The drug treatment of delayed ejaculation
Introduction
The term delayed ejaculation (DE) (also called retarded ejaculation, or inhibited ejaculation) has been used to describe “a marked delay in or inability to achieve ejaculation. The man reports difficulty or inability to ejaculate despite of the presence of adequate sexual stimulation and the desire to ejaculate” (1). In DSM-5 definition the condition must persist for a minimum duration of approximately six months with no specific duration of ejaculation latency. The condition is only a problem if it causes significant distress for the patient or his partner. In most cases, the diagnosis is made by self-report of the individual. Of all the male sexual dysfunctions, DE is the least understood, least common and least studied (2). This condition can be lifelong (primary) or acquired (secondary). Also it may be global, that is, occurring in all sexual scenarios or situational, occurring only in specific sexual scenarios (e.g., with partner but not masturbation, or with one partner but not another). DE was shown to be associated with significant reduction in health-related quality of life as well as self-esteem, anxiety, and depression and has been linked to reduced sexual satisfaction and relationship dissatisfaction and discord. Proposed etiological factors include many medical, psychological and lifestyle factors (2-4). DE is not easy to treat because it is poorly understood (2,5). Treatment should be etiology specific, and may include patient and their partner psychosexual therapy, drug therapy or integrated treatment. Drug treatment of DE includes many agents with varying degrees of success. Currently, no drug has been approved by FDA for DE. A variety of drugs are identified for potential use in this condition. These drugs include testosterone, cabergoline, bupropion, amantadine, cyproheptadine, yohimbine, bethanechol, buspirone and others. However, no drugs have been approved by regulatory agencies for this indication. In a recent survey to evaluate the current opinion and clinical management of DE by members of the Sexual Medicine Society of North America (SMSNA), cabergoline and bupropion were the most commonly selected first line treatments for DE (5). There is a consensus that DE is still a poorly understood disorder with inconsistent practice patterns seen among members of the SMSNA. Here, we reviewed how DE is treated pharmacologically. We also highlighted specific settings where drugs could be introduced to medical practice.
Testosterone (T) therapy
Rationale
The rationale for using T in the treatment of DE came from the following observations: (I) the expression of androgen receptors in smooth muscles of the male genital tract (6), pelvic autonomic pathways (7), the spinal nucleus of the bulbocavernosus muscle (8), and in the medial preoptic area of the hypothalamus (9) is suggesting that Ttestosterone has a role in regulating of the ejaculatory process at different levels; (II) there is some experimental evidence indicating that T treatment shortens the ejaculatory latency (10) indicating that T plays a facilitatory role in the control of ejaculatory reflex; and (III) in humans, subjects with DE showed the highest (26%) prevalence of hypogonadism (11) and reduced T plasma level (T <10.4 nmol/L) is significantly associated with mild and moderate forms of DE (3). These testosterone levels retained association with DE even after adjustment for patient’s hypoactive sexual desire. Moreover, significant effects of small effect size are observed indicating elevated T levels in premature ejaculation (PE), and decreased T levels in DE patients. This effect demonstrated linear function of severity of ejaculatory dysfunction, so that patients with the most severe PE displayed the highest T values, and those patients with the most severe DE display the lowest T levels (11).
Findings
In a multicenter, double-blind, randomized, placebo-controlled, 16-week trial with T solution 2% (n=18) versus placebo (n=24), Paduch et al. (12) reported that the perceived delay of ejaculation was comparable between the placebo and T solution 2% groups. Similarly, nonsignificant difference in the composite Male Sexual Health Questionnaire-Ejaculatory Dysfunction-Short Form score was reported at week -16 among androgen-deficient DE patients.
Conclusions
Treatment of T-deficient DE patients with a 2% solution of T is not associated with improved perceived delay of ejaculation. These negative results might be an accurate reflection of reality and androgen deficiency is not the sole contributor to DE or they may be related to small sample size, short intervention period, and below threshold level of serum T required for ejaculatory function which is not yet known. Further studies are awaited.
Cabergoline
Rationale
The rationale for a pharmacological approach to treating DE by cabergoline [a dopamine (DA) agonist on D2 receptors] came from the following observations: (I) DA has been recognized as a pro-sexual neurotransmitter (13,14); (II) DA agonists have been demonstrated to facilitate both animal (15) and human sexual behavior (16); (III) acute changes in the normal physiological levels of prolactin may also modify sexual motivation and function (17); (IV) increased prolactin concentrations by protirelin (anterior pituitary gland stimulator) administration produced significantly longer ejaculation latency during the first sequence of sexual activity in healthy men, whereas, cabergoline-induced hypoprolactinemia significantly enhanced all parameters of sexual drive and function, including ejaculation latency (17); and (V) cabergoline has been shown to activate the 5-HT2B (agonists) receptors (18). Activation of 5-HT2B receptors may have effects on the ejaculation depending on the dose of the agonist (19).
Findings
Research on cabergoline for treatment of DE is hard to find. A retrospective study presented at the 2012 annual meeting of the American Urological Association (20) evaluated the efficacy of cabergoline (0.5 mg twice/week) in the treatment of 72 anorgasmic men showed improvement in 50 men (69%). Twenty six of these 50 men (52%) returned to normal orgasm after this therapy. The mean age of the patients was 63 whereas the mean duration of therapy for non-responders and responders was 214 and 296 days, respectively. However, the duration of therapy and the concomitant testosterone replacement therapy were associated with significant response to treatment. In another retrospective study, Kacker et al. (21) treated four testosterone deficient men with cabergoline (0.5 mg twice weekly) for persistent difficulty reaching orgasm despite treatment for testosterone deficiency. The authors treated these men on the basis of high-normal or mildly elevated prolactin (mean 19.2 ng/mL). One of these men (25%) reported improved orgasmic function. Although these studies are promising in male anorgasmia, it is still uncertain if this drug will do the same in DE. Orgasm is a mental/emotional process that primarily occurs in the brain. On the other hand, ejaculation is the expulsion of semen to outside involving both emission and the ejaculation proper (a physical process). Usually the two conditions occur simultaneously in men (21,22).
Conclusions
There is weak scientific evidence to suggest that cabergoline could be beneficial for some cases of delayed orgasm. Large-scale randomized controlled trials are necessary to further define the role of the drug in DE apart from hyperprolactinemia. However, special note must be taken that the drawbacks of cabergoline treatment include a higher risk of cardiac valve regurgitation and heart failure (23) due to activation of 5-HT2B receptors on valvular interstitial cells which is mitogenic (18), increasing valve leaflet area and causing the poor valve closure. Longer treatment duration and higher dose of cabergoline is associated with these risks (23). These side effects have never been demonstrated in episodic small dosing for sexual dysfunction.
Bupropion
Rationale
Bupropion is an atypical antidepressant belonging to the chemical class of aminoketones. It is known as a DA and norepinephrine (NE) reuptake inhibitor used as smoking cessation and antidepressant drug with a lower incidence of male sexual dysfunction. The rationale for using bupropion in DE comes from the following observations: (I) it has been shown that chronic bupropion administration at high doses alters the function of the spinal generator for ejaculation (SGE) in rats (24); (II) bupropion produces concentration- dependent increases in the contractile response to nerve stimulation in the rat vas deferens and significantly increases the pressor response to noradrenaline suggesting facilitatory action of the ejaculatory reflex (25); (III) in vitro bupropion increases by eight-fold the NE potency in inducing contractions of the epididymal duct from untreated rats. The ability of bupropion to enhance epididymal duct contractions to NE is due to its action as a NE transporter blocker as bupropion did not change duct contraction to methoxamine (26); (IV) bupropion has been demonstrated to induce premature ejaculation in 46 years old man after the use of 150 mg bupropion per day for depression and premature ejaculation disappeared two weeks after stopping of bupropion (27,28); and (V) bupropion showed prosexual effects when compared with the other antidepressants and no patient taking bupropion complained of delayed orgasm (29). Most of the studies have noted that bupropion is not only as effective as other antidepressants but has the advantage of a lower impact on the sexual function. Other studies have found that bupropion can even enhance sexual function in certain individuals (30). In men treated for depression, DE and orgasmic dysfunction are the most significant sexual complications (31). The DE and anorgasmia with antidepressants have been attributed to increased serotonergic tone. This occurs due to suppression of ejaculation at the level of the hypothalamus (32). Noradrenergic tone on the other hand stimulates ejaculation, which concurs with the finding that noradrenergic antidepressants such as bupropion have no or milder degree of sexual dysfunction as compared with selective serotonin re-uptake inhibitors (SSRIs). In SSRIs-induced DE, the patient can be prescribed buproprion (up to 300 mg/day) as an alternative antidepressant or the drug can be used as an augmentation therapy in patients receiving SSRIs and experiencing DE (33).
