
Development and validation of a nomogram for predicting the overall survival of prostate cancer patients: a large population-based cohort study
Introduction
Prostate cancer (PC) is the second most common malignant tumor diagnosed among men and remains the fifth leading cause of cancer-related deaths worldwide (1,2). An estimated 268,490 new cases and 34,500 cancer-related deaths are reported annually in the United States (1). The 5-year survival rate declines to 31% for PC patients with metastatic disease (3). Therefore, it is imperative to identify PC patients with poor prognoses, so as to implement management regimens to improve their quality of life.
Previous research have identified the factors associated with the prognosis of PC, including expression of the prostate specific antigen (PSA) (4,5). Younger age at diagnosis and being married have also been associated with improved prognosis and survival in PC patients (6-10). To predict patient prognosis more accurately, several models incorporating multiple prognostic factors have been built. Hu et al. developed a prognostic prediction model based on 22 autophagy-related genes expressed in PC patients (11). Han et al. conducted a prognostic nomogram for progression-free survival of 255 PC patients (12). However, the clinical applicability of these models is limited by the need to collect clinical samples and predictive ability. Furthermore, the performance of these prediction models validated in different subgroups has not been investigated.
Herein, a nomogram was developed to predict the long-term survival (3, 5, and 10 years) in 152,796 individuals based on data from the Surveillance, Epidemiology, and End Results (SEER) database. Internal validation and subgroup validation based on marital status and race were performed to assess the predictive performance of the nomogram. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-498/rc).
Methods
Study design and population
This was the development and validation of the nomogram based on a retrospective cohort. Data of PC cases were obtained from the SEER 18 Regs Custom Data (with additional treatment fields) of the National Cancer Institute (http://seer.cancer.gov/), which included cases diagnosed between 2005 and 2010. The SEER registries collect data on patient demographics, primary tumor site, tumor morphology, stage at diagnosis, first course of treatment, and patient vital statistics at follow up (13). The diagnosis of PC was confirmed in accordance with the International Classification of Diseases-Oncology 3 (ICD-O-3) 2008 site codes C8000, C8010, C8012, C8014, C8015, C8021, C8032, C 8041, C8042, C8045, C8140, C8141, C8143, C8200, C8201, C8210, C8246, C8255, C8260, C8310, C8323, C8380, C8480, C8481, C8490, C8500, C8501, C8521, C8523, C8550, C8551, C8560, C8570, C8571, C8574. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The inclusion criteria of this study were as follows: (I) PC patients; and (II) age more than 18 years old. The exclusion criteria were as follows: (I) unknown baseline characteristics (age, race, and marital status); (II) unknown American Joint Committee on Cancer (AJCC) stage [tumor/node/metastasis (T/N/M)], Gleason score, and PSA status; (III) missing or unknown survival status and survival months; and (IV) patients diagnosed with more than one primary cancer.
Data extraction
Demographic data from the SEER database were collated, including age at diagnosis, race (white, black, and others), and marital status (married and un-married). The pathological data collected included the following: pathological stage (distant/localized/regional), AJCC T stage (T1–T4), N stage (N0 and N1), M stage (M0, and M1), PSA levels (ng/mL), Gleason score, and bone metastasis. The survival months and survival status were obtained. The 3-, 5-, and 10-year survival of the PC patients were regarded as the outcomes. The follow up duration was 10 years, and follow-up was terminated when death occurred.
Statistical analysis
All statistical analyses were performed using the R (4.0.3) software. Tests for normality were conducted using the Kolmogorov-Smirnov test. Measurement data were described as mean ± standard deviation (mean ± SD) or median [interquartile range (IQR)]. The t-test or Mann-Whitney U test was used for intergroup comparisons. The statistical significance levels were all two-sided. A P value <0.05 was considered statistically significant.
The patients were randomly divided into a training set (n=107,657) and a testing set (n=46,139). In the training set, univariate and multivariate Cox regression analyses were conducted to identify predictive factors and develop the predictive nomogram. Internal validation was performed using the testing set. The C-index was calculated to assess the predictive performance of the presented nomogram. Subgroup validations based on marital status and race were conducted. The hazard ratio (HR) and 95% confidence interval (CI) were calculated. The time-dependent receiver operating characteristic (ROC) curves at different time points (3, 5, and 10 years) in the whole population and subgroups based on marital status and race were drawn, and the corresponding areas under the ROC curve (AUC) were calculated to assess the predictive effect of the nomogram.
