
The clinical efficacy and limitations of dutasteride-regulated abiraterone metabolism in abiraterone-resistant patients: a prospective single-arm clinical trial in Chinese patients
Introduction
Prostate cancer is the second most common cancer in men worldwide (1). Androgen precursors, mainly originating from the adrenal gland, stimulate the development of castration-resistant prostate cancer (CRPC) after androgen deprivation therapy (ADT) (2-5). Abiraterone has been used to treat metastatic CRPC (mCRPC) by targeting the steroidogenic enzyme cytochrome P450 17A1 (CYP17A) (6-10). Moreover, abiraterone causes a significant reduction in circulating dehydroepiandrosterone (DHEA) level and prolongs overall survival (11). However, abiraterone resistance is inevitable, and limited clinical approaches are currently available for further disease management.
A novel metabolic pathway of steroidal medicine in patients has been discovered (12-14). Abiraterone could be catalyzed by the steroidogenic enzyme 3β-hydroxysteroid dehydrogenase 1 (3βHSD1) to generate ∆4-abiraterone (D4A), which has more potent antitumor activity (13). However, D4A is converted to 5α-abiraterone by steroid-5α-reductase (SRD5A), which prevents D4A accumulation in patients. The metabolite 5α-abiraterone (5α-Abi) binds to the androgen receptor (AR) directly and acts as a mild agonist (12). Thus, the conversion from D4A to 5α-Abi has been speculated to be a potential mechanism of abiraterone resistance (12).
It has been reported recently that abiraterone metabolism accelerated and abiraterone concentration decreased in patients as disease progression, indicating the involvement of abiraterone metabolism in drug resistance (15). However, increasing abiraterone dose failed to elevate abiraterone concentration and overcome abiraterone resistance in patients (16). Inhibition of abiraterone metabolism seems to be an alternative way to increase abiraterone concentration.
Dutasteride is a potent SRD5A inhibitor used for the treatment of benign prostatic hyperplasia (17). The REDUCE clinical trial has shown that dutasteride reduces the incidence of prostate cancer (18). However, there are concerns that dutasteride might increase the risk of high-grade cancer, which prevents its application in prostate cancer management. It has been reported that dutasteride (3.5 mg/day) inhibits the conversion of D4A to 5α-Abi in patients by suppressing the activity of SRD5A (12). Although dutasteride has been used in CRPC patients together with abiraterone (NCT01393730), the clinical efficacy of dutasteride in abiraterone-resistant patients has not been evaluated. Also, the side effects of the combination of dutasteride and abiraterone has not been investigated in Chinese patients. The potential benefits of dutasteride-regulated abiraterone metabolism rely on the reduction of the AR agonist 5α-Abi, the increase of plasma abiraterone and D4A levels, and the prolongation of the half-life of abiraterone in patients. A previous case report indicated the potential benefits of dutasteride in 2 abiraterone-resistant patients (19). Hence, we conducted a single-arm, open-label clinical trial to investigate the clinical efficacy and safety of dutasteride in abiraterone-resistant patients with mCRPC. We present the following article in accordance with the TREND reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-507/rc).
Methods
Study design
This investigation was conducted according to Declaration of Helsinki principles (as revised in 2013). Informed consent was obtained from patients. The study was approved by institutional review board of Tongji Hospital (No. 2018-LCYJ-003). Patients with mCRPC were eligible if they had abiraterone tolerance according to prostate-specific antigen (PSA) progression or radiographic progression as defined by the Prostate Cancer Clinical Trials Working Group 2 (PCWG2), an estimated life expectancy of ≥12 months, and an ECOG of ≤3. Patients were excluded if they had previously received next-generation AR inhibitors such as enzalutamide or apalutamide, had a special histologic type such as small cell carcinoma or sarcoma of prostate, had a history of aggressive cancer within 3 years before screening, or had total bilirubin (TB) ≥ 1.5 × institutional upper limit of normal (ULN) or alanine aminotransferase (ALT) ≥ 2.5 × institutional ULN.
