
Prognostic indicators for survival in renal cell carcinoma with venous thrombus and development of predictive nomograms
Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all adult malignancies. In the past 10 years, the incidence rate of RCC has gradually increased. As many as half of the cases in developed countries are accidentally discovered, which is attributed to the popularization of and improvements in the means of detection. According to reports, the mortality rate of RCC in highly developed countries has declined since 1990 (1,2). Despite the rapid development of diagnosis and treatment methods for RCC, some patients are still diagnosed with advanced tumours with venous tumour thrombus (VTT), which poses a great challenge to urological surgeons. According to reports, approximately 4–10% of patients with RCCs develop VTT (3). Surgery is the main therapeutic means for these patients, and some patients achieve long-term survival after radical nephrectomy (RN) and thrombectomy (4). Limited by their sample sizes, previous studies showed great differences when describing the prognosis and prognostic factors of patients with RCCs and VTT, and the survival time of patients varied from several months to more than ten years (5-8). Prognostic models play an important role in assisting clinicians predicting prognosis based on personal experience. The prognostic models established by previous studies are based on patients who underwent nephrectomy and thrombectomy, but some patients didn’t receive surgery and previous models may have limited value for them (9,10). This study aimed to identify the independent prognostic factors for patients with RCC and VTT using large-sample data and to predict the survival of patients by establishing nomograms. We present the following article in accordance with the TRIPOD reporting checklist (11-13) (available at https://tau.amegroups.com/article/view/10.21037/tau-22-128/rc).
Methods
Source of patients
The process of patient screening and data analysis was shown in Figure 1. The SEER database of the National Cancer Institute collects cancer diagnosis, treatment, and survival data for approximately 30% of the US patients and is an important population-based resource (14). The clinical data of patients with renal malignant tumours diagnosed from 2000–2018 were downloaded from SEERstat software. The relevant guidelines and regulations of the SEER database were referred to when performing all methods.
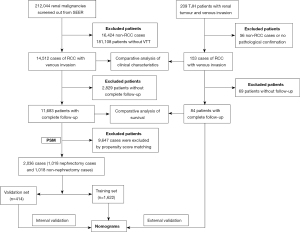
The inclusion criteria were as follows: primary renal tumours; known follow-up time; and pathological confirmation of malignancy. The exclusion criteria were as follows: non-RCC histologic classification; without VTT.
A total of 212,044 patients with renal malignant tumours were identified according to the criteria above, and further screening was conducted to exclude 16,424 cases of non-RCC, such as squamous cell carcinoma, urothelial carcinoma, and lymphoma 181,108 patients without VTT were also screened out. Finally, 14,512 cases of RCCs with VTT were obtained.
Furthermore, the clinical data of 209 patients with renal tumours and VTT admitted to Tongji Hospital (TJH) from January 2004 to December 2020 were collected. After screening, 153 cases pathologically confirmed RCC and VTT were obtained. Among them, 84 patients had complete clinical data and received full follow-up.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
All tumor, node and metastasis (TNM) stages were reclassified to the eighth edition. Categorical variables were integrated, and the cases were divided into the nephrectomy + thrombectomy group and the non-nephrectomy management group according to the surgical approach. Patients who underwent local tumour resection only or biopsy only were combined into the non- nephrectomy management group (hereinafter referred to as the non- nephrectomy group). Patients who underwent cytoreductive nephrectomy + thrombectomy, nephroureterectomy + thrombectomy, radical nephrectomy + thrombectomy or radical nephrectomy + thrombectomy + other organ resection were combined into the nephrectomy + thrombectomy group (hereinafter referred to as the nephrectomy group).
Data analysis and plotting were conducted with R 3.5.3 (www.r-project.org), and a comparative analysis was conducted between 14,512 SEER cases and 153 TJH cases. Continuous variables that fit a non-normal distribution, such as age and tumour size, were analysed by Kruskal-Walis test, and Fisher’s exact test was used for categorical variables (Table 1). Kaplan-Meier survival analysis was conducted with the “survival” package of R.