Findings
There have been few reports (34-36) on the use of bupropion in DE among non-depressed men. Modell et al. (33) treated ten non-depressed men complaining of orgasmic delay or inhibition with bupropion (150 or 300 mg/day). The authors noted significant improvements over baseline with both doses in overall sexual satisfaction, ability to achieve an erection, and delay in reaching orgasm/ejaculation in 70% of patients. Side effects such as insomnia, anxiety, irritability and headache were generally mild and transient occurred with greater frequency on the higher dose and in no case necessitated drug discontinuation. Abdel-Hamid and Saleh (34) reported on bupropion therapy in a series of 19 lifelong DE men. In that study, bupropion resulted in 25% decrease in mean IELT, and an increased the percentage rating control over ejaculation as ‘‘fair to good’’ from 0–21%. There was also a significant improvement in the intercourse satisfaction and the orgasmic domains of IIEF and depression score from baseline. The authors concluded that bupropion-SR in a daily dosage of 150 mg seemed to be of limited benefit in about of 1 fourth of lifelong DE patients. Bidaki et al. (35) treated successfully 35-year-old opiate addict man complaining of decreased libido and DE with bupropion (75 mg/daily). Although laboratory tests showed primary hypogonadism (high level of LH and low T level), T undecanoate (25 mg weekly IM) treatment did not result in improved DE. On the other hand, Martínez-Raga et al. (36) reported anorgasmia associated with ejaculatory failure in 36-year-old man who received bupropion SR for smoking cessation. The patient developed this sexual dysfunction six days after initiating the medication, while still on a 150 mg/day dose. The authors reported that the sexual dysfunction had resolved three days after stopping bupropion. Although it remains unclear if this side effect was due to the medication itself, it might be mediated through bupropion’s serotoninergic effects.
Conclusions
Although preliminary clinical data seem to suggest that bupropion has beneficial clinical effects in certain DE patients, there is not sufficient scientific evidence to suggest that it could stand alone as a drug therapy for all patients with DE. Further studies are needed.
Amantadine
Rationale
Amantadine hydrochloride is a weak glutamatergic antagonist that works by inhibiting the N-methyl-D-aspartate (NMDA) receptor, binding to it to avoid its excessive excitation by the glutamate neurotransmitter. It was approved by the US FDA in 1976 as an antiviral drug to treat Influenza A. It is also used for treating Parkinsonism, extrapyramidal symptoms and many other disorders (37). It possesses DA enhancing activity by facilitating presynaptic DA release and inhibiting DA reuptake post-synaptically. Amantadine does not act directly on DA receptors but enhances DA release indirectly via antagonism of the NMDA (38,39). Based on the principle that increasing DA neurotransmission may enhance sexual function, amantadine-treated male rats showed a higher sexual response and decreased ejaculation latency time than vehicle-treated rats (40-42). The primary site of action of this drug is possibly supraspinal because amantadine-induced seminal emissions were impaired by spinal cord transection (40). On the other hand, Devaangam et al. (43) demonstrated that amantadine in all aspects failed to antagonize clomipramine-induced sexual dysfunction in male rats. Even the sexual competence of male rats treated with ½ therapeutic dose of clomipramine failed to regain their sexual competence in the presence of amantadine.
Findings
The effects of amantadine on the human ejaculatory or orgasmic functions are conflicting. While the drug has been shown in a number of small case series to reverse DE/or delayed orgasm in men treated with antidepressants (44-46), it showed no change in ejaculatory function in schizophrenic men treated with neuroleptics at a dosage of 100 mg/day for 6 weeks (47). Reported effective doses have ranged between 100–400 mg taken either on a daily or as-needed basis. Shrivastava et al. (45) successfully treated six male patients, who were experiencing ejaculatory difficulty while taking paroxetine, with amantadine. Amantadine was given in divided doses of 200 mg twice daily and 400 mg daily. There were no other side effects or drug interactions reported. In the largest case series, amantadine improved sexual functioning in approximately 42% of the 19 patients complaining of antidepressant-induced sexual dysfunction. Fifty three percent of these patients were originally complaining of orgasmic delay or anorgasmia. In this study, one patient on amantadine discontinued it secondary to depression, which resolved within 48 hr of discontinuation.
Conclusions
There is insufficient evidence to recommend amantadine for treatment of DE. Further studies are needed.
Cyproheptadine
Rationale
Cyproheptadine is a piperidine derivative first-generation antihistamine. Cyproheptadine also has mild anticholinergic and antiserotonergic properties (possibly through blocking 5-HT1A and 5-HT2A receptors) (48). Animal data suggested that cyproheptadine potentiates the prejunctional inhibitory effect of 5-HT and attenuates its stimulatory effect in the rat and guinea-pig vas deferens (49,50) suggesting peripheral effects. Additionally, it facilitates sexual behavior of the male rat possibly through antiserotonergic effects (51). Moreover, cyproheptadine as a 5-HT2A antagonist is usually recommended and is the most widely used antidote in the treatment of serotonin syndrome (52,53) suggesting its efficacy as an antiserotonergic agent. The mechanism of antidepressants-induced DE and delayed orgasm is thought to involve stimulation of post synaptic 5-HT2A and 5-HT2C receptors by the increased synaptic levels of serotonin (54). Antidepressants that antagonize these particular 5-HT receptors subtypes, such as mirtazapine and nefazodone, have a lower propensity to cause DE (55). The prosexual effects of cyproheptadine are likely due to the antiserotonergic properties of the drug, rather than its antihistaminic effects, because diphenhydramine (an antihistaminic) is ineffective in treating sexual dysfunction (46,56).
Findings
Several case reports and case series showed efficacy of cyproheptadine in reversal of DE or anorgasmia supposed to be caused by antidepressants (57-63), monoamine oxidase inhibitors (64) or imipramine (65). Effective doses range from 2–16 mg. The drug is effective when taken either on an as-needed basis (1–2 hours before planned intercourse) or on a daily basis (nightly before bed time). In the largest case series (46), cyproheptadine improved sexual function in approximately 48% of the 25 patients complaining of antidepressant-induced sexual dysfunction. Fifty three percent of these patients were originally complaining of orgasmic delay or anorgasmia. The mean duration of treatment with cyproheptadine was 2.5 months. Sedation and weight gain were associated with cyproheptadine.
Conclusions
The role for cyproheptadine in the treatment of DE awaits more research with double blind randomized design. However, its sedative effects are likely to diminish its overall efficacy.
α1-adrenergic receptor agonists
Rationale
Several studies have noted a tissue-specific distribution of α1-adrenergic receptors (AR) subtype among genital organs participating in seminal emission. The human vas deferens (VD) is rich in α1-ARs with the α1L subtype predominating in the longitudinal and the α1A subtype in circular muscle layers (66-69), whereas the α1A subtype predominates in the human prostate, SV and urethra (70). On the other hand, human detrusor (which is responsible for bladder neck closure during ejaculation) showed a predominance of the α1D subtype (71). It is assumed that stimulation of α1A-AR induces smooth muscle cell (SMC) contraction of the VD, SV and prostate, facilitating the ejaculation. Additionally, increased noradrenergic tone, either by blockade of presynaptic α2-AR by stimulation of postsynaptic α1-AR, results in the activation of the SGE leading to facilitated ejaculation (72).
