crossref] [PubMed]
- Nolazco C, Bellora O, López M, et al. Prevalence of sexual dysfunctions in Argentina. Int J Impot Res 2004;16:69-72. [Crossref] [PubMed]
- Andersen I, Heitmann BL, Wagner G. Obesity and sexual dysfunction in younger Danish men. J Sex Med 2008;5:2053-60. [Crossref] [PubMed]
- Paduch DA, Polzer P, Morgentaler A, et al. Clinical and demographic correlates of ejaculatory dysfunctions other than premature ejaculation: A prospective, observational study. J Sex Med 2015;12:2276-86. [Crossref] [PubMed]
- Papaharitou S, Athanasiadis L, Nakopoulou E, et al. Erectile dysfunction and premature ejaculation are the most frequently self-reported sexual concerns: profiles of 9,536 men calling a helpline. Eur Urol 2006;49:557-63. [Crossref] [PubMed]
- Simonelli C, Tripodi F, Cosmi V, et al. What do men and women ask a helpline on sexual concerns? Results of an Italian telephone counselling service. Int J Clin Pract 2010;64:360-70. [Crossref] [PubMed]
- Tomlinson JM, Fernandes LC, Wylie KR. An e-mail and telephone helpline for sexual problems - results of a 2-year survey of men's sexual concerns. Int J Clin Pract 2011;65:1085-91. [Crossref] [PubMed]
- Rosen RC, Althof S. Impact of premature ejaculation: the psychological, quality of life, and sexual relationship consequences. J Sex Med 2008;5:1296-307. [Crossref] [PubMed]
- Rowland DL. Psychological impact of premature ejaculation and barriers to its recognition and treatment. Curr Med Res Opin 2011;27:1509-18. [Crossref] [PubMed]
- Rowland D, van Diest S, Incrocci L, et al. Psychosexual factors that differentiate men with inhibited ejaculation from men with no dysfunction or another sexual dysfunction. J Sex Med 2005;2:383-9. [Crossref] [PubMed]
- Xia JD, Han YF, Pan F, et al. Clinical characteristics and penile afferent neuronal function in patients with primary delayed ejaculation. Andrology 2013;1:787-92. [Crossref] [PubMed]
- Moreira ED Jr, Brock G, Glasser DB, et al. Help-seeking behaviour for sexual problems: the global study of sexual attitudes and behaviors. Int J Clin Pract 2005;59:6-16. [Crossref] [PubMed]
- Shindel A, Nelson C, Brandes S. Urologist practice patterns in the management of premature ejaculation: a nationwide survey. J Sex Med 2008;5:199-205. [Crossref] [PubMed]
- Yang DY, Ko K, Lee WK, et al. Urologist's Practice Patterns Including Surgical Treatment in the Management of Premature Ejaculation: A Korean Nationwide Survey. World J Mens Health 2013;31:226-31. [Crossref] [PubMed]
- Gott M, Hinchliff S. Barriers to seeking treatment for sexual problems in primary care: a qualitative study with older people. Fam Pract 2003;20:690-5. [Crossref] [PubMed]
- Gott M, Galena E, Hinchliff S, et al. "Opening a can of worms": GP and practice nurse barriers to talking about sexual health in primary care. Fam Pract 2004;21:528-36. [Crossref] [PubMed]
- Clark RD, Williams AA. Patient preferences in discussing sexual dysfunctions in primary care. Fam Med 2014;46:124-8. [PubMed]
- Ribeiro S, Alarcão V, Simões R, et al. General practitioners' procedures for sexual history taking and treating sexual dysfunction in primary care. J Sex Med 2014;11:386-93. [Crossref] [PubMed]
- Sadovsky R. The role of the primary care clinician in the management of erectile dysfunction. Rev Urol 2002;4 Suppl 3:S54-63. [PubMed]
- Jungwirth A, Diemer T, Dohle GR, et al. European Association of Urology Guidelines on Male Infertility: the 2015 version. Available online: http://uroweb.org/wp-content/uploads/17-Male-Infertility_LR1.pdf