Treatment and follow up
Dutasteride (0.5 mg/day) was administered together with abiraterone (1,000 mg/day) and prednisone (5 mg/twice daily) to mCRPC patients with abiraterone resistance in this single-arm clinical trial (ChiCTR1800015510). Visits occurred and PSA was measured every 4 weeks. Radiographic examinations included the bone scan and computed tomography (CT) were performed every 3 months after the treatment. The Eastern Cooperative Oncology Group (ECOG) performance status was used to assess the general situation of the patients. Based on the pharmacokinetics of abiraterone metabolism, blood samples were collected approximately 3 hours after abiraterone administration (18). Plasma concentrations of abiraterone and its metabolites were determined with liquid chromatography-tandem mass spectrometry (LC-MS) as described previously (19). The extent of the worst pain over the previous 24 hours was assessed by the Brief Pain Inventory-Short Form (BPI-SF; on a scale of 0 to 10, 0 indicates no pain, 1–3 indicates mild pain, 4–6 indicates moderate pain, and 7–10 indicates severe pain). The Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 were used to assess the safety of the treatment, and grade 3 or grade 4 toxicities were defined as severe adverse events. The conditions for study termination included disease progression (on the basis of PSA concentration, radiographic imaging, and clinical findings), unacceptable adverse events, death, and withdrawal of consent.
End points
The primary endpoints were to evaluate PSA response (defined as ≥50% decline from baseline, PSA50). PSA progression was defined as a 25% increase above the nadir along with an increase in absolute value of 2 ng/mL and confirmed 3 weeks later. For patients without an initial decline in PSA, PSA progression was defined as a 25% increase from the baseline value along with an increase in absolute value of 2 ng/mL or more after 12 weeks of treatment. Radiographic progression was defined by PCWG2 for bone disease and by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 for soft tissue and visceral disease and was assessed by 2 senior radiologists. The secondary objectives were to assess symptom relief and safety of dutasteride according to BPI-SF and CTCAE5.0.
Sample size
We calculated that a sample of 42 patients (with 40 events) would provide the trial with 80% power to detect the difference regarding the primary outcome at a one-sided alpha of 0.025, assuming that the median PSA progression free survival (PSA-PFS) time was 3 months for patients without treatment and 4.8 months for patients with dutasteride and abiraterone treatment. The accrual period was about 2 years and the follow-up time was about 1 year. The survival distribution was assumed to be exponentially distributed. Assuming a 15% loss to follow-up, the trial was designed to enroll approximately 50 patients.
Statistics
The comparison of multi-group paired variables was conducted using the Friedman’s Two-Way Analysis. PSA-PFS was calculated using the Kaplan-Meier method. Statistical analyses between groups were performed using the Student’s t-test or the chi-square test according to the data distribution feature. Correlations were determined using Pearson analysis; All data analyses were performed using IBM SPSS Statistics 22.0 for Windows (IBM Corp, Inc., Armonk, NY, USA), and the significance level was set at P<0.05.
Results
Patient characteristics
Twenty-two patients with mCRPC were enrolled in the study between April 2018 and June 2020 at Shanghai Tongji Hospital, and 19 patients finished the treatment and follow-up (Figure 1). The clinical characteristics of the patients at baseline are presented in Table 1. The median age of the 19 patients was 75 years (range, 54–84 years). All patients received ADT and abiraterone treatment. Five (26%) of these patients had previously undergone docetaxel therapy. All patients had bone metastatic disease, and 2 patients (11%) had a combination of bone and lymph node metastatic disease at the time of study enrollment. The median baseline PSA and Gleason score are listed in Table 1. Six (32%) patients were primarily resistant to abiraterone, and the remaining patients responded to abiraterone for 4–37 months (Table 2).
Table 1
| Variables | Number (n=19) |
|---|---|
| Age (years), median (range) | 75 (54 to 84) |
| Gleason, n [%] | |
| <8 | 3 [16] |
| ≥8 | 16 [84] |
| Metastatic sites, n [%] | |
| Lymph node | 2 [11] |
| Bone | 19 [100] |
| Previous therapy, n [%] | |
| ADT method | |
| LHRHa | 14 [74] |
| Orchiectomy | 5 [26] |
| Docetaxel | 5 [26] |
| ECOG, n [%] | |
| 0–1 | 8 [42] |
| 2–3 | 11 [58] |
| Primary PSA (ng/mL), median (range) | 51.50 (5.71–1,255.00) |
ADT, androgen deprivation therapy; LHRHa, luteinizing hormone-releasing hormone analogue; ECOG, Eastern Cooperative Oncology Group; PSA, prostate specific antigen.