Results
Patient characteristics
A total of 153,796 patients were eventually included in this study, with an average age of 64.99±9.10 years. The patients were divided into a training cohort (n=107,657) and a testing cohort (n=46,139). The detailed procedure for patient selection is presented in Figure 1. There was no significant difference in any of the variables between the training and testing sets (P>0.05), suggesting that the data from the two groups were comparable (Table 1). Regarding ethnicity, there were 23,052 (14.99%) blacks, 122,449 (79.62%) whites, and 8,295 (5.39%) patients of other ethnicities. Of the 153,796 patients, 118,051 (76.76%) were married at diagnosis and 35,745 (23.24%) patients were un-married. The mean follow-up time of the whole patient cohort was 101.52±29.44 months. There were 31,161 (20.26%) deceased patients and 122,635 (79.74%) patients were alive at last follow-up.
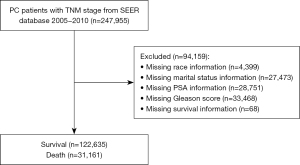
Table 1
| Variables | Total (n=153,796) | Training set (n=107,657) | Testing set (n=46,139) | Statistics | P |
|---|---|---|---|---|---|
| Age at diagnosis (year) | 64.99±9.10 | 64.97±9.09 | 65.04±9.12 | t=1.450 | 0.147 |
| Race | χ2=0.245 | 0.885 | |||
| Black | 23,052 (14.99) | 16,168 (15.02) | 6,884 (14.92) | ||
| White | 122,449 (79.62) | 85,683 (79.59) | 36,766 (79.69) | ||
| Others | 8,295 (5.39) | 5,806 (5.39) | 2,489 (5.39) | ||
| Marital status | χ2=0.096 | 0.756 | |||
| Married | 118,051 (76.76) | 82,612 (76.74) | 35,439 (76.81) | ||
| Un-married | 35,745 (23.24) | 25,045 (23.26) | 10,700 (23.19) | ||
| Pathological stage | χ2=0.001 | 0.977 | |||
| Distant | 3,654 (2.38) | 2,557 (2.38) | 1,097 (2.38) | ||
| Localized/regional | 150,142 (97.62) | 105,100 (97.62) | 45,042 (97.62) | ||
| T stage | χ2=7.184 | 0.066 | |||
| T1 | 56,679 (36.85) | 39,691 (36.87) | 16,988 (36.82) | ||
| T2 | 81,134 (52.75) | 56,639 (52.61) | 24,495 (53.09) | ||
| T3 | 14,278 (9.28) | 10,117 (9.40) | 4,161 (9.02) | ||
| T4 | 1,705 (1.11) | 1,210 (1.12) | 495 (1.07) | ||
| N stage | χ2=0.492 | 0.483 | |||
| N0 | 150,734 (98.01) | 105,496 (97.99) | 45,238 (98.05) | ||
| N1 | 3,062 (1.99) | 2,161 (2.01) | 901 (1.95) | ||
| M stage | χ2=0.034 | 0.854 | |||
| M0 | 150,196 (97.66) | 105,142 (97.66) | 45,054 (97.65) | ||
| M1 | 3,600 (2.34) | 2,515 (2.34) | 1,085 (2.35) | ||
| PSA (ng/mL) | 62 [46, 97] | 62 [46, 97] | 62 [46, 96] | Z=−1.107 | 0.268 |
| Gleason score | 6.74±0.95 | 6.74±0.95 | 6.73±0.94 | t=−1.84 | 0.065 |
| Bone metastasis | χ2=0.007 | 0.934 | |||
| No | 151,279 (98.36) | 105,897 (98.37) | 45,382 (98.36) | ||
| Yes | 2,517 (1.64) | 1,760 (1.63) | 757 (1.64) | ||
| Survival month | 101.52±29.44 | 101.49±29.50 | 101.60±29.29 | t=0.710 | 0.479 |
| Survival status | χ2=0.082 | 0.775 | |||
| Alive | 122,635 (79.74) | 85,865 (79.76) | 36,770 (79.69) | ||
| Dead | 31,161 (20.26) | 21,792 (20.24) | 9,369 (20.31) |
The data are expressed as mean ± SD or n (%) or M [Q1, Q3]. PC, prostate cancer; SD, standard deviation; T, tumor; N, node; M, metastasis; PSA, prostate-specific antigen.