Table 1
| Characteristics | SEER (n=14,512) | TJH (n=153) | P |
|---|---|---|---|
| Age (years), median [IQR] | 64.00 [56.00, 72.00] | 58.00 [50.00, 63.00] | <0.001 |
| Size (mm), median [IQR] | 84.00 [60.00, 110.00] | 89.00 [70.75, 110.50] | 0.029 |
| Race, n (%) | <0.001 | ||
| Black | 1,051 (7.2) | 0 (0.0) | |
| Other | 975 (6.7) | 153 (100.0) | |
| Unknown | 50 (0.3) | 0 (0.0) | |
| White | 12,433 (85.7) | 0 (0.0) | |
| Sex, n (%) | 0.067 | ||
| Female | 4,648 (32.0) | 38 (24.8) | |
| Male | 9,864 (68.0) | 115 (75.2) | |
| Laterality, n (%) | <0.001 | ||
| Bilateral | 47 (0.3) | 1 (0.7) | |
| Left | 7,018 (48.4) | 47 (33.3) | |
| Right | 7,447 (51.3) | 93 (66.0) | |
| T, n (%) | <0.001 | ||
| T3a | 9,786 (67.4) | 33 (24.6) | |
| T3a or T3b | 1,876 (12.9) | 0 (0.0) | |
| T3b | 1,204 (8.3) | 66 (49.3) | |
| T3b or T3c | 860 (5.9) | 0 (0.0) | |
| T3c | 652 (4.5) | 14 (10.4) | |
| T4 | 134 (0.9) | 21 (15.7) | |
| N, n (%) | <0.001 | ||
| N0 | 11,351 (78.2) | 73 (54.5) | |
| N1 | 2,389 (16.5) | 51 (38.1) | |
| NX | 772 (5.3) | 10 (7.5) | |
| M, n (%) | 0.003 | ||
| M0 | 10,681 (73.6) | 83 (61.9) | |
| M1 | 3,831 (26.4) | 51 (38.1) | |
| Location of VTT, n (%) | <0.001 | ||
| Above diaphragm or invading the IVC wall | 676 (4.7) | 17 (11.1) | |
| Below the diaphragm | 1,204 (8.3) | 98 (64.1) | |
| In the IVC | 860 (5.9) | 0 (0.0) | |
| In the RV | 5,420 (37.3) | 38 (24.8) | |
| In the RV or IVC below the diaphragm | 6,352 (43.8) | 0 (0.0) | |
| Histology, n (%) | <0.001 | ||
| ccRCC | 9,616 (66.3) | 72 (47.1) | |
| ChRCC | 223 (1.5) | 1 (0.7) | |
| Other | 4,103 (28.3) | 65 (42.5) | |
| PRCC | 570 (3.9) | 15 (9.8) | |
| Grade, n (%) | <0.001 | ||
| Grade I | 400 (2.8) | 9 (5.9) | |
| Grade II | 3,602 (24.8) | 38 (24.8) | |
| Grade III | 5,134 (35.4) | 35 (22.9) | |
| Grade IV | 2,488 (17.1) | 23 (15.0) | |
| Unknown | 2,888 (19.9) | 48 (31.4) | |
| Radiation, n (%) | <0.001 | ||
| None/unknown | 13,394 (92.3) | 153 (100.0) | |
| Yes | 1,118 (7.7) | 0 (0.0) | |
| Systemic treatments, n (%) | 0.677 | ||
| No/unknown | 11,817 (81.4) | 127 (83.0) | |
| Yes | 2,695 (18.6) | 26 (17.0) | |
| Surgery, n (%) | <0.001 | ||
| Nephrectomy | 12,516 (86.2) | 88 (57.5) | |
| Non-nephrectomy | 1,996 (13.8) | 65 (42.5) |
RCC, renal cell carcinoma; VTT, venous tumor thrombus; IQR, interquartile range; IVC, inferior vena cava; RV, renal vein; ccRCC, clear cell renal cell carcinoma; ChRCC, chromophobe renal cell carcinoma; PRCC, papillary renal cell carcinoma; TJH, Tongji Hospital.
Propensity score matching (PSM), as a statistical tool, aims to help strengthen the causality in observational data by reducing the inherent selection bias (15). The ‘matchit’ function of the ‘MatchIt’ package was used to match patients with complete survival information from the TJH and SEER database in a 1:2 ratio by the “nearest” method. Kaplan-Meier survival analysis was performed again on the two matched groups. All clinical and pathologic features were used as caliper.
The thrombectomy procedure is extremely complicated. Whether the surgeon recommends nephrectomy + thrombectomy is obviously affected by the clinical characteristics of patients. Therefore, propensity score matching was performed in the SEER cases. The “nearest” method was used to match 1,018 cases in the non- nephrectomy group and 1,018 cases in the nephrectomy group in a ratio of 1:1, all clinical features were used as caliper. The difference in clinical characteristics between the two groups was eliminated after matching (Table 2).