- Richardson D, Goldmeier D. BASHH Special Interest Group for Sexual Dysfunction. Recommendations for the management of retarded ejaculation: BASHH Special Interest Group for Sexual Dysfunction. Int J STD AIDS 2006;17:7-13. [Crossref] [PubMed]
- Corona G, Mannucci E, Petrone L, et al. Psychobiological correlates of delayed ejaculation in male patients with sexual dysfunctions. J Androl 2006;27:453-8. [Crossref] [PubMed]
- Zegarra Montes LZ, Sanchez Mejia AA, Loza Munarriz CA, et al. Semen and urine culture in the diagnosis of chronic bacterial prostatitis. Int Braz J Urol 2008;34:30-7, discussion 38-40. [Crossref] [PubMed]
- Symonds T, Perelman MA, Althof S, et al. Development and validation of a premature ejaculation diagnostic tool. Eur Urol 2007;52:565-73. [Crossref] [PubMed]
- Patrick DL, Giuliano F, Ho KF, et al. The Premature Ejaculation Profile: validation of self-reported outcome measures for research and practice. BJU Int 2009;103:358-64. [Crossref] [PubMed]
- Althof S, Rosen R, Symonds T, et al. Development and validation of a new questionnaire to assess sexual satisfaction, control, and distress associated with premature ejaculation. J Sex Med 2006;3:465-75. [Crossref] [PubMed]
- Rosen RC, Catania JA, Althof SE, et al. Development and validation of four-item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology 2007;69:805-9. [Crossref] [PubMed]
- Ciocca G, Limoncin E, Mollaioli D, et al. Integrating psychotherapy and pharmacotherapy in the treatment of premature ejaculation. Arab J Urol 2013;11:305-12. [Crossref] [PubMed]
- Jannini EA, Ciocca G, Limoncin E, et al. Premature ejaculation: old story, new insights. Fertil Steril 2015;104:1061-73. [Crossref] [PubMed]
- Althof SE. Psychological treatment strategies for rapid ejaculation: rationale, practical aspects, and outcome. World J Urol 2005;23:89-92. [Crossref] [PubMed]
- Cooper K, Martyn-St James M, Kaltenthaler E, et al. Behavioral therapies for management of premature ejaculation: A systematic review. Sex Med 2015;3:174-88. [Crossref] [PubMed]
- Sharlip I. Diagnosis and treatment of premature ejaculation: the physician's perspective. J Sex Med 2005;2 Suppl 2:103-9. [Crossref] [PubMed]
- McMahon CG, Jannini E, Waldinger M, et al. Standard operating procedures in the disorders of orgasm and ejaculation. J Sex Med 2013;10:204-29. [Crossref] [PubMed]
- Serefoglu EC, Saitz TR, Trost L, et al. Premature ejaculation: do we have effective therapy? Transl Androl Urol 2013;2:45-53. [PubMed]
- Chung E, Gilbert B, Perera M, et al. Premature ejaculation: A clinical review for the general physician. Aust Fam Physician. 2015;44:737-43. [PubMed]
- Melnik T, Althof S, Atallah AN, et al. Psychosocial interventions for premature ejaculation. Cochrane Database Syst Rev 2011;8:CD008195. [PubMed]
- Giuliano F, Clément P. Serotonin and premature ejaculation: from physiology to patient management. Eur Urol 2006;50:454-66. [Crossref] [PubMed]
- Modi NB, Dresser MJ, Simon M, et al. Single- and multiple-dose pharmacokinetics of dapoxetine hydrochloride, a novel agent for the treatment of premature ejaculation. J Clin Pharmacol 2006;46:301-9. [Crossref] [PubMed]
- McMahon CG, Althof SE, Kaufman JM, et al. Efficacy and safety of dapoxetine for the treatment of premature ejaculation: integrated analysis of results from five phase 3 trials. J Sex Med 2011;8:524-39. [Crossref] [PubMed]
- Porst H, McMahon CG, Althof SE, et al. Baseline characteristics and treatment outcomes for men with acquired or lifelong premature ejaculation with mild or no erectile dysfunction: integrated analyses of two phase 3 dapoxetine trials. J Sex Med 2010;7:2231-42. [Crossref] [PubMed]