Selection of predictive factors and construction of a predictive nomogram
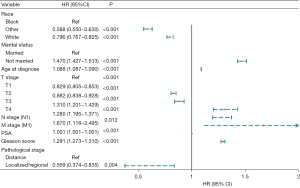
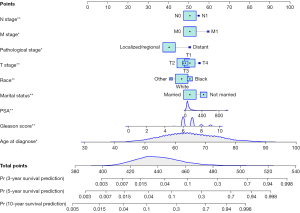
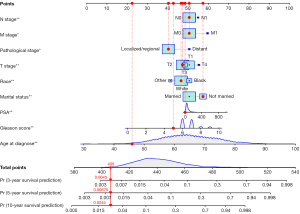
Univariate Cox regression analyses of the training dataset revealed that age at diagnosis, race, marital status, TNM stage, PSA, Gleason score, pathological stage, and bone metastasis were significant predictive factors (P<0.05; Table 2). Furthermore, multivariate Cox stepwise regression analyses demonstrated that age at diagnosis, race, marital status, TNM stage, PSA, Gleason score, and pathological stage were significantly associated with the survival of patients with PC (P<0.05; Figure 2). Based on these predictors, a nomogram for survival prediction in PC patients was established (Figure 3).
Table 2
| Variables | β | Z | SE | P | HR (95% CI) |
|---|---|---|---|---|---|
| Race | |||||
| Black | Ref | ||||
| White | −0.211 | −11.706 | 0.018 | <0.001 | 0.810 (0.782–0.839) |
| Others | −0.257 | −7.455 | 0.034 | <0.001 | 0.774 (0.723–0.828) |
| Marital status | |||||
| Married | Ref | ||||
| Un-married | 0.527 | 36.274 | 0.015 | <0.001 | 1.693 (1.646–1.742) |
| Age at diagnosis | 0.098 | 122.220 | 0.001 | <0.001 | 1.103 (1.102–1.105) |
| T stage | |||||
| T1 | Ref | ||||
| T2 | −0.453 | −31.341 | 0.014 | <0.001 | 0.636 (0.618–0.654) |
| T3 | −0.240 | −9.792 | 0.025 | <0.001 | 0.786 (0.749–0.825) |
| T4 | 1.158 | 28.677 | 0.040 | <0.001 | 3.183 (2.940–3.445) |
| N stage (N1) | 1.330 | 43.694 | 0.030 | <0.001 | 3.780 (3.561–4.012) |
| M stage (M1) | 2.510 | 108.407 | 0.023 | <0.001 | 12.301 (11.756–12.872) |
| PSA | 0.003 | 113.714 | <0.001 | <0.001 | 1.003 (1.003–1.003) |
| Gleason score | 0.515 | 80.333 | 0.006 | <0.001 | 1.674 (1.653–1.695) |
| Pathological stage | |||||
| Distance | Ref | – | – | – | – |
| Localized/regional | −2.494 | −108.33 | 0.023 | <0.001 | 0.083 (0.079–0.086) |
| Bone metastasis (yes) | 2.498 | 92.462 | 0.027 | <0.001 | 12.160 (11.533–12.821) |
SE, standard error; HR, hazard ratio; CI, confidence interval; T, tumor; N, node; M, metastasis; PSA, prostate-specific antigen; Ref, reference.
Predictive performance of the nomogram
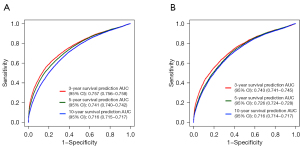
The C-indexes of the nomogram in the training and testing sets were 0.782 (95% CI: 0.779–0.785) and 0.782 (95% CI: 0.777–0.787), respectively. In the training set, the AUCs for the prognostic nomogram at 3, 5, and 10 years were 0.757 (95% CI: 0.756–0.758), 0.741 (95% CI: 0.740–0.742), and 0.716 (95% CI: 0.715–0.717), respectively. In the testing set, the AUCs for the prognostic nomogram at 3, 5, and 10 years were 0.743 (95% CI: 0.741–0.745), 0.726 (95% CI: 0.724–0.728), and 0.716 (95% CI: 0.714–0.717), respectively. These results demonstrated that the nomogram exhibited a good predictive performance (Figure 4).