Table 2
| Characteristics | Before match | After match | |||||
|---|---|---|---|---|---|---|---|
| Nephrectomy (n=6,101) | Non-nephrectomy (n=1,018) | P | Nephrectomy (n=1,018) | Non-nephrectomy (n=1,018) | P | ||
| Age (years), median [IQR] | 63.00 [56.00, 71.00] | 65.00 [57.00, 73.00] | 0.001 | 65.00 [58.00, 72.00] | 65.00 [57.00, 73.00] | 0.547 | |
| Size (mm), median [IQR] | 85.00 [62.00, 110.00] | 82.00 [54.25, 110.75] | 0.004 | 80.00 [60.00, 105.00] | 82.00 [54.25, 110.75] | 0.745 | |
| Race, n (%) | 0.003 | 0.996 | |||||
| Black | 412 (6.8) | 102 (10.0) | 101 (9.9) | 102 (10.0) | |||
| Other | 432 (7.1) | 77 (7.6) | 79 (7.8) | 77 (7.6) | |||
| Unknown | 36 (0.6) | 4 (0.4) | 3 (0.3) | 4 (0.4) | |||
| White | 5,221 (85.6) | 835 (82.0) | 835 (82.0) | 835 (82.0) | |||
| Sex, n (%) | 0.029 | 0.963 | |||||
| Female | 1,886 (30.9) | 350 (34.4) | 348 (34.2) | 350 (34.4) | |||
| Male | 4,215 (69.1) | 668 (65.6) | 670 (65.8) | 668 (65.6) | |||
| Laterality, n (%) | <0.001 | 0.062 | |||||
| Bilateral | 8 (0.1) | 17 (1.7) | 6 (0.6) | 17 (1.7) | |||
| Left | 2,871 (47.1) | 471 (46.3) | 466 (45.8) | 471 (46.3) | |||
| Right | 3,222 (52.8) | 530 (52.1) | 546 (53.6) | 530 (52.1) | |||
| T, n (%) | <0.001 | 0.879 | |||||
| T3a | 4,656 (76.3) | 654 (64.2) | 668 (65.6) | 654 (64.2) | |||
| T3b | 929 (15.2) | 236 (23.2) | 225 (22.1) | 236 (23.2) | |||
| T3c | 504 (8.3) | 119 (11.7) | 118 (11.6) | 119 (11.7) | |||
| T4 | 12 (0.2) | 9 (0.9) | 7 (0.7) | 9 (0.9) | |||
| N, n (%) | <0.001 | 0.539 | |||||
| N0 | 5,168 (84.7) | 604 (59.3) | 607 (59.6) | 604 (59.3) | |||
| N1 | 799 (13.1) | 344 (33.8) | 353 (34.7) | 344 (33.8) | |||
| NX | 134 (2.2) | 70 (6.9) | 58 (5.7) | 70 (6.9) | |||
| M, n (%) | <0.001 | 0.715 | |||||
| M0 | 4,686 (76.8) | 381 (37.4) | 390 (38.3) | 381 (37.4) | |||
| M1 | 1,415 (23.2) | 637 (62.6) | 628 (61.7) | 637 (62.6) | |||
| Location of VTT, n (%) | <0.001 | 0.805 | |||||
| Above diaphragm or invading the IVC wall | 516 (8.5) | 128 (12.6) | 125 (12.3) | 128 (12.6) | |||
| Below the diaphragm | 929 (15.2) | 236 (23.2) | 225 (22.1) | 236 (23.2) | |||
| In the RV | 4,656 (76.3) | 654 (64.2) | 668 (65.6) | 654 (64.2) | |||
| Histology, n (%) | <0.001 | <0.001 | |||||
| ccRCC | 4,729 (77.5) | 535 (52.6) | 726 (71.3) | 535 (52.6) | |||
| ChRCC | 101 (1.7) | 16 (1.6) | 14 (1.4) | 16 (1.6) | |||
| Other | 1,014 (16.6) | 417 (41.0) | 227 (22.3) | 417 (41.0) | |||
| PRCC | 257 (4.2) | 50 (4.9) | 51 (5.0) | 50 (4.9) | |||
| Grade, n (%) | <0.001 | <0.001 | |||||
| Grade I | 95 (1.6) | 32 (3.1) | 15 (1.5) | 32 (3.1) | |||
| Grade II | 1,488 (24.4) | 172 (16.9) | 171 (16.8) | 172 (16.9) | |||
| Grade III | 2,485 (40.7) | 202 (19.8) | 397 (39.0) | 202 (19.8) | |||
| Grade IV | 1,330 (21.8) | 55 (5.4) | 300 (29.5) | 55 (5.4) | |||
| Unknown | 703 (11.5) | 557 (54.7) | 135 (13.3) | 557 (54.7) | |||
| Radiation, n (%) | <0.001 | 0.03 | |||||
| None/unknown | 5,743 (94.1) | 836 (82.1) | 873 (85.8) | 836 (82.1) | |||
| Yes | 358 (5.9) | 182 (17.9) | 145 (14.2) | 182 (17.9) | |||
| Systemic treatments, n (%) | <0.001 | 0.015 | |||||
| No/unknown | 4,956 (81.2) | 580 (57.0) | 635 (62.4) | 580 (57.0) | |||
| Yes | 1,145 (18.8) | 438 (43.0) | 383 (37.6) | 438 (43.0) | |||
RCC, renal cell carcinoma; VTT, venous tumor thrombus; IVC, inferior vena cava; IQR, interquartile range; RV, renal vein; ccRCC, clear cell renal cell carcinoma; ChRCC, chromophobe renal cell carcinoma; PRCC, papillary renal cell carcinoma.
The cases obtained by PSM were randomly divided into a training set and a validation at a ratio of 8:2, and there was no significant difference in characteristics between the training set and the validation set (Table S1).