- Mirone V, Arcaniolo D, Rivas D, et al. Results from a prospective observational study of men with premature ejaculation treated with dapoxetine or alternative care: the PAUSE study. Eur Urol 2014;65:733-9. [Crossref] [PubMed]
- Moncada I. The importance of follow-up in patients with premature ejaculation. J Sex Med 2011;8 Suppl 4:353-9. [Crossref] [PubMed]
- Janssen PK, Touw D, Schweitzer DH, et al. Nonresponders to daily paroxetine and another SSRI in men with lifelong premature ejaculation: a pharmacokinetic dose-escalation study for a rare phenomenon. Korean J Urol 2014;55:599-607. [Crossref] [PubMed]
- Jiann BP, Huang YJ. Assessing satisfaction in men with premature ejaculation after dapoxetine treatment in real-world practice. Int J Clin Pract 2015;69:1326-33. [Crossref] [PubMed]
- McMahon CG, Giuliano F, Dean J, et al. Efficacy and safety of dapoxetine in men with premature ejaculation and concomitant erectile dysfunction treated with a phosphodiesterase type 5 inhibitor: randomized, placebo-controlled, phase III study. J Sex Med 2013;10:2312-25. [Crossref] [PubMed]
- Corty EW, Guardiani JM. Canadian and American sex therapists' perceptions of normal and abnormal ejaculatory latencies: how long should intercourse last? J Sex Med 2008;5:1251-6. [Crossref] [PubMed]
- Mondaini N, Fusco F, Cai T, et al. Dapoxetine treatment in patients with lifelong premature ejaculation: the reasons of a "Waterloo". Urology 2013;82:620-4. [Crossref] [PubMed]
- Park HJ, Park NC. Discontinuation of dapoxetine treatment in patients with premature ejaculation: a 2-year prospective observational study. Transl Androl Urol 2015;4:AB142.
- Corona G, Ricca V, Bandini E, et al. Selective serotonin reuptake inhibitor-induced sexual dysfunction. J Sex Med 2009;6:1259-69. [Crossref] [PubMed]
- Mann JJ, Emslie G, Baldessarini RJ, et al. ACNP Task Force report on SSRIs and suicidal behavior in youth. Neuropsychopharmacology 2006;31:473-92. [Crossref] [PubMed]
- Black K, Shea C, Dursun S, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci 2000;25:255-61. [PubMed]
- Wyllie MG, Powell JA. The role of local anaesthetics in premature ejaculation. BJU Int 2012;110:E943-8. [Crossref] [PubMed]
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 1999;84:50-6. [Crossref] [PubMed]
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 1993;270:83-90. [Crossref] [PubMed]
- Burnett AL. Nitric oxide regulation of penile erection: biology and therapeutic implications. J Androl 2002;23:S20-6. [PubMed]
- Fugl-Meyer K, Fugl-Meyer AR. Sexual disabilities are not singularities. Int J Impot Res 2002;14:487-93. [Crossref] [PubMed]
- Basile Fasolo C, Mirone V, Gentile V, et al. Premature ejaculation: prevalence and associated conditions in a sample of 12,558 men attending the andrology prevention week 2001—a study of the Italian Society of Andrology (SIA). J Sex Med 2005;2:376-82. [Crossref] [PubMed]
- Rosenberg MT, Sadovsky R. Identification and diagnosis of premature ejaculation. Int J Clin Pract 2007;61:903-8. [Crossref] [PubMed]
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319-26. [Crossref] [PubMed]
- McMahon CG. Screening for erectile dysfunction in men with lifelong premature ejaculation-Is the Sexual Health Inventory for Men (SHIM) reliable? J Sex Med 2009;6:567-73. [Crossref] [PubMed]
- Tang Y, Wang Y, Zhu H, et al. Bias in evaluating erectile function in lifelong premature ejaculation patients with the International Index of Erectile Function-5. J Sex Med 2015;12:2061-9. [Crossref] [PubMed]
- Jannini EA, McMahon C, Chen J, et al. The controversial role of phosphodiesterase type 5 inhibitors in the treatment of premature ejaculation. J Sex Med 2011;8:2135-43. [Crossref] [PubMed]