Validation for the predictive performance of nomogram in different subgroups based on marital status and race
For subgroup validation based on marital status, the C-index of the nomogram was 0.784 (95% CI: 0.778–0.790) among married patients and 0.757 (95% CI: 0.747–0.767) among non-married patients. In the married group, the AUCs for the nomogram at 3, 5, and 10 years were 0.747 (95% CI: 0.745–0.749), 0.739 (95% CI: 0.737–0.741), and 0.713 (95% CI: 0.710–0.715), respectively. In non-married patients, the AUCs for the nomogram at 3, 5, and 10 years were 0.730 (95% CI: 0.726–0.734), 0.725 (95% CI: 0.721–0.728), and 0.711 (95% CI: 0.707–0.715), respectively. These results demonstrated that the nomogram had a good predictive performance in populations with different marital status (Figure 5).
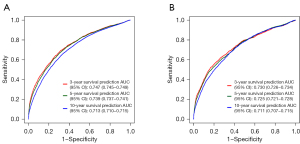
For subgroup validation based on ethnicity, the C-index of the nomogram was 0.788 (95% CI: 0.782–0.794) among whites, 0.750 (95% CI: 0.738–0.762) in blacks, and 0.783 (95% CI: 0.761–0.805) in other races. In the white population, the AUCs for the nomogram at 3, 5, and 10 years were 0.746 (95% CI: 0.745–0.748), 0.739 (95% CI: 0.737–0.741), and 0.716 (95% CI: 0.714–0.718), respectively. Among the black population, the AUCs for the nomogram at 3, 5, and 10 years were 0.739 (95% CI: 0.734–0.743), 0.733 (95% CI: 0.728–0.737), and 0.707 (95% CI: 0.702–0.712), respectively. In other races, the AUCs for the nomogram at 3, 5, and 10 years were 0.782 (95% CI: 0.775–0.789), 0.760 (95% CI: 0.753–0.767), and 0.739 (95% CI: 0.731–0.747), respectively. These results suggested that the nomogram had a good predictive performance regardless of ethnicity (Figure 6).

Example
To further validate the nomogram, we examined a divorced white patient who was diagnosed with PC at the age of 46 years. He had a pathological stage classification of localize/regional, a PSA level of 13 ng/100 mL, a Gleason score of 5, and T2/M0/N0 stage tumor. According to our nomogram, the patient had a total score of 406 points, and the predicted risk of death within 3, 5, and 10 years were 0.0045, 0.00875, and 0.0244, respectively. The patient’s actual survival time was 137 months, which was consistent with the prediction from the nomogram (Figure 7).
Discussion
In this study, a novel prediction nomogram for the long-term survival (3-, 5-, and 10-year survival) of PC patients was developed and validated. The selected predictive factors included age at diagnosis, race, marital status, TNM stage, PSA levels, Gleason score, and pathological stage. The predictive tool for survival in PC patients was established based on the above predictors, with a C-index of 0.782 (95% CI: 0.779–0.785) and validation with the testing set showed a C-index of 0.782 (95% CI: 0.777–0.787). The AUCs of the model at 3, 5, and 10 years were 0.757 (95% CI: 0.756–0.758), 0.741 (95% CI: 0.740–0.742), and 0.716 (95% CI: 0.715–0.717), respectively. Validation with the testing set showed AUCs of 0.743 (95% CI: 0.741–0.745), 0.726 (95% CI: 0.724–0.728), and 0.716 (95% CI: 0.714–0.717) for the 3-, 5-, and 10-year survival, respectively. Additionally, subgroup validations based on marital status and race showed good predictive performances of the nomogram.
This research showed that patients in the state of being married had a better prognosis than patients who were un-married. The association between marital status and survival may be attributed to the following plausible explanations: (I) married cases are likely to be in a better economic situation and have a higher degree of education compared to unmarried patients, and this may be associated with improved adherence to treatments (14); (II) unmarried patients are more likely to develop metastatic disease compared to married cases (15); and (III) there is a positive relationship between marriage and the possibility of early diagnosis of all types of cancer, and indeed, unmarried patients diagnosed with cancer are at higher risk of progression to advanced cancer and typically present with a shorter life expectancy compared to married patients (16). Hence, more effort should be focused on improving the long-term survival of unmarried individuals. Our results showed that increased age at diagnosis is associated with poor prognosis among PC patients, which is consistent with a previous study (6). It was noted that black patients had poorer prognosis compared to white patients. A previous study found that the CAG repeat length was shorter in black patients compared to white patients, and this was thought to be the reason for their higher risk of death (17).