Survival analysis of OS and CSS was performed on the training set. First, univariate Cox regression was conducted, and then variables with significant differences were selected for multivariate Cox regression. Both univariate and multivariate Cox regression were analysed by the “coxph” function of the “survival” package. The key prognostic factors identified by multivariate Cox regression were used to build the nomograms for OS and CSS. Then, the nomograms were validated in the training set, validation set, and TJH cohort. First, the C-index was calculated and applied to evaluate the predictive accuracy. Then, bootstrap validation (1,000 resamples) was conducted, and calibration plots were created. Finally, time-dependent ROC curves were generated to compare the ability of the nomograms to predict survival with that of prognostic models trained in all SEER cases and based on TNM staging. The nomograms were constructed with the “rms” package (16), and the ROC curves were drawn with the “survivalROC” package.
Results
Comparison of SEER and Tongji cohort
The baseline characteristics of 14,512 SEER patients and 153 TJH patients were compared (Table 1). The two groups were mainly male, with 9,864 (68.0%) and 115 (75.2%) male patients in the two groups, respectively. The median age of the SEER cohort was 64-year-old, and that of TJH patients was 58-year-old; the TJH patients were significantly younger than the SEER patients (P<0.001). The SEER patients had more left renal tumours [7,018 (48.4%) vs. 47 (33.3%), P<0.001]; clear cell RCC (ccRCC) was the main histological type in both groups, but the proportions of each subtype were different between the two groups (P<0.001). The TJH cases were more advanced than the SEER cases according to TNM stage [the eighth edition of the (American Joint Committee on Cancer) TNM staging criteria were used here], and the TJH patients also had tumour thrombus with a higher location (P<0.001); There was no significant difference in the proportion of patients who received systemic treatments (including chemotherapy, immunotherapy and targeted therapy) between the two groups. The SEER patients were followed-up for 1–226 months, with a median follow-up of 41 months. At the last follow-up, there were 9,036 deaths, 6,934 (76.7%) patients died of RCC. Eighty-four TJH cases were followed-up for 2–120 months, with a median follow-up of 16 months. 26 patients died by the last follow-up, and all of them died of RCC. Survival analysis was performed on the two groups. The 1-, 3-, and 5-year OS and CSS rates of the SEER patients were 79.0%, 60.9%, and 50.2%, and 81.0%, 65.3%, and 57.1%, respectively. The median OS and CSS were 60 and 87 months, respectively. The 1-, 2- and 3-year OS rates of the TJH patients were the same as the CSS rates, which were 77.7%, 61.5%, and 39.5%, respectively. The median OS and CSS were both 32 months. The log-rank test showed that there was no significant difference in OS (P=0.72) and CSS (P=0.66) between SEER and TJH cohorts. After balancing the characteristics of the patients (Table S2), there was still no significant difference in the OS (P=0.096) and CSS (P=0.37) between the two groups (Figure S1).
Subset of SEER cases
The differences in variables between the non-nephrectomy group and the nephrectomy group among the 2,036 SEER cases obtained after PSM were eliminated (Table 2). The Kaplan-Meier method was applied to analyse the influence of surgery on the OS and CSS of the SEER patients before and after matching. As a result, the difference in survival caused by surgery was reduced after matching (Figure S2).
A total of 2,036 SEER cases obtained after PSM were randomly divided into a training set (1,622 cases) and a validation set (414 cases) at an 8:2 ratio. There were no significant differences in the variables between the two groups (Table S1).
Cox regression analysis of OS/CSS
Univariate Cox regression analysis was performed for each variable in the training set. Univariate Cox regression analysis showed that the significant prognostic factors of OS were age, race, histologic classification, tumour size, nuclear grade, T stage, N stage, M stage, location of tumour thrombus, surgery, radiotherapy and systemic treatments; the significant prognostic factors of CSS were race, histological classification, tumour size, nuclear grade, T stage, N stage, M stage, location of tumour thrombus, surgery, radiotherapy and systemic treatments. Multivariate Cox regression analysis was performed on OS and CSS (the tumour thrombus of 11 T4 stage patients in the training cohort were all above the diaphragm, and the T stage coincided with the tumour thrombus classification, so the T stage was excluded from the multivariate analysis). Nine key prognostic factors of OS were found: age (P<0.001), location of VTT (P=0.039), tumour size (P=0.002), histological classification (P<0.001), nuclear grade (P<0.001), N stage (P<0.001), M stage (P<0.001), surgery (P<0.001), and systemic treatments (P<0.001); 8 key risk factors related to CSS were identified: location of VTT (P=0.021), tumour size (P=0.007), histological classification (P<0.001), nuclear grade (P<0.001), N stage (P<0.001), M stage (P<0.001), surgery (P<0.001) and systemic treatments (P<0.001). In the analysis of OS and CSS, the papillary RCC showed worse prognosis than ccRCC. The location of tumour thrombus and tumour size were independent prognostic factor of RCC patients with VTT, whereas systemic treatments was a risk factor in univariate analysis but a protective factor in multivariate analysis; a high age was a risk factor for OS but not for CSS (Table 3 and Table S3).