Findings
Several α1-adrenergic agonists have been used for the treatment of DE and anejaculation (the severest form of DE). These drugs include pseudoephedrine, ephedrine, midodrine and imipramine (a tricyclic with α1-adrenergic activity). A systematic review including 136 patients that were treated with α-agonists for reversal of anejaculation reported a 21% success rate as defined by induction of antegrade or retrograde ejaculation (73). The overall success rate of α-agonists to induce antegrade ejaculation was 12% that can be explained by the severe damage to the ejaculatory system in most of the treated patients. Indeed, most of these patients were diagnosed as spinal cord injury (SCI) (68%) and retroperitoneal lymph node dissection (20.7%) and 5.4% as idiopathic cases. The drug most used for reversal of anejaculation was imipramine (38% of the patients).However; imipramine is significantly inferior to midodrine. Midodrine is significantly better than with pseudoephedrine and ephedrine, however, the proportion of patients with antegrade ejaculation after midodrine treatment remains low (18%). Midodrine is usually given in flexible doses, starting with 7.5 mg in patients with tetraplegia and 15 mg in those with paraplegia. The dose of midodrine can be increased to 30 mg/day (74) and it also can be used as adjuvant to penile vibratory stimulation (PVS) (73).
Other case series of men with SCI and anejaculation have suggested that oral midodrine and PVS report an antegrade and retrograde ejaculation success rate ranging from 12% to 50%, and up to 44% for antegrade ejaculation alone (75-77). A study with a robust design and sample of 20 men with anejaculation associated with SCI evaluated the effects of midodrine (78). In this small sample study, treatment with midodrine and PVS did not result in a better rate of antegrade ejaculation in ten men than in ten men treated with a placebo and PVS. The drug was well-tolerated. Another study (79) with a robust design reported success rate of 58% among anejaculation men. However, these later findings are questionable because the author of this primary study had six retracted clinical studies in the past three years (79).
Conclusions
Although α1-adrenergic agonists are widely used in the treatment of DE and anejaculation, their success is limited. The idea is intriguing and still awaits prospective testing in a large sample size enrolling different grades of DE.
Yohimbine
Rationale
Yohimbine is a central α2-adrenergic receptor antagonist that has been used for treatment of erectile dysfunction before the era of phosphodiesterase-5 inhibitors. α2-adrenergic receptor antagonist block the (classically presynaptic) α2-receptors, resulting in facilitation of NE release from the presynaptic neuron or activation of the postsynaptic neuron, in the case of postsynaptic α2-receptors (80). Yohimbine is not only an α2-adrenergic antagonist but also a 5-HT1A agonist (81-83). Animal studies have demonstrated that yohimbine has pro- ejaculatory effects (84-87). These effects occur quite independently from the effects on arousal/motivation (84), may be continued for at least 5 hr after administration (88), intracerebroventricular administration is as efficient as systemic administration suggesting that yohimbine works within the central nervous system (85,89) and happen with lower doses compared with higher doses suggesting biphasic effects (90). Clonidine, primarily an α2-adrenoceptor agonist, induced suppression of sexual behavior which was reversed by yohimbine (91,92). Concerning the 5-HT1A agonist effect of yohimbine, it has been demonstrated that stimulation of 5-HT1A is associated with reduced number of pre-ejaculatory intromissions and thereby the ejaculation latency (93). Therefore, it is likely that the pro- ejaculatory effects of yohimbine can be attributed to actions at both the 5-HT1A receptors and the α2-adrenoceptors.
Findings
In a retrospective study of 21 patients complaining of antidepressant-induced sexual dysfunction, yohimbine was found to be superior to both cyproheptadine and amantadine in improving the sexual function (46). Seventeen patients (81.0%) were shown to respond to yohimbine. The average dose for yohimbine was 16.2±4.0 mg/day with a mean duration of treatment 1.4±1.1 months. The most encountered side effect reported with yohimbine was agitation, which contributed to drug discontinuation in three patients. Another study (94) on 29 men with orgasmic dysfunction of different etiology that were provided yohimbine at 20 mg and were allowed to increase the dose at home up to 50 mg. Approximately 55% of these patients succeeded to reach orgasm and were able to ejaculate either during masturbation or sexual intercourse. A further 3 men (10.4%) ejaculated by using yohimbine and a vibrator together. Of the 19 men, 11 (58%) ejaculated with the first dose whereas the remaining 8 men (42%) required dose escalation at home. Side effects included dartos contraction, a rise in the pulse and blood pressure, tremor, pleasurable tingling, palpitations, malaise, nausea and headache.
Conclusions
Yohimbine may be useful in the treatment of DE, however final conclusions still awaits large sample size as well as prospective double blind placebo controlled testing.
Buspirone
Rationale
Buspirone is a mixed agonist/antagonist at both 5HT1A and DA2 receptor sites respectively (95). The drug is originally an azapirone that was approved by the US FDA for the treatment of generalized anxiety disorder. The rationale for its use in DE may be related to 5HT1A agonist effects or α2-adrenergic antagonist effects of one of buspirone’s major metabolites, 1-pyrimidinylpiperazine (96). 5-HT1A receptor agonists have been demonstrated to decrease the threshold of ejaculatory behavior in laboratory animals (97-99). In other words, the mechanism of action of buspirone in treating DE may be through reduced serotonergic tone via stimulation of presynaptic 5-HT1A receptor. In contrast, these effects could be antagonized by with the DA2 receptor antagonist effect of buspirone (99,100). It appears that the effects of on sexual behavior may be possible at different parts of the central nervous system (99). This area of research is in need of further effort.
Findings
A retrospective study was undertaken of 16 patients treated with adjunctive buspirone in the context of sexual dysfunction associated with the use of SSRls (101). Sexual functioning was rated as much or very much improved in 11 patients (69%). Treatment was generally very well tolerated. However, several patients that had become less irritable after treatment with an SSRI, reported increased irritability. In another prospective study placebo-controlled trial designed to explore the efficacy of buspirone as add-on treatment for patients not responding to an SSRI alone, Landén et al. (102) evaluated the possible influence of buspirone on sexual dysfunction in depressed patients treated with SSRIs. Among a total of 119 patents (82 women, 37 men), 12 men (32.4%) reported orgasmic dysfunction. Buspirone (flexible dosage, 20–60 mg/day) or placebo was added to the SSRI for 4 weeks. During treatment, approximately half of subjects treated with buspirone reported an improved orgasmic function; however, the difference between buspirone and placebo was more pronounced in women than in men.
Conclusions
Given the small numbers of studies, the small sample size, no conclusions could be drawn at this time regarding the role of buspirone in DE. Further studies are warranted.
Oxytocin
Rationale
Oxytocin (OT), is a nine amino acid CNS neuropeptide produced by posterior pituitary gland. It is involved in a wide variety of physiological and pathological functions including sexual activity, penile erection and ejaculation (103). The rationale for its use in DE may include the followings: (I) animal data suggest that sexual cues and behaviors stimulate central and peripheral OT neurotransmission which, in turn, facilitate copulation (104-106); (II) OT and its receptor have been identified in the human and animal male genital tract suggesting a role in regulation of contractility of these organs (107-111); (III) low dose systemic (intravenous or intraperitoneal) injection of oxytocin facilitates ejaculation in the laboratory animals (112-116); (IV) the pro-ejaculatory effects of systemic OT may be mediated via actions on peripheral OT or vasopressin (AVP) receptors that have been demonstrated in the testis, epididymis, vas deferens, prostate and penis of humans and animals, where they regulate the contractility of smooth muscle cells involved in ejaculatory process (117,118); (V) administration of OT receptor antagonists could inhibit or block these effects of OT multiple sites of action (118,119); (VI) treatment with OT restores the ejaculatory response but has no effect on the intensity of sexual excitement in male rats receiving fluoxetine indicating that SSRIs probably suppress ejaculatory responses by interrupting the action of OT (120); and (VII) men volunteers reported a feeling of decreased arousal and pleasure at orgasm on suppression of the systemic OT pulse by naloxone (121). In contrast to the findings of pro-ejaculatory effect in male rats, in the male prairie vole, OT causes an immediate cessation of all sexual activity which continues to remain so for at least 24 hr (122).