Table 2
| Respond | Patient No. | Abi treatment | Abi + Dut | |||
|---|---|---|---|---|---|---|
| Max PSA reduction (%) | PFS (days) | Initial PSA | Max PSA reduction (%) | |||
| Patients with PSA decline | #1 | −96.8 | 1,121 | 5.71 | −1.9 | |
| #2 | −99 | 549 | 10.24 | −3.4 | ||
| #3 | −85.6 | 329 | 1255 | −5.1 | ||
| #4 | −76 | 184 | 28.33 | −13.6 | ||
| #5 | −94.2 | 118 | 283 | −17 | ||
| #6 | NA | 0 | 102.1 | −27 | ||
| #7 | NA | 0 | 33.8 | −32 | ||
| Patients without PSA decline | #1 | −82.7 | 294 | 7.36 | 115 | |
| #9 | −68 | 173 | 12.11 | 102 | ||
| #10 | NA | 0 | 118.8 | 78.5 | ||
| #11 | −87.7 | 235 | 10.34 | 69.6 | ||
| #12 | −84.2 | 347 | 51.45 | 59.6 | ||
| #13 | −98.5 | 196 | 22.37 | 50.6 | ||
| #14 | NA | 0 | 17.4 | 43 | ||
| #15 | NA | 0 | 51.5 | 29.7 | ||
| #16 | −86 | 119 | 289.1 | 23.5 | ||
| #17 | −45 | 119 | 100 | 23.3 | ||
| #18 | −54 | 125 | 3372 | 14.5 | ||
| #19 | NA | 0 | 12.7 | 3.5 | ||
Abi, abiraterone; Dut, dutasteride; PSA, prostate specific antigen; PFS, progression free survival; N/A, not applicable.
Patient response to the combination therapy
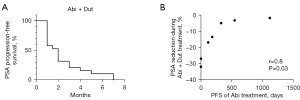
The median treatment duration was 4.0 months (range, 3–18 months), a maximum reduction in PSA levels was observed in 7 (37%, range, −2% to −32%; Table 2) out of 19 patients. One abiraterone-resistant patient (5%) achieved a 30% PSA decline with the combination therapy of dutasteride and abiraterone (Table 2). The median PSA-PFS of the combination therapy was 2.0 months (range, 1–7 months; Figure 2A). Further analysis in the 7 patients who showed declined PSA after the combination therapy indicated a reverse correlation between response to abiraterone treatment and response to the combination therapy. Patients with a longer PSA-PFS during previous abiraterone treatment showed less reduction in PSA levels during the course of the combination therapy (Figure 2B; Table 2). Patients #6 and #7, who had primary resistance to abiraterone, experienced the most striking PSA decline after the combination therapy (−27% and −32%, respectively), indicating the existence of abiraterone metabolism-related drug resistance in patients (Table 2).
ECOG performance status and bone pain symptoms were evaluated after 3 months of combination treatment. No significant differences were observed before and after the combination treatment in ECOG performance (Table 3). The relief of bone pain was observed in 6 patients, but no significant improvement was confirmed overall, possibly due to the limited patient numbers (Table 3). Dutasteride halted the progression in ECOG performance and bone pain in abiraterone-resistant patients, although the effect was mild. These results showed that a mild efficacy of the combination therapy, and the study was terminated.
Table 3
| Events | Pre-treatment | 1st month | 2nd month | 3rd month | Test statistics | P |
|---|---|---|---|---|---|---|
| ECOG, median (IQR) | 2.0 (1 to 2) | 2.0 (1 to 2) | 2.0 (1 to 3) | 2.0 (1 to 3) | 5.462 | 0.141 |
| Bone pain n (%) | 4.385 | 0.223 | ||||
| No | 6 (31.6) | 7 (36.8) | 7 (36.9) | 9 (47.4) | ||
| Mild | 6 (31.6) | 9 (47.4) | 7 (36.8) | 5 (26.3) | ||
| Moderate | 7 (36.8) | 3 (15.8) | 5 (26.3) | 4 (21.1) | ||
| Severe | 0 (0.0) | 0 (0.0) | 0 (0) | 1 (5.3) |
ECOG, Eastern Cooperative Oncology Group performance status; IQR, interquartile range.
Side effects
A total of 19 adverse events were observed in patients receiving the combination therapy of dutasteride and abiraterone. Adverse events included nausea/vomiting (n=5, 26%), fluid retention and edema (n=5, 26%), novel anemia (n=4, 21%), fatigue (n=3, 16%), and constipation (n=2, 11%; Table 4). No grade 3 or grade 4 toxicities were observed in our study. This indicated that the combination therapy was well-tolerated in the Chinese population.