This investigation demonstrated that PSA, Gleason score, TNM stage, and pathological stage were associated with long-term survival among PC patients. The TNM staging system has been widely used to assess the prognosis of cancer patients (18). It is worth noting that PC patients with T4 stage tumors have a higher risk of death than those with T1 stage tumors, while patients with T2 or T3 stage tumors have a better prognosis compared to those with T1 stage tumors. Some studies indicated that shortened overall survival may be related to the presence of bone metastasis among PC patients (19-21). Indeed, Lu et al. have mentioned that PC patients with stage T4 cancer have the highest risk of bone metastasis, and T2 or T3 patients have a lower risk of being diagnosed with bone metastasis than T1 patients (22). Further in-depth studies are warranted to explore the underlying reasons behind the differences in survival. PSA is a sensitive indicator for the evaluation of the therapeutic effect or even for the prognostic assessment of PC patients (23,24). Previous studies suggested that there is a negative correlation between PSA levels and risk of death (25,26), which is consistent with our results showing that higher levels of PSA is a risk factor for survival among PC patients. Consistent with a previous study (27), the results herein demonstrated that the Gleason scoring system, as the most widely used pathological grading criteria for PC, is a prognostic predictor for survival in PC patients.
Several models have been developed to estimate the survival of PC patients. Schmidt et al. reported the success of using 4-miRNA [(miR-23a-3p × miR-10b-5p)/(miR-133a × miR-374b-5p)] for the prediction of outcomes in PC patients (28). However, the inconvenience of clinical collection of predictive indicators used in this latter model would increase the economic burden of patients and restrict its clinical application. Our nomogram was established using clinical features that are easily collected in the clinical setting. Another model proposed by Wang et al., combines the method of treatment, hyperintensity within the prostate on diffusion-weighted imaging (DWI), and the metastasis burden of pelvic lymph nodes to assess the survival of PC patients (29). However, the predictive performance of this system was not evaluated and the small sample size (n=121) may weaken the reliability of this model. In contrast, the model constructed herein, was based on a larger sample size (n=16,775) and had a moderate predictive power after internal validation and subgroup analysis.
The nomogram designed in this report may be used effectively in predicting the long-term survival of PC patients. However, there were several limitations to this study. First, specific information of patients related to the survival of PC, such as certain biological indicators and behavioral habits, could not be collected from the SEER database. Second, long-term survival predicted by this nomogram may be affected by new treatment approaches, and more rigorously designed studies are needed to validate this model in the future. Third, there was no external validation in our study and future investigations should include this to verify the predictive ability of the presented model.
Conclusions
This study demonstrated that age, race, marital status, TNM stage, PSA, Gleason score, and pathological stage are associated with the survival of PC patients. Based on these predictors, a nomogram for the long-term survival (3-, 5-, and 10-year survival) of PC patients was developed and validated, with good predictive performance. This nomogram may be useful in the management of PC patients.