Findings
Review of literature revealed conflicting results regarding the effects of OT on the ejaculation time or time to orgasm in men. In a prospective randomized controlled study at a private assisted reproduction technology center included 103 consecutive healthy men, OT (16 IU) were administered intranasally to 49 subjects (study group) before masturbation to ejaculation (123). The results showed that the mean time to ejaculation was slightly shorter in the OT group compared with the controls (n=53), however, the difference was nonsignificant. In the OT group, no major side effects were registered. Minor side effects such as feeling flushed, slight transient headache, or a strange peculiar taste disappeared in all subjects within 10 min after application of the hormone. In another double-blind, placebo-controlled, cross-over trial, Burri et al. (124) investigated the acute effects of intranasal application of OT (24 IU) on endocrine parameters and sexual function in ten healthy men. All subjects reported having achieved an orgasm in the 10-min sequence of sexual activity in the experimental sessions with significant shorter ejaculation time after placebo (5.3±0.6 min) than after OT (6.3±0.6 min). In contrast, Ishak el al. (125) treated successfully a case of treatment-resistant male anorgasmia with intracoital administration of intranasal OT (20–24 IU). In another case report, MacDonald and Feifel (126) treated attention deficit/hyperactivity disorder (ADHD) man complaining of social anxiety and avoidance with 20 IU intranasal OT twice daily. OT improved the sexual function in this man with satisfied with orgasm changed on the Arizona Sexual Experience Scale, from 3 (somewhat satisfying) to 2 (very satisfying).
Conclusions
Currently, no conclusion could be drawn, the effects of intranasal OT may require proper dose-response studies, and this research might need to include control subjects for peripheral effects, by administering OT peripherally and by blocking peripheral actions with antagonists. It appears that very little of the huge amounts applied intranasally reach the cerebrospinal fluid and peripheral concentrations are increased to supraphysiologic levels, with likely effects on diverse targets including and the genital tract (127). It has been demonstrated that whereas OT plasma concentrations peaked at 15 min after intranasal administration and decreased after 75 min, CSF concentrations took up to 75 min to reach a significant level. Moreover, there was no correlation between OT plasma and CSF concentrations (128).
Bethanechol
Rationale
Bethanechol is muscarinic receptor agonists that are fairly selective for muscarinic receptors (possibly for M3 receptors) with little effect on nicotinic receptors. It is supposed to have mixed central and peripheral cholinergic and adrenergic effects (129). The drug is used to treat urinary retention and other disorders. Several studies have demonstrated that muscarinic receptor agonists decrease the ejaculation latency of male rat sexual behavior (130-134), while muscarinic antagonist’s administration produced a dose-dependent increase in ejaculation latency (131). Additionally, it has been suggested that an imbalance between cholinergic and adrenergic function may be responsible for the tricyclic antidepressant-induced orgasmic dysfunction (135).
Findings
Case reports (135-137) and one randomized, double-blind, placebo-controlled, two-period crossover study (12 patients) (129) have shown the benefit of bethanechol in treating DE associated with antidepressants. The doses of the drug in DE are 10–20 mg as needed or 30–100 mg daily in a divided dose. Its potential side effects of bethanechol might include diarrhea, cramps, and diaphoresis.
Conclusions
Although there is only one randomized, double-blind, placebo-controlled trial that reported efficacy of bethanechol in treating antidepressants -associated DE (evidence level 1), the sample size was small. Definitely, it is difficult to draw solid conclusion waiting for further randomized clinical trials.
Other drugs
Other drugs that may be of value in the treatment of DE include, apomorphine, pramipexole, ropinirole, quinelorane, anandamide (AEA), and reboxetine. Apomorphine, a non-selective DA receptor agonist, has been shown to facilitate the ejaculatory response possibly through the activation of D(2)-like and 5-HT(2C) receptors, respectively (138-141). This proejaculatory effect is likely mediated at supraspinal, spinal or peripheral levels (139-141). To the best of our knowledge, no clinical study has ever been published to test this hypothesis to date.
Quinelorane (LY163502), is another D2 DA receptor agonist that has been demonstrated to facilitate ejaculation in animals (142,143), however, it may inhibit the erectile mechanisms (142). DA agonists such as pramipexole and ropinirole are used in the treatment of Parkinson’s disease. Case series and retrospective patient surveys have shown strong association between these drugs and the occurrence of hypersexuality, one of the severe impulse control disorders, suggesting a pro-sexual effect (144). Additionally, DA receptor is known to facilitate ejaculation in laboratory animals (145,146).
Anandamide (N-arachidonoylethanolamine, AEA) is an essential fatty acid neurotransmitter derived from the non-oxidative metabolism of arachidonic acid and is considered as a cannabinoid receptor (CB1) agonist. It is the first endogenous ligand of central CB1, was isolated from porcine brain in 1992 (147). It showed pro-ejaculatory effects in laboratory animals possibly through the activation of CB1 receptors (148-150).
Reboxetine is a selective noradrenaline reuptake inhibitor with little effects on other neurotransmitter systems (151). It has been reported to reverse SSRI-induced DE possibly due to increased activity of noradrenaline (152).
Herbal preparations
A variety of herbal preparations have been consumed along thousands of years across numerous cultures for stimulation of sexual activity. These herbs may include Ferula hermonis (153), Chinese herbal extract (154), Ginkgo biloba extract (155), Mucuna pruriens Linn. Seed (156), Eurycoma longifolia (157), Pedalium murex Linn. Fruits (158), Maca root (159), and many others. The common action for all of these preparations is shortening in ejaculation latency in male rats whereas its place of these herbs in the therapeutic armamentarium of DE is not yet known.
Which drug to choose
For many pharmacological treatment options the evidence is still limited for small trials, case series or case reports. Further studies are warranted. Given the variety of drugs available for managing DE (Table 1), which one to choose might be a complicated issue in the clinical setting. The choice of management option should be guided by the etiologic factor, illness characteristics (DE or anejaculation), patient preferences, and physician’s comfort with different. For a patient who had suffered antidepressant-related DE, shifting to another antidepressant such as bupropion or adding cabergoline if there is hyperprolactinemia may be the prudent option. An individual with idiopathic DE, symptomatic management with one drug or a combination of drugs to target more than one therapeutic target may be an option of choice. Hence, the selection of drug would be best tailored to the needs of individual patient and the specific circumstances of the case. To conclude, successful drug treatment of DE is still in its infancy. The clinicians need to be aware of the pathogenesis of DE and the pharmacological basis underlying the use of different drugs to extend better care to the patients. Various drugs are available to address such problem, however the evidence of their efficacy is still limited and the choice of drugs needs to be individualized to each specific case.
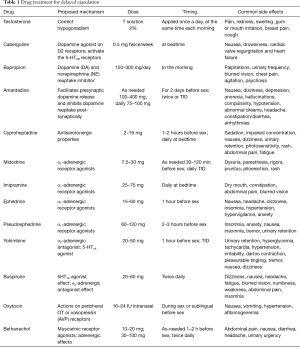
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edition. Washington, DC: American Psychiatric Association, 2013.