Table 3
| Characteristics | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| Age (per year) | 1.01 | 1–1.01 | 0.007 | 1.02 | 1.01–1.02 | <0.001 | |
| Systemic treatments | |||||||
| No | 1 (reference) | 1 (reference) | |||||
| Yes | 1.92 | 1.7–2.16 | <0.001 | 0.71 | 0.62–0.82 | <0.001 | |
| Grade | <0.001 | <0.001 | |||||
| Grade I | 1 (reference) | 1 (reference) | |||||
| Grade II | 0.95 | 0.57–1.57 | 0.833 | 1.29 | 0.78–2.15 | 0.323 | |
| Grade III | 1.35 | 0.83–2.20 | 0.231 | 1.50 | 0.91–2.46 | 0.109 | |
| Grade IV | 2.11 | 1.29–3.47 | 0.003 | 1.71 | 1.03–2.84 | 0.040 | |
| Unknown | 3.07 | 1.89–5.00 | <0.001 | 1.98 | 1.21–3.24 | 0.007 | |
| Histology | <0.001 | <0.001 | |||||
| ccRCC | 1 (reference) | 1 (reference) | |||||
| ChRCC | 0.56 | 0.29–1.07 | 0.081 | 0.84 | 0.43–1.62 | 0.599 | |
| Other | 2.35 | 2.08–2.67 | <0.001 | 1.53 | 1.33–1.76 | <0.001 | |
| PRCC | 1.76 | 1.33–2.32 | <0.001 | 1.44 | 1.08–1.92 | 0.013 | |
| M | |||||||
| M0 | 1 (reference) | 1 (reference) | |||||
| M1 | 4.42 | 3.82–5.11 | <0.001 | 3.39 | 2.82–4.09 | <0.001 | |
| N | <0.001 | <0.001 | |||||
| N0 | 1 (reference) | 1 (reference) | |||||
| N1 | 3.39 | 2.98–3.86 | <0.001 | 1.95 | 1.69–2.26 | <0.001 | |
| NX | 2.59 | 2.08–3.24 | <0.001 | 1.62 | 1.28–2.04 | <0.001 | |
| Race | 0.014 | 0.895 | |||||
| Black | 1 (reference) | 1 (reference) | |||||
| Other | 0.76 | 0.57–1.01 | 0.058 | 0.97 | 0.72–1.31 | 0.862 | |
| Unknown | 1.26 | 0.31–5.11 | 0.742 | 1.09 | 0.27–4.49 | 0.902 | |
| White | 0.73 | 0.61–0.88 | 0.001 | 1.05 | 0.86–1.28 | 0.616 | |
| Radiation | |||||||
| None/unknown | 1 (reference) | 1 (reference) | |||||
| Yes | 1.92 | 1.66–2.23 | <0.001 | 1.17 | 1.00–1.36 | 0.050 | |
| Size (per 1 mm) | 1.01 | 1.01–1.01 | <0.001 | 1.00 | 1.00–1.00 | 0.002 | |
| Surgery | |||||||
| Nephrectomy | 1 (reference) | 1 (reference) | |||||
| Non-nephrectomy | 1.37 | 1.21–1.54 | <0.001 | 1.44 | 1.24–1.67 | <0.001 | |
| Location of VTT | <0.001 | 0.039 | |||||
| Above diaphragm or invading the IVC wall | 1 (reference) | 1 (reference) | |||||
| Below the diaphragm | 0.76 | 0.62–0.92 | 0.006 | 0.89 | 0.73–1.09 | 0.273 | |
| In the RV | 0.46 | 0.38–0.54 | <0.001 | 0.80 | 0.66–0.96 | 0.014 | |
ccRCC, clear cell renal cell carcinoma; ChRCC, chromophobe renal cell carcinoma; PRCC, papillary renal cell carcinoma; VTT, venous tumor thrombus; IVC, inferior vena cava; RV, renal vein.