Acknowledgments
Funding: The study was supported by Suzhou Science and Technology Planning Project (No. SLJ2021002).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-498/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-498/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Sandhu S, Moore CM, Chiong E, et al. Prostate cancer. Lancet 2021;398:1075-90. [Crossref] [PubMed]
- Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin 2022; Epub ahead of print. [Crossref] [PubMed]
- Liu D, Kuai Y, Zhu R, et al. Prognosis of prostate cancer and bone metastasis pattern of patients: a SEER-based study and a local hospital based study from China. Sci Rep 2020;10:9104. [Crossref] [PubMed]
- Parol M, Gzil A, Bodnar M, et al. Systematic review and meta-analysis of the prognostic significance of microRNAs related to metastatic and EMT process among prostate cancer patients. J Transl Med 2021;19:28. [Crossref] [PubMed]
- Pettersson A, Robinson D, Garmo H, et al. Age at diagnosis and prostate cancer treatment and prognosis: a population-based cohort study. Ann Oncol 2018;29:377-85. [Crossref] [PubMed]
- Yasui M, Uemura K, Yoneyama S, et al. Predictors of poor response to secondary alternative antiandrogen therapy with flutamide in metastatic castration-resistant prostate cancer. Jpn J Clin Oncol 2016;46:1042-6. [Crossref] [PubMed]
- Liu Y, Xia Q, Xia J, et al. The impact of marriage on the overall survival of prostate cancer patients: A Surveillance, Epidemiology, and End Results (SEER) analysis. Can Urol Assoc J 2019;13:E135-9. [PubMed]
- Abdollah F, Sun M, Thuret R, et al. The effect of marital status on stage and survival of prostate cancer patients treated with radical prostatectomy: a population-based study. Cancer Causes Control 2011;22:1085-95. [Crossref] [PubMed]
- Deng Y, Bi R, Zhu Z, et al. A Surveillance, Epidemiology and End Results database analysis of the prognostic value of organ-specific metastases in patients with advanced prostatic adenocarcinoma. Oncol Lett 2019;18:1057-70. [Crossref] [PubMed]
- Hu D, Jiang L, Luo S, et al. Development of an autophagy-related gene expression signature for prognosis prediction in prostate cancer patients. J Transl Med 2020;18:160. [Crossref] [PubMed]
- Han Y, Wen X, Chen D, et al. Survival analysis and a novel nomogram model for progression-free survival in patients with prostate cancer. J Oncol 2022;2022:6358707. [Crossref] [PubMed]
- Cronin KA, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer 2014;120:3755-7. [Crossref] [PubMed]
- Cohen SD, Sharma T, Acquaviva K, et al. Social support and chronic kidney disease: an update. Adv Chronic Kidney Dis 2007;14:335-44. [Crossref] [PubMed]
- Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol 2013;31:3869-76. [Crossref] [PubMed]
- Buja A, Lago L, Lago S, et al. Marital status and stage of cancer at diagnosis: A systematic review. Eur J Cancer Care (Engl) 2018;27: [Crossref] [PubMed]
- Giovannucci E, Stampfer MJ, Krithivas K, et al. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci U S A 1997;94:3320-3. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Som A, Tu SM, Liu J, et al. Response in bone turnover markers during therapy predicts overall survival in patients with metastatic prostate cancer: analysis of three clinical trials. Br J Cancer 2012;107:1547-53. [Crossref] [PubMed]
- Tait C, Moore D, Hodgson C, et al. Quantification of skeletal metastases in castrate-resistant prostate cancer predicts progression-free and overall survival. BJU Int 2014;114:E70-3. [Crossref] [PubMed]
- Fizazi K, Massard C, Smith M, et al. Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol 2015;68:42-50. [Crossref] [PubMed]
- Lu YJ, Duan WM. Establishment and validation of a novel predictive model to quantify the risk of bone metastasis in patients with prostate cancer. Transl Androl Urol 2021;10:310-25. [Crossref] [PubMed]
- Tikkinen KAO, Dahm P, Lytvyn L, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a clinical practice guideline. BMJ 2018;362:k3581. [Crossref] [PubMed]
- Van Poppel H, Roobol MJ, Chapple CR, et al. Prostate-specific antigen testing as part of a risk-adapted early detection strategy for prostate cancer: European Association of Urology Position and Recommendations for 2021. Eur Urol 2021;80:703-11. [Crossref] [PubMed]
- Ikuemonisan J, Lediju O, Togun A, et al. Association between preoperative prostate-specific antigen levels and mortality in high- and intermediate-grade prostate cancer patients who received radical prostatectomy: Findings from the SEER database. Prostate Int 2021;9:72-7. [Crossref] [PubMed]
- Van den Broeck T, van den Bergh RCN, Arfi N, et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: A systematic review. Eur Urol 2019;75:967-87. [Crossref] [PubMed]
- Guo X, Zhang C, Guo Q, et al. The homogeneous and heterogeneous risk factors for the morbidity and prognosis of bone metastasis in patients with prostate cancer. Cancer Manag Res 2018;10:1639-46. [Crossref] [PubMed]
- Schmidt L, Fredsøe J, Kristensen H, et al. Training and validation of a novel 4-miRNA ratio model (MiCaP) for prediction of postoperative outcome in prostate cancer patients. Ann Oncol 2018;29:2003-9. [Crossref] [PubMed]
- Wang Y, Wu G, Fan L, et al. The prognostic nomogram including MRI for locally advanced prostate cancer treated by radical prostatectomy. Prostate 2021;81:463-8. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