- Althof SE. Psychological interventions for delayed ejaculation/orgasm. Int J Impot Res 2012;24:131-6. [Crossref] [PubMed]
- Corona G, Mannucci E, Petrone L, et al. Psychobiological correlates of delayed ejaculation in male patients with sexual dysfunctions. J Androl 2006;27:453-8. [Crossref] [PubMed]
- McMahon CG, Jannini E, Waldinger M, et al. Standard operating procedures in the disorders of orgasm and ejaculation. J Sex Med 2013;10:204-29. [Crossref] [PubMed]
- Butcher MJ, Welliver RC Jr, Sadowski D, et al. How is delayed ejaculation defined and treated in North America? Andrology 2015;3:626-31. [Crossref] [PubMed]
- Lewis RW, Mills TM. Effect of androgens on penile tissue. Endocrine 2004;23:101-5. [Crossref] [PubMed]
- Keast JR. Effects of testosterone on pelvic autonomic pathways: progress and pitfalls. J Auton Nerv Syst 2000;79:67-73. [Crossref] [PubMed]
- Dabaja AA, Wosnitzer MS, Mielnik A, et al. Bulbocavernosus muscle area measurement: a novel method to assess androgenic activity. Asian J Androl 2014;16:618-22. [Crossref] [PubMed]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav 2005;86:356-68. [Crossref] [PubMed]
- Piekarski DJ, Place NJ, Zucker I. Facilitation of male sexual behavior in Syrian hamsters by the combined action of dihydrotestosterone and testosterone. PLoS One 2010;5:e12749. [Crossref] [PubMed]
- Corona G, Jannini EA, Mannucci E, et al. Different testosterone levels are associated with ejaculatory dysfunction. J Sex Med 2008;5:1991-8. [Crossref] [PubMed]
- Paduch DA, Polzer PK, Ni X, et al. Testosterone Replacement in Androgen-Deficient Men With Ejaculatory Dysfunction: A Randomized Controlled Trial. J Clin Endocrinol Metab 2015;100:2956-62. [Crossref] [PubMed]
- Krüger TH, Hartmann U, Schedlowski M. Prolactinergic and dopaminergic mechanisms underlying sexual arousal and orgasm in humans. World J Urol 2005;23:130-8. [Crossref] [PubMed]
- Alwaal A, Breyer BN, Lue TF. Normal male sexual function: emphasis on orgasm and ejaculation. Fertil Steril 2015;104:1051-60. [Crossref] [PubMed]
- Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav 2007;52:45-55. [Crossref] [PubMed]
- Montorsi F, Perani D, Anchisi D, et al. Apomorphine-induced brain modulation during sexual stimulation: a new look at central phenomena related to erectile dysfunction. Int J Impot Res 2003;15:203-9. [Crossref] [PubMed]
- Krüger TH, Haake P, Haverkamp J, et al. Effects of acute prolactin manipulation on sexual drive and function in males. J Endocrinol 2003;179:357-65. [Crossref] [PubMed]
- Setola V, Hufeisen SJ, Grande-Allen KJ, et al. 3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol 2003;63:1223-9. [Crossref] [PubMed]
- Yonezawa A, Yoshizumi M, Ebiko M, et al. Ejaculatory response induced by a 5-HT2 receptor agonist m-CPP in rats: differential roles of 5-HT2 receptor subtypes. Pharmacol Biochem Behav 2008;88:367-73. [Crossref] [PubMed]
- Hsieh TC, Hollander AB, Walters RC, et al. Cabergoline for the treatment of male anorgasmia. J Urol 2012;187:e605. (Abstract). [Crossref]
- Kacker R, Morgentaler A, Conners W. Salvage pharmacotherapy for delayed orgasm after treatment for testosterone deficiency. The New England Section of American Urological Association, 83rd Annual Meeting October 16-19, 2014, Newport, Rhode island, USA. Available online: http://meeting.neaua.org/abstracts/2014/P14.cgi, accessed at January 22,2016.
- Perelman MA. Patient highlights. Delayed ejaculation. J Sex Med 2013;10:1189-90. [Crossref] [PubMed]
- Tran T, Brophy JM, Suissa S, et al. Risks of Cardiac Valve Regurgitation and Heart Failure Associated with Ergot- and Non-Ergot-Derived Dopamine Agonist Use in Patients with Parkinson's Disease: A Systematic Review of Observational Studies. CNS Drugs 2015;29:985-98. [Crossref] [PubMed]
- Hueletl-Soto ME, Carro-Juárez M, Rodríguez-Manzo G. Effects of bupropion on the ejaculatory response of male rats. Int J Impot Res 2014;26:205-12. [Crossref] [PubMed]
- Killian LM, Docherty JR. Cardiovascular stimulant actions of bupropion in comparison to cocaine in the rat. Eur J Pharmacol 2014;735:32-7. [Crossref] [PubMed]
- Cavariani MM, de Almeida Kiguti LR, de Lima Rosa J, et al. Bupropion treatment increases epididymal contractility and impairs sperm quality with no effects on the epididymal sperm transit time of male rats. J Appl Toxicol 2015;35:1007-16. [Crossref] [PubMed]
- Kravos M. Bupropion associated premature ejaculation. Pharmacopsychiatry 2010;43:156-7. [Crossref] [PubMed]
- Evrensel A, Ceylan ME. Bupropion associated premature ejaculation. Turk Psikiyatri Derg 2014;25:290. [PubMed]
- Modell JG, Katholi CR, Modell JD, et al. Comparative sexual side effects of bupropion, fluoxetine, paroxetine, and sertraline. Clin Pharmacol Ther 1997;61:476-87. [Crossref] [PubMed]
- Pereira VM, Arias-Carrión O, Machado S, et al. Bupropion in the depression-related sexual dysfunction: a systematic review. CNS Neurol Disord Drug Targets 2014;13:1079-88. [Crossref] [PubMed]
- Labbate LA, Grimes JB, Hines A, et al. Bupropion treatment of serotonin reuptake antidepressant-associated sexual dysfunction. Ann Clin Psychiatry 1997;9:241-5. [Crossref] [PubMed]
- Waldinger MD. The neurobiological approach to premature ejaculation. J Urol 2002;168:2359-67. [Crossref] [PubMed]
- Modell JG, May RS, Katholi CR. Effect of bupropion-SR on orgasmic dysfunction in nondepressed subjects: a pilot study. J Sex Marital Ther 2000;26:231-40. [Crossref] [PubMed]
- Abdel-Hamid IA, Saleh E. Primary lifelong delayed ejaculation: characteristics and response to bupropion. J Sex Med 2011;8:1772-9. [Crossref] [PubMed]
- Bidaki R, Mirhoseini H, Rezahosseini O, et al. Generalized Acquired Retarded ejaculation in a young man with opium dependency: A rare case report. Health Med 2012;6:4066.