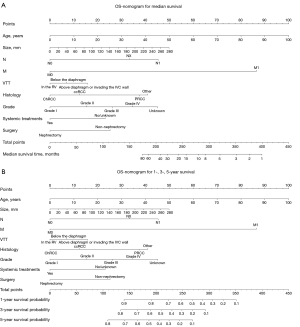
Establishment and validation of nomogram
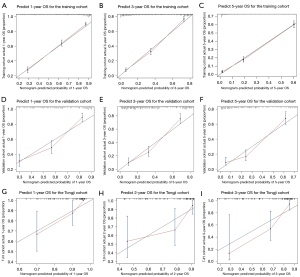
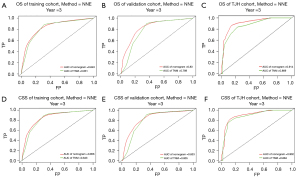
The key prognostic factors screened by multivariate Cox regression analysis were applied to establish nomograms to predict the median OS and CSS and 1-, 3-, and 5-year OS and CSS rates (Figure 2 and Figure S3). Variables of every patient can found corresponding scores in the “point” line on the nomograms, and all scores of variables are added to obtain a total score of this patient. The median survival time or survival probability corresponding to the total score is the expected median survival time or survival probability of that patient. A total of 1,622 cases from the training set, 414 cases from the validation set and 84 TJH cases were applied to validate the nomograms internally and externally. The C-indexes of the nomogram for predicting OS in the training set, validation set, TJH cohort were 0.762 (95% CI: 0.746–0.778), 0.718 (95% CI: 0.687–0.749), and 0.819 (95% CI: 0.745–0.893), respectively; and those of the nomogram for predicting CSS in the training set, validation set, and TJH cohort were 0.776 (95% CI: 0.760–0.792), 0.724 (95% CI: 0.693–0.755), and 0.818 (95% CI: 0745–0.891), respectively. The calibration curves are all close to a straight line with a slope of 1 (Figure 3 and Figure S4). Another prognostic model was established based on TNM stage in the training set. Time-dependent ROC curves were applied to compare the accuracy of the nomograms and TNM model for predicting OS and CSS. The areas under the curve (AUCs) of the nomogram for predicting the 3-year OS of the training set, validation set, and TJH cohort were 0.843, 0.830, and 0.914, respectively, and those for predicting the 3-year CSS were 0.856, 0.833, and 0.902, respectively. The AUCs of the TNM model for predicting the 3-year OS rates were 0.831, 0.789, and 0.869, and those for predicting the 3-year CSS were 0.843, 0.805, and 0.884, respectively. The AUCs obtained from the nomogram were greater than those obtained from the TNM model (Figure 4). All the validations proved that the nomograms established based on key prognostic factors have reliable accuracy in predicting the survival of both Chinese and American patients who developed RCCs with VTT.
Discussion
VTT is a characteristic of RCC progression. RCC with VTT was classified as T3 in the AJCC staging system. Approximately one-third of patients with tumour thrombus have distant metastasis (17,18). Patients with RCC and VTT have poor prognosis, the progression of surgical technique improved the 5-year OS of patients with non-metastatic RCC and VTT from 17% to 40%, but it is still not optimistic, especially in patients with metastases (19). Therefore, it is important to identify the prognostic factors of these patients and predict their survival. Then, we will discuss these controversial prognostic factors respectively.
A focus of discussion is always how the location of VTT affects patients’ prognosis. Some researchers believe that the location of VTT does not affect survival significantly (6,8,19-25). Some other studies support that the location of VTT has a significant impact on survival; in other words, these authors agree with the 2017 AJCC TNM staging system (7,26-30). There are also studies that support that invasion of the venous wall is an important risk factor (5,27,29). Some research indicates that the location of VTT is an independent prognostic factor in non-metastatic RCC patients but not in metastatic patients (3,29). The above studies were carried out in patients who underwent nephrectomy and thrombectomy, and there is a lack of data for patients who did not undergo nephrectomy and thrombectomy. In our study, before the establishment of the prognosis models, PSM was conducted between the nephrectomy group and the non-surgical group, and we found that the location of VTT indeed affect survival, but not significantly as tumour size, N stage and M stage.
VTT is usually accompanied by metastasis. Regardless of whether there is VTT, distant metastasis is an important risk factor (31-34). The 2019 EAU guidelines recommend that RN and thrombectomy should be performed in patients without distant metastasis regardless of the location of VTT, but there is no clear recommendation for metastatic cases (4). It remains controversial whether cytoreductive nephrectomy (CN) should be performed for patients with metastatic RCC and VTT. Some studies with few cases believed that CN can improve quality of life or improve OS, so it should be performed (28,35-37), nevertheless the CARMENA study showed that CN does not improve the survival of mRCC patients (38). In our study, metastatic cases accounted for a large proportion of all cases obtained by PSM (61.4%), and the results demonstrate that nephrectomy and thrombectomy predict favourable outcomes in patients with RCC and VTT.
When studying the influence of pathological classification on the prognosis of patients with RCC and VTT, Kim et al. found histological classification to be an important prognostic factor, and patients with type II papillary RCC had a significantly worse prognosis than those with ccRCC (39). Ciancio et al. found that the prognosis of patients with ccRCC and VTT are better than that of patients with non-ccRCC (20). Kaushik et al. found that non-ccRCC is associated with some unfavourable pathological features, but after matching with ccRCC patients, there was no significant difference in survival and recurrence between the two groups (40). In our study, patients with papillary RCC and other non-ccRCC had worse outcomes than those with ccRCC. In the TJH cohort, 7 of the 15 PRCC were further classified and all of them were type II, which may indicate that the majority of PRCC patients with VTT are type II.
The maximum diameter of the renal tumour (tumour size) is the basis for T1-T2 staging, but this parameter is not applied in T3 staging. In the studies from Chen and Tang, tumour size had no significant effect on the survival of patients with RCC and VTT (5,7). However, tumour size was an independent prognostic factor in other studies (9,19,27,41,42). In our study, a greater tumour size indicated a worse prognosis.