- Dresser MJ, Desai D, Gidwani S, et al. Dapoxetine, a novel treatment for premature ejaculation, does not have pharmacokinetic interactions with phosphodiesterase-5 inhibitors. Int J Impot Res 2006;18:104-10. [Crossref] [PubMed]
- Rowland DL, Patrick DL, Rothman M, et al. The psychological burden of premature ejaculation. J Urol 2007;177:1065-70. [Crossref] [PubMed]
- Giuliano F, Patrick DL, Porst H, et al. Premature ejaculation: results from a five-country European observational study. Eur Urol 2008;53:1048-57. [Crossref] [PubMed]
- Burri A, Giuliano F, McMahon C, et al. Female partner's perception of premature ejaculation and its impact on relationship breakups, relationship quality, and sexual satisfaction. J Sex Med 2014;11:2243-55. [Crossref] [PubMed]
- Dinsmore WW, Wyllie MG. PSD502 improves ejaculatory latency, control and sexual satisfaction when applied topically 5 min before intercourse in men with premature ejaculation: results of a phase III, multicentre, double-blind, placebo-controlled study. BJU Int 2009;103:940-9. [Crossref] [PubMed]
- Dinsmore WW, Hackett G, Goldmeier D, et al. Topical eutectic mixture for premature ejaculation (TEMPE): a novel aerosol-delivery form of lidocaine-prilocaine for treating premature ejaculation. BJU Int 2007;99:369-75. [Crossref] [PubMed]
- Waldinger MD, Zwinderman AH, Olivier B, et al. The majority of men with lifelong premature ejaculation prefer daily drug treatment: an observation study in a consecutive group of Dutch men. J Sex Med 2007;4:1028-37. [Crossref] [PubMed]
- Rowland D, Cooper S. Practical tips for sexual counseling and psychotherapy in premature ejaculation. J Sex Med 2011;8:342-52. [Crossref] [PubMed]
- Perelman M. Retarded ejaculation. Curr Sex Health Rep 2004;1:95-101. [Crossref]
- McMahon CG. Management of ejaculatory dysfunction. Intern Med J 2014;44:124-31. [Crossref] [PubMed]
- Hertlein KM, Weeks GR, Gambescia N. editors. Systemic Sex Therapy, Second Edition. New York and London: Routledge, 2015.
- Kamischke A, Nieshlag E. Treatment of retrograde ejaculation and anejaculation. Hum Reprod Update 1999;5:448-74. [Crossref] [PubMed]
- Colpi G, Weidner W, Jungwirth A, et al. EAU guidelines on ejaculatory dysfunction. Eur Urol 2004;46:555-8. [Crossref] [PubMed]
- Ohl DA, Quallich SA, Sønksen J, et al. Anejaculation: an electrifying approach. Semin Reprod Med 2009;27:179-85. [Crossref] [PubMed]
- Kamischke A, Nieschlag E. Update on medical treatment of ejaculatory disorders. Int J Androl 2002;25:333-44. [Crossref] [PubMed]
- Middleton RG, Urry RL. The Young-Dees operation for the correction of retrograde ejaculation. J Urol 1986;136:1208-9. [PubMed]
- Reynolds JC, McCall A, Kim ED, et al. Bladder neck collagen injection restores antegrade ejaculation after bladder neck surgery. J Urol 1998;159:1303. [Crossref] [PubMed]
- Kurbatov D, Russo GI, Galstyan GR, et al. Correction of retrograde ejaculation in patients with diabetes mellitus using endourethral collagen injection: preliminary results. J Sex Med 2015;12:2126-9. [Crossref] [PubMed]
- Goktas S, Kibar Y, Kilic S, et al. Recovery of abnormal ejaculation by intermittent tamsulosin treatment. J Urol 2006;175:650-2; discussion 652-3. [Crossref] [PubMed]
- Seyam R. A systematic review of the correlates and management of nonpremature ejaculatory dysfunction in heterosexual men. Ther Adv Urol 2013;5:254-97. [Crossref] [PubMed]
- Crich JP, Jequier AM. Infertility in men with retrograde ejaculation: the action of urine on sperm motility, and a simple method for achieving antegrade ejaculation. Fertil Steril 1978;30:572-6. [Crossref] [PubMed]