- Martínez-Raga J, Sabater A, Cervera G. Anorgasmia in a patient treated with bupropion SR for smoking cessation. J Clin Psychopharmacol 2004;24:460-1. [Crossref] [PubMed]
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry 2013;22:55-60. [PubMed]
- Kornhuber J, Weller M, Schoppmeyer K, et al. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J Neural Transm Suppl 1994;43:91-104. [PubMed]
- Mizoguchi K, Yokoo H, Yoshida M, et al. Amantadine increases the extracellular dopamine levels in the striatum by re-uptake inhibition and by N-methyl-D-aspartate antagonism. Brain Res 1994;662:255-8. [Crossref] [PubMed]
- Baraldi M, Bertolini A. Penile erections induced by amantadine in male rats. Life Sci 1974;14:1231-5. [Crossref] [PubMed]
- Ferraz MR, Santos R. Amantadine stimulates sexual behavior in male rats. Pharmacol Biochem Behav 1995;51:709-14. [Crossref] [PubMed]
- Ferraz MM, Fontanella JC, Damasceno F, et al. Chronic amantadine treatment enhances the sexual behavior of male rats. Pharmacol Biochem Behav 2007;86:616-21. [Crossref] [PubMed]
- Devaangam SS, Satyanarayana S, Eswar Kumar K, et al. The effect of amantadine on clomipramine induced sexual dysfunction in male rats. Oman Med J 2011;26:404-9. [Crossref] [PubMed]
- Balogh S, Hendricks SE, Kang J. Treatment of fluoxetine-induced anorgasmia with amantadine. J Clin Psychiatry 1992;53:212-3. [PubMed]
- Shrivastava RK, Shrivastava S, Overweg N, et al. Amantadine in the treatment of sexual dysfunction associated with SSRIs. J Clin Psychopharmacol 1995;15:83-4. [Crossref] [PubMed]
- Keller Ashton A, Hamer R, Rosen RC. Serotonin reuptake inhibitor-induced sexual dysfunction and its treatment: a large-scale retrospective study of 596 psychiatric outpatients. J Sex Marital Ther 1997;23:165-75. [Crossref] [PubMed]
- Valevski A, Modai I, Zbarski E, et al. Effect of amantadine on sexual dysfunction in neuroleptic treated male schizophrenic patients. Clin Neuropharmacol 1998;21:355-7. [PubMed]
- Remy DC, Raab AW, Rittle KE, et al. A comparison of the antiserotonin, antihistamine, and anticholinergic activity of cyproheptadine with analogues having furan nuclei fused to the 10,11-vinylene bridge. J Med Chem 1977;20:836-8. [Crossref] [PubMed]
- Moritoki H, Fukuda H, Kanaya J, et al. Ketanserin potentiates the prejunctional inhibitory effect of 5-hydroxytryptamine on rat vas deferens. J Pharm Pharmacol 1986;38:737-41. [Crossref] [PubMed]
- Yoshida S, Kuga T. Probable pre- and postsynaptic modifications by 5-hydroxytryptamine of contractile responses to electrical stimulation of isolated guinea-pig vas deferens. Jpn J Pharmacol 1986;41:315-23. [Crossref] [PubMed]
- Menéndez Abraham E, Morán Viesca P, Velasco Plaza A, et al. Modifications of the sexual activity in male rats following administration of antiserotoninergic drugs. Behav Brain Res 1988;30:251-8. [Crossref] [PubMed]
- Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med 1998;16:615-9. [Crossref] [PubMed]
- Ables AZ, Nagubilli R. Prevention, recognition, and management of serotonin syndrome. Am Fam Physician 2010;81:1139-42. [PubMed]
- Mir S, Taylor D. Sexual adverse effects with new antidepressants. Psychiatric Bulletin 1998;22:438-41. [Crossref]
- Outhoff K. Antidepressant-induced sexual dysfunction. South Afr Fam Pract 2009;51:298-302. [Crossref]
- Harvey KV, Balon R. Clinical implications of antidepressant drug effects on sexual function. Ann Clin Psychiatry 1995;7:189-201. [Crossref] [PubMed]
- Riley AJ, Riley EJ. Cyproheptadine and antidepressant induced anorgasmia. Br J Psychiatry 1986;148:217-8. [Crossref] [PubMed]
- Jeffries JJ, Walker C. Cyproheptadine and drug-induced anorgasmia. Can J Psychiatry 1987;32:79. [PubMed]
- McCormick S, Olin J, Brotman AW. Reversal of fluoxetine-induced anorgasmia by cyproheptadine in two patients. J Clin Psychiatry 1990;51:383-4. [PubMed]
- Feder R. Reversal of antidepressant activity of fluoxetine by cyproheptadine in three patients. J Clin Psychiatry 1991;52:163-4. [PubMed]
- Arnott S, Nutt D. Successful treatment of fluvoxamine-induced anorgasmia by cyproheptadine. Br J Psychiatry 1994;164:838-9. [Crossref] [PubMed]
- Cohen AJ. Fluoxetine-induced yawning and anorgasmia reversed by cyproheptadine treatment. J Clin Psychiatry 1992;53:174. [PubMed]
- Lauerma H. Successful treatment of citalopram-induced anorgasmia by cyproheptadine. Acta Psychiatr Scand 1996;93:69-70. [Crossref] [PubMed]
- Decastro RM. Reversal of MAOI-induced anorgasmia with cyproheptadine. Am J Psychiatry 1985;142:783. [Crossref] [PubMed]
- Steele TE, Howell EF. Cyproheptadine for imipramine-induced anorgasmia. J Clin Psychopharmacol 1986;6:326-7. [Crossref] [PubMed]
- Hedlund H, Andersson KE, Larsson B. Effect of drugs interacting with adrenoreceptors and muscarinic receptors in the epididymal and prostatic parts of the human isolated vas deferens. J Auton Pharmacol 1985;5:261-70. [Crossref] [PubMed]
- Olivier B, Chan JS, Pattij T, et al. Psychopharmacology of male rat sexual behavior: modeling human sexual dysfunctions? Int J Impot Res 2006;18:S14-23. [Crossref] [PubMed]
- Jen PY, Dixon JS, Gosling JA. Colocalisation of neuropeptides, nitric oxide synthase and immunomarkers for catecholamines in nerve fibres of the adult human vas deferens. J Anat 1999;195:481-9. [Crossref] [PubMed]
- de Almeida Kiguti LR, Pupo AS. Investigation of the effects of α1-adrenoceptor antagonism and L-type calcium channel blockade on ejaculation and vas deferens and seminal vesicle contractility in vitro. J Sex Med 2012;9:159-68. [Crossref] [PubMed]
- Hisasue S, Furuya R, Itoh N, et al. Ejaculatory disorder caused by alpha-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission. Int J Urol 2006;13:1311-6. [Crossref] [PubMed]
- Malloy BJ, Price DT, Price RR, et al. Alpha1-adrenergic receptor subtypes in human detrusor. J Urol 1998;160:937-43. [Crossref] [PubMed]
- Carro-Juárez M, Rodríguez-Manzo G. alpha-Adrenergic agents modulate the activity of the spinal pattern generator for ejaculation. Int J Impot Res 2006;18:32-8. [Crossref] [PubMed]
- Kamischke A, Nieschlag E. Update on medical treatment of ejaculatory disorders. Int J Androl 2002;25:333-44. [Crossref] [PubMed]
- Soler JM, Previnaire JG. Ejaculatory dysfunction in spinal cord injury men is suggestive of dyssynergic ejaculation. Eur J Phys Rehabil Med 2011;47:677-81. [PubMed]
- Soler JM, Previnaire JG, Plante P, et al. Midodrine improves ejaculation in spinal cord injured men. J Urol 2007;178:2082-6. [Crossref] [PubMed]
- Courtois FJ, Charvier KF, Leriche A, et al. Blood pressure changes during sexual stimulation, ejaculation and midodrine treatment in men with spinal cord injury. BJU Int 2008;101:331-7. [Crossref] [PubMed]
- Courtois F, Charvier K, Leriche A, et al. Perceived physiological and orgasmic sensations at ejaculation in spinal cord injured men. J Sex Med 2008;5:2419-30. [Crossref] [PubMed]
- Leduc BE, Fournier C, Jacquemin G, et al. Midodrine in patients with spinal cord injury and anejaculation: A double-blind randomized placebo-controlled pilot study. J Spinal Cord Med 2015;38:57-62. [Crossref] [PubMed]
- Safarinejad MR. Midodrine for the treatment of organic anejaculation but not spinal cord injury: a prospective randomized placebo-controlled double-blind clinical study. Int J Impot Res 2009;21:213-20. [Crossref] [PubMed]