Regarding the differences between our institution and SEER database cases, we believe that it may be mainly caused by differences in socioeconomic factors. For example, patients in our institution is relatively younger, which may be mainly because our patients have been screened by local hospitals, and some elderly patients are more likely to choose conservative treatment in local hospitals. Another example is that patients in our institution have larger tumors and more advanced stage, which may be related to the lower frequency of routine physical examinations. In addition, these demographics and clinicopathological parameters were also significantly correlated with receiving surgery, suggesting that urologists prefer to operate on patients with favourable conditions.
There are also some limitations in our study, due to the limitations of the database, we failed to obtain baseline information about the patients; physical condition such as the Eastern Cooperative Oncology Group (ECOG) score, body mass index (BMI), comorbidities, approaches of surgery, and concentration of haemoglobin and albumin. In addition, systemic treatments in the SEER database include chemotherapy, immunotherapy and targeted therapy. The systemic treatments and radiotherapy regimens, type of drugs and start times can’t be identified which may bias the results of the study, and the clinical values of these features may be limited.
Conclusions
Patients developed RCC and VTT in the United States and central China had comparable outcomes. Age at diagnosis, tumour size, location of VTT, histological classification, nuclear grade, N stage, M stage, surgery, and systemic treatments are the nine key prognostic factors for RCC patients with VTT. The nomograms established based on key prognostic factors could predict the survival of patients in America and central China with reliable accuracy.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (No. 81702989).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-128/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-128/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519-30. [Crossref] [PubMed]
- Gu L, Li H, Wang Z, et al. A systematic review and meta-analysis of clinicopathologic factors linked to oncologic outcomes for renal cell carcinoma with tumor thrombus treated by radical nephrectomy with thrombectomy. Cancer Treat Rev 2018;69:112-20. [Crossref] [PubMed]
- Blute ML, Leibovich BC, Lohse CM, et al. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int 2004;94:33-41. [Crossref] [PubMed]
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur Urol 2019;75:799-810. [Crossref] [PubMed]
- Chen X, Li S, Xu Z, et al. Clinical and oncological outcomes in Chinese patients with renal cell carcinoma and venous tumor thrombus extension: single-center experience. World J Surg Oncol 2015;13:14. [Crossref] [PubMed]
- Sidana A, Goyal J, Aggarwal P, et al. Determinants of outcomes after resection of renal cell carcinoma with venous involvement. Int Urol Nephrol 2012;44:1671-9. [Crossref] [PubMed]
- Tang Q, Song Y, Li X, et al. Prognostic Outcomes and Risk Factors for Patients with Renal Cell Carcinoma and Venous Tumor Thrombus after Radical Nephrectomy and Thrombectomy: The Prognostic Significance of Venous Tumor Thrombus Level. Biomed Res Int 2015;2015:163423. [Crossref] [PubMed]
- Niedworok C, Dörrenhaus B, Vom Dorp F, et al. Renal cell carcinoma and tumour thrombus in the inferior vena cava: clinical outcome of 98 consecutive patients and the prognostic value of preoperative parameters. World J Urol 2015;33:1541-52. [Crossref] [PubMed]
- Abel EJ, Masterson TA, Karam JA, et al. Predictive Nomogram for Recurrence following Surgery for Nonmetastatic Renal Cell Cancer with Tumor Thrombus. J Urol 2017;198:810-6. [Crossref] [PubMed]
- Gu L, Wang Z, Chen L, et al. A proposal of post-operative nomogram for overall survival in patients with renal cell carcinoma and venous tumor thrombus. J Surg Oncol 2017;115:905-12. [Crossref] [PubMed]
- Agha RA, Borrelli MR, Vella-Baldacchino M, et al. The STROCSS statement: Strengthening the Reporting of Cohort Studies in Surgery. Int J Surg 2017;46:198-202. [Crossref] [PubMed]
- Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1-73. [Crossref] [PubMed]
- Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594. [Crossref] [PubMed]
- Cronin KA, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer 2014;120:3755-7. [Crossref] [PubMed]
- D'Agostino RB Jr, D'Agostino RB Sr. Estimating treatment effects using observational data. JAMA 2007;297:314-6. [Crossref] [PubMed]
- Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. [Crossref] [PubMed]