Table 4
| Adverse events | Number (%) |
|---|---|
| Fluid retention and edema | 5 (26.3) |
| Nausea/vomiting | 5 (26.3) |
| Novel anemia | 4 (21.1) |
| Fatigue | 3 (15.8) |
| Constipation | 2 (10.5) |
Discussion
Novel approaches are urgently required to meet the clinical challenges associated with abiraterone resistance (5,15,20). It is hypothesized that dutasteride might enhance abiraterone efficacy by regulating abiraterone metabolism (12). Here, we conducted a clinical trial to investigate the efficacy of dutasteride in abiraterone-resistant mCRPC patients and found that the combination therapy with dutasteride and abiraterone resulted in a mild and transient effect in the East Asian population.
The steroidogenic enzyme SRD5A participates in abiraterone metabolism and catalyzes D4A to 5α-Abi, leading to a decline in plasma abiraterone and D4A and an accumulation of plasma 5α-Abi (13). Studies investigating the crystal structures of SRD5A have revealed how the polymorphisms in SRD5A1 and SRD5A2 genes affect enzyme activity (21,22). Dutasteride is a potent SRD5A inhibitor that is able to block the generation of 5α-Abi in patients (12). Here, our results indicated a transient and mild effect of dutasteride in abiraterone-resistant patients, which might be unique to Chinese patients. Population genome sequencing projects have indicated relatively low SRD5A activity in the Chinese population. For example, the SRD5A2 gene rs523349 (L89V), encoding a gain-of-function variant, is more dominant in African (77%) and Caucasian (72%) populations but not in the Chinese population (43%) (23). A relatively low 5α-Abi level in Chinese patients has been reported previously (19,24).
In this study, the most dramatic reductions in PSA levels were observed in 2 patients with primary resistance to abiraterone, which indicated the existence of drug metabolism-related resistance in the patients. To overcome abiraterone resistance by regulating drug metabolism, novel targets should be considered to regulate abiraterone metabolism more thoroughly. The steroidogenic enzyme 3βHSD1 catalyzes the first step of abiraterone metabolism to generate D4A (13). The clinical significance of 3βHSD1 in disease progression and treatment response has been reported recently (25-27). Targeting 3βHSD1 might be a promising strategy to regulate abiraterone metabolism and steroidogenesis simultaneously for prostate cancer treatment.
The timing of the combination therapy might also affect its efficacy. Dutasteride was added after previous resistance to abiraterone in our trial. Multiple resistant mechanisms, including an increase in the prevalence of AR splice variant 7 and the incidence of neuroendocrine prostate cancer, might be attributable to abiraterone resistance (28-30). Increasing progesterone levels after abiraterone treatment might also fuel disease progression via multiple mechanisms. Dutasteride is well tolerated and leads to limited side effects (18). Administration of dutasteride to patients at the beginning of abiraterone treatment, but not after the development of abiraterone resistance, is feasible and might result in a better clinical response.
The limitations of this study included a lack of patient diversity and a small sample size. Only Chinese patients were recruited in this single-center, single-arm trial. Therefore, it is necessary to conduct a similar clinical trial in patients from Western countries, where the population has robust SRD5A activity. This trial ended early with 22 but not 50 patients as designed after abiraterone metabolites were detected in these patients. Less 5α-abiraterone was generated in Chinese patients, making dutasteride losing its target.
Conclusions
Overall, our data confirmed the existence of drug metabolism-related resistance in patients with mCRPC. Combination therapy with dutasteride and abiraterone resulted in a mild and transient clinical benefit to abiraterone-resistant Chinese patients.
Acknowledgments
We thank the patients and their families for participating in this study. We are also grateful to the members of Shanghai Tongji Hospital for their support.
Funding: The study was supported by New Frontier Technology Joint Research Project of Shanghai Municipal Hospital (No. SHDC 12019112), Natural Science Foundation of Shanghai Municipal Science and Technology Committee (No. 21ZR1458300 and No. 22ZR1456800).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-507/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-507/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-507/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This investigation was conducted according to Declaration of Helsinki principles (as revised in 2013). Informed consent was obtained from patients. The study was approved by institutional review board of Tongji Hospital (No. 2018-LCYJ-003)
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Sharifi N, Auchus RJ. Steroid biosynthesis and prostate cancer. Steroids 2012;77:719-26. [Crossref] [PubMed]
- Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008;68:4447-54. [Crossref