- Smith ER, Lee RL, Schnur SL, et al. Alpha 2-adrenoceptor antagonists and male sexual behavior: I. Mating behavior. Physiol Behav 1987;41:7-14. [Crossref] [PubMed]
- Winter JC, Rabin RA. Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. J Pharmacol Exp Ther 1992;263:682-9. [PubMed]
- Millan MJ, Newman-Tancredi A, Audinot V, et al. Agonist and antagonist actions of yohimbine as compared to fluparoxan at α2-adrenergic receptors (AR)s, serotonin (5-HT)1A, 5-HT1B, 5-HT1D and dopamine D2 and D3 receptors. Significance for the modulation of frontocortical monoaminergic transmission in depressive states. Synapse 2000;35:79-95. [Crossref] [PubMed]
- Shannon HE, Lutz EA. Yohimbine produces antinociception in the formalin test in rats: involvement of serotonin1A receptors. Psychopharmacology 2000;149:93-7. [Crossref] [PubMed]
- Smith ER, Lee RL, Schnur SL, et al. Alpha 2-adrenoceptor antagonists and male sexual behavior: II. Erectile and ejaculatory reflexes. Physiol Behav 1987;41:15-9. [Crossref] [PubMed]
- Sala M, Braida D, Leone MP, et al. Central effect of yohimbine on sexual behavior in the rat. Physiol Behav 1990;47:165-73. [Crossref] [PubMed]
- Yonezawa A, Ando R, Watanabe C, et al. Alpha 2-adrenoceptor antagonists: effects on ejaculation, penile erection and pelvic thrusting behavior in dogs. Pharmacol Biochem Behav 2001;70:141-7. [Crossref] [PubMed]
- Carro-Juáreza M, Rodríguez-Manzo G. Yohimbine reverses the exhaustion of the coital reflex in spinal male rats. Behav Brain Res 2003;141:43-50. [Crossref] [PubMed]
- Yonezawa A, Yoshizumii M, Ebiko M, et al. Long-lasting effects of yohimbine on the ejaculatory function in male dogs. Biomed Res 2005;26:201-6. [Crossref] [PubMed]
- Saito TR, Hokao R, Aoki S, et al. Central effects of yohimbine on copulatory behavior in aged male rats. Jikken Dobutsu 1991;40:337-41. [PubMed]
- Yonezawa A, Kawamura S, Ando R, et al. Biphasic effects of yohimbine on the ejaculatory response in the dog. Life Sci 1991;48:PL103-9. [Crossref] [PubMed]
- Yonezawa A, Andoh R, Tadano T, et al. Evidence for central alpha2-adrenergic mechanism of clonidine-induced ejaculatory disturbance in dogs. J Pharmacobiodyn 1986;9:1032-5. [Crossref] [PubMed]
- Yonezawa A, Kawamura S, Ando R, et al. Chronic clonidine treatment and its termination: effects on penile erection and ejaculation in the dog. Life Sci 1992;51:1999-2007. [Crossref] [PubMed]
- Ahlenius S, Larsson K. Specific involvement of central 5-HT1A receptors in the mediation of male rat ejaculatory behavior. Neurochem Res 1997;22:1065-70. [Crossref] [PubMed]
- Adeniyi AA, Brindley GS, Pryor JP, et al. Yohimbine in the treatment of orgasmic dysfunction. Asian J Androl 2007;9:403-7. [Crossref] [PubMed]
- Kleven MS, Assié MB, Koek W. Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT(2A/2C) antagonist anxiolytics. II. Drug discrimination and behavioral observation studies in rats. J Pharmacol Exp Ther 1997;282:747-59. [PubMed]
- Berlin I, Chalon S, Payan C, et al. Evaluation of the alpha 2-adrenoceptor blocking properties of buspirone and ipsapirone in healthy subjects. Relationship with the plasma concentration of the common metabolite 1-(2-pyrimidinyl)-piperazine. Br J Clin Pharmacol 1995;39:243-9. [Crossref] [PubMed]
- Mathes CW, Smith ER, Popa BR, et al. Effects of intrathecal and systemic administration of buspirone on genital reflexes and mating behavior in male rats. Pharmacol Biochem Behav 1990;36:63-8. [Crossref] [PubMed]
- Lee RL, Smith ER, Mas M, et al. Effects of intrathecal administration of 8-OH-DPAT on genital reflexes and mating behavior in male rats. Physiol Behav 1990;47:665-9. [Crossref] [PubMed]
- Rehman J, Kaynan A, Christ G, et al. Modification of sexual behavior of Long-Evans male rats by drugs acting on the 5-HT1A receptor. Brain Res 1999;821:414-25. [Crossref] [PubMed]
- Matuszewich L, Lorrain DS, Trujillo R, et al. Partial antagonism of 8-OH-DPAT'S effects on male rat sexual behavior with a D2, but not a 5-HT1A, antagonist. Brain Res 1999;820:55-62. [Crossref] [PubMed]
- Norden MJ. Buspirone treatment of sexual dysfunction associated with selective serotonin re-uptake inhibitors. Depression 1994;2:109-12. [Crossref]
- Landén M, Eriksson E, Agren H, et al. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol 1999;19:268-71. [Crossref] [PubMed]
- Viero C, Shibuya I, Kitamura N, et al. REVIEW: Oxytocin: Crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther 2010;16:e138-56. [Crossref] [PubMed]
- Hillegaart V, Alster P, Uvnäs-Moberg K, et al. Sexual motivation promotes oxytocin secretion in male rats. Peptides 1998;19:39-45. [Crossref] [PubMed]
- Fuchs AR, Cubile L, Dawood MY. Effects of mating on levels of oxytocin and prolactin in the plasma of male and female rabbits. J Endocrinol 1981;90:245-53. [Crossref] [PubMed]
- Gil M, Bhatt R, Picotte KB, et al. Sexual experience increases oxytocin receptor gene expression and protein in the medial preoptic area of the male rat. Psychoneuroendocrinology 2013;38:1688-97. [Crossref] [PubMed]
- Frayne J, Nicholson HD. Localization of oxytocin receptors in the human and macaque monkey male reproductive tracts: evidence for a physiological role of oxytocin in the male. Mol Hum Reprod 1998;4:527-32. [Crossref] [PubMed]
- Filippi S, Vannelli GB, Granchi S, et al. Identification, localization and functional activity of oxytocin receptors in epididymis. Mol Cell Endocrinol 2002;193:89-100. [Crossref] [PubMed]
- Filippi S, Morelli A, Vignozzi L, et al. Oxytocin mediates the estrogen-dependent contractile activity of endothelin-1 in human and rabbit epididymis. Endocrinology 2005;146:3506-17. [Crossref] [PubMed]
- Zhang XH, Filippi S, Vignozzi L, et al. Identification, localization and functional in vitro and in vivo activity of oxytocin receptor in the rat penis. J Endocrinol 2005;184:567-76. [Crossref] [PubMed]
- Isola M, Cossu M, DE, Lisa A, et al. Oxytocin immunoreactivity in the human urethral (Littrè's) glands. J Reprod Dev 2010;56:94-7. [Crossref] [PubMed]
- Stoneham MD, Everitt BJ, Hansen S, et al. Oxytocin and sexual behaviour in the male rat and rabbit. J Endocrinol 1985;107:97-106. [Crossref] [PubMed]
- Arletti R, Benelli A, Bertolini A. Sexual behavior of aging male rats is stimulated by oxytocin. Eur J Pharmacol 1990;179:377-81. [Crossref] [PubMed]
- Filippi S, Vignozzi L, Vannelli GB, et al. Role of oxytocin in the ejaculatory process. J Endocrinol Invest 2003;26:82-6. [PubMed]
- Palmer CW, Amundson SD, Brito LF, et al. Use of oxytocin and cloprostenol to facilitate semen collection by electroejaculation or transrectal massage in bulls. Anim Reprod Sci 2004;80:213-23. [Crossref] [PubMed]
- Gil M, Bhatt R, Picotte KB, et al. Oxytocin in the medial preoptic area facilitates male sexual behavior in the rat. Horm Behav 2011;59:435-43. [Crossref] [PubMed]
- Corona G, Jannini EA, Vignozzi L, et al. The hormonal control of ejaculation. Nat Rev Urol 2012;9:508-19. [Crossref] [PubMed]
- Argiolas A, Collu M, Gessa GL, et al. The oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin inhibits male copulatory behaviour in rats. Eur J Pharmacol 1988;149:389-92. [Crossref] [PubMed]
- Clément P, Bernabé J, Compagnie S, et al. Inhibition of ejaculation by the non-peptide oxytocin receptor antagonist GSK557296: a multi-level site of action. Br J Pharmacol 2013;169:1477-85. [Crossref] [PubMed]