- Skinner DG, Pfister RF, Colvin R. Extension of renal cell carcinoma into the vena cava: the rationale for aggressive surgical management. J Urol 1972;107:711-6. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Lambert EH, Pierorazio PM, Shabsigh A, et al. Prognostic risk stratification and clinical outcomes in patients undergoing surgical treatment for renal cell carcinoma with vascular tumor thrombus. Urology 2007;69:1054-8. [Crossref] [PubMed]
- Ciancio G, Manoharan M, Katkoori D, et al. Long-term survival in patients undergoing radical nephrectomy and inferior vena cava thrombectomy: single-center experience. Eur Urol 2010;57:667-72. [Crossref] [PubMed]
- Klatte T, Pantuck AJ, Riggs SB, et al. Prognostic factors for renal cell carcinoma with tumor thrombus extension. J Urol 2007;178:1189-95; discussion 1195. [Crossref] [PubMed]
- Staehler G, Brkovic D. The role of radical surgery for renal cell carcinoma with extension into the vena cava. J Urol 2000;163:1671-5. [Crossref] [PubMed]
- Ljungberg B, Stenling R, Osterdahl B, et al. Vein invasion in renal cell carcinoma: impact on metastatic behavior and survival. J Urol 1995;154:1681-4. [Crossref] [PubMed]
- Zapała Ł, Sharma S, Kunc M, et al. Analysis of Clinicopathological Factors Influencing Survival in Patients with Renal Cell Carcinoma and Venous Tumor Thrombus. J Clin Med 2021;10:3852. [Crossref] [PubMed]
- Shiff B, Breau RH, Mallick R, et al. Prognostic significance of extent of venous tumor thrombus in patients with non-metastatic renal cell carcinoma: Results from a Canadian multi-institutional collaborative. Urol Oncol 2021;39:836.e19-27. [Crossref] [PubMed]
- Chan AA, Abel EJ, Carrasco A, et al. Impact of preoperative renal artery embolization on surgical outcomes and overall survival in patients with renal cell carcinoma and inferior vena cava thrombus. J Urol 2011;185:e707-8. [Crossref]
- Rodriguez Faba O, Linares E, Tilki D, et al. Impact of Microscopic Wall Invasion of the Renal Vein or Inferior Vena Cava on Cancer-specific Survival in Patients with Renal Cell Carcinoma and Tumor Thrombus: A Multi-institutional Analysis from the International Renal Cell Carcinoma-Venous Thrombus Consortium. Eur Urol Focus 2018;4:435-41. [Crossref] [PubMed]
- Abel EJ, Spiess PE, Margulis V, et al. Cytoreductive Nephrectomy for Renal Cell Carcinoma with Venous Tumor Thrombus. J Urol 2017;198:281-8. [Crossref] [PubMed]
- Klaver S, Joniau S, Suy R, et al. Analysis of renal cell carcinoma with subdiaphragmatic macroscopic venous invasion (T3b). BJU Int 2008;101:444-9. [PubMed]
- Kirkali Z, Van Poppel H. A critical analysis of surgery for kidney cancer with vena cava invasion. Eur Urol 2007;52:658-62. [Crossref] [PubMed]
- Gettman MT, Boelter CW, Cheville JC, et al. Charlson co-morbidity index as a predictor of outcome after surgery for renal cell carcinoma with renal vein, vena cava or right atrium extension. J Urol 2003;169:1282-6. [Crossref] [PubMed]
- Libertino JA, Zinman L, Watkins E Jr. Long-term results of resection of renal cell cancer with extension into inferior vena cava. J Urol 1987;137:21-4. [Crossref] [PubMed]
- Moinzadeh A, Libertino JA. Prognostic significance of tumor thrombus level in patients with renal cell carcinoma and venous tumor thrombus extension. Is all T3b the same? J Urol 2004;171:598-601. [Crossref] [PubMed]
- Haferkamp A, Bastian PJ, Jakobi H, et al. Renal cell carcinoma with tumor thrombus extension into the vena cava: prospective long-term followup. J Urol 2007;177:1703-8. [Crossref] [PubMed]
- Lenis AT, Burton CS, Golla V, et al. Cytoreductive nephrectomy in patients with metastatic renal cell carcinoma and venous thrombus-Trends and effect on overall survival. Urol Oncol 2019;37:577.e9-577.e16. [Crossref] [PubMed]
- Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 2001;358:966-70. [Crossref] [PubMed]
- Westesson KE, Klink JC, Rabets JC, et al. Surgical outcomes after cytoreductive nephrectomy with inferior vena cava thrombectomy. Urology 2014;84:1414-9. [Crossref] [PubMed]
- Méjean A, Ravaud A, Thezenas S, et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N Engl J Med 2018;379:417-27. [Crossref] [PubMed]
- Kim KH, You D, Jeong IG, et al. Type II papillary histology predicts poor outcome in patients with renal cell carcinoma and vena cava thrombus. BJU Int 2012;110:E673-8. [Crossref] [PubMed]
- Kaushik D, Linder BJ, Thompson RH, et al. The impact of histology on clinicopathologic outcomes for patients with renal cell carcinoma and venous tumor thrombus: a matched cohort analysis. Urology 2013;82:136-41. [Crossref] [PubMed]
- Wagner B, Patard JJ, Méjean A, et al. Prognostic value of renal vein and inferior vena cava involvement in renal cell carcinoma. Eur Urol 2009;55:452-9. [Crossref] [PubMed]
- Martínez-Salamanca JI, Huang WC, Millán I, et al. Prognostic impact of the 2009 UICC/AJCC TNM staging system for renal cell carcinoma with venous extension. Eur Urol 2011;59:120-7. [Crossref] [PubMed]