- Cantor JM, Binik YM, Pfaus JG. Chronic fluoxetine inhibits sexual behaviour in the male rat: reversal with oxytocin. Psychopharmacology (Berl) 1999;144:355-62. [Crossref] [PubMed]
- Murphy MR, Checkley SA, Seckl JR, et al. Naloxone inhibits oxytocin release at orgasm in man. J Clin Endocrinol Metab 1990;71:1056-8. [Crossref] [PubMed]
- Mahalati K, Okanoya K, Witt DM, et al. Oxytocin inhibits male sexual behaviour in prairie voles. Pharmacol Biochem Behav 1991;39:219-22. [Crossref] [PubMed]
- Walch K, Eder R, Schindler A, et al. The effect of single-dose oxytocin application on time to ejaculation and seminal parameters in men. J Assist Reprod Genet 2001;18:655-9. [Crossref] [PubMed]
- Burri A, Heinrichs M, Schedlowski M, et al. The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology 2008;33:591-600. [Crossref] [PubMed]
- Ishak WW, Berman DS, Peters A. Male anorgasmia treated with oxytocin. J Sex Med 2008;5:1022-4. [Crossref] [PubMed]
- MacDonald K, Feifel D. Dramatic improvement in sexual function induced by intranasal oxytocin. J Sex Med 2012;9:1407-10. [Crossref] [PubMed]
- Leng G, Ludwig M. Intranasal Oxytocin: Myths and Delusions. Biol Psychiatry 2016;79:243-50. [Crossref] [PubMed]
- Striepens N, Kendrick KM, Hanking V, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep 2013;3:3440. [Crossref] [PubMed]
- Bernik M, Vieira AH, Nunes PV. Bethanecol chloride for treatment of clomipramine-induced orgasmic dysfunction in males. Rev Hosp Clin Fac Med Sao Paulo 2004;59:357-60. [Crossref] [PubMed]
- Retana-Marquez S, Salazar ED, Velazquez-Moctezuma J. Muscarinic and nicotinic influences on masculine sexual behavior in rats: effects of oxotremorine, scopolamine, and nicotine. Pharmacol Biochem Behav 1993;44:913-7. [Crossref] [PubMed]
- Gómez-Martínez LE, Cueva-Rolón R. Muscarinic receptor antagonism at the spinal cord level causes inhibitory effects on male rat sexual behavior. Behav Brain Res 2009;203:247-55. [Crossref] [PubMed]
- Retana-Marquez S, Velazquez-Moctezuma J. Evidence that the M1 muscarinic receptor subtype mediates the effects of oxotremorine on masculine sexual behavior. Neuropsychopharmacology 1993;9:267-70. [Crossref] [PubMed]
- Durán I, Gil L, Cueva-Rolón R. Masculine copulatory behavior is facilitated by intrathecally administered muscarine. Exp Brain Res 2000;134:490-6. [Crossref] [PubMed]
- Vargas VM, Torres D, Corona F, et al. Cholinergic facilitation of erection and ejaculation in spinal cord-transected rats. Int J Impot Res 2004;16:86-90. [Crossref] [PubMed]
- Sorscher SM, Dilsaver SC. Antidepressant-induced sexual dysfunction in men: due to cholinergic blockade? J Clin Psychopharmacol 1986;6:53-5. [Crossref] [PubMed]
- Yager J. Bethanechol chloride can reverse erectile and ejaculatory dysfunction induced by tricyclic antidepressants and mazindol: case report. J Clin Psychiatry 1986;47:210-1. [PubMed]
- Segraves RT. Reversal by bethanechol of imipramine-induced ejaculatory dysfunction. Am J Psychiatry 1987;144:1243-4. [Crossref] [PubMed]
- Paglietti E, Quarantotti BP, Mereu G, et al. Apomorphine and L-DOPA lower ejaculation threshold in the male rat. Physiol Behav 1978;20:559-62. [Crossref] [PubMed]
- Pehek EA, Thompson JT, Hull EM. The effects of intrathecal administration of the dopamine agonist apomorphine on penilereflexes and copulation in the male rat. Psychopharmacology (Berl) 1989;99:304-8. [Crossref] [PubMed]
- Yonezawa A, Yoshizumi M, Ise SN, et al. Synergistic actions of apomorphine and m-chlorophenylpiperazine on ejaculation, but not penile erection in rats. Biomed Res 2009;30:71-8. [Crossref] [PubMed]
- Sanna F, Piludu MA, Corda MG, et al. Dopamine is involved in the different patterns of copulatory behaviour of Roman high and low avoidance rats: studies with apomorphine and haloperidol. Pharmacol Biochem Behav 2014;124:211-9. [Crossref] [PubMed]
- Bitran D, Thompson JT, Hull EM, et al. Quinelorane (LY163502), a D2 dopamine receptor agonist, facilitates seminal emission, but inhibits penile erection in the rat. Pharmacol Biochem Behav 1989;34:453-8. [Crossref] [PubMed]
- Charles MA, McGinnis MY. Effects of LY163502, a D2 dopaminergic agonist, on the sexual behavior of male rats. Pharmacol Biochem Behav 1992;43:1087-92. [Crossref] [PubMed]
- Moore TJ, Glenmullen J, Mattison DR. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern Med 2014;174:1930-3. [Crossref] [PubMed]
- Ahlenius S, Larsson K. Effects of the dopamine D3 receptor ligand 7-OH-DPAT on male rat ejaculatory behavior. Pharmacol Biochem Behav 1995;51:545-7. [Crossref] [PubMed]
- Kitrey ND, Clément P, Bernabé J, et al. Microinjection of the preferential dopamine receptor D3 agonist 7-hydroxy-N,N-di-n-propylaminotetralin hydrobromide into the hypothalamic medial preoptic area induced ejaculation in anesthetized rats. Neuroscience 2007;149:636-41. [Crossref] [PubMed]
- Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992;258:1946-9. [Crossref] [PubMed]
- Gorzalka BB, Morrish AC, Hill MN. Endocannabinoid modulation of male rat sexual behavior. Psychopharmacology (Berl) 2008;198:479-86. [Crossref] [PubMed]
- Rodríguez-Manzo G, Canseco-Alba A. Anandamide reduces the ejaculatory threshold of sexually sluggish male rats: possible relevance for human lifelong delayed ejaculation disorder. J Sex Med 2015;12:1128-35. [Crossref] [PubMed]
- Canseco-Alba A, Rodríguez-Manzo G. Anandamide transforms noncopulating rats into sexually active animals. J Sex Med 2013;10:686-93. [Crossref] [PubMed]
- Clayton AH, Zajecka J, Ferguson JM, et al. Lack of sexual dysfunction with the selective noradrenaline reuptake inhibitor reboxetine during treatment for major depressive disorder. Int Clin Psychopharmacol 2003;18:151-6. [PubMed]
- O'Flynn R, Michael A. Reboxetine-induced spontaneous ejaculation. Br J Psychiatry 2000;177:567-8. [Crossref] [PubMed]
- Zanoli P, Rivasi M, Zavatti M, et al. Activity of single components of Ferula hermonis on male rat sexual behavior. Int J Impot Res 2005;17:513-8. [Crossref] [PubMed]
- Zanoli P, Benelli A, Zavatti M, et al. Improved sexual behavior in male rats treated with a Chinese herbal extract: hormonal and neuronal implications. Asian J Androl 2008;10:937-45. [Crossref] [PubMed]
- Yeh KY, Pu HF, Kaphle K, et al. Ginkgo biloba extract enhances male copulatory behavior and reduces serum prolactin levels in rats. Horm Behav 2008;53:225-31. [Crossref] [PubMed]
- Suresh S, Prithiviraj E, Prakash S. Dose- and time-dependent effects of ethanolic extract of Mucuna pruriens Linn. seed on sexual behaviour of normal male rats. J Ethnopharmacol 2009;122:497-501. [Crossref] [PubMed]
- Zanoli P, Zavatti M, Montanari C, et al. Influence of Eurycoma longifolia on the copulatory activity of sexually sluggish and impotent male rats. J Ethnopharmacol 2009;126:308-13. [Crossref] [PubMed]
- Sharma V, Thakur M, Dixit VK. A comparative study of ethanolic extracts of Pedalium murex Linn. fruits and sildenafil citrate on sexual behaviors and serum testosterone level in male rats during and after treatment. J Ethnopharmacol 2012;143:201-6. [Crossref] [PubMed]
- Lentz A, Gravitt K, Carson CC, et al. Acute and chronic dosing of Lepidium meyenii (Maca) on male rat sexual behavior. J Sex Med 2007;4:332-9; discussion 339-40. [Crossref] [PubMed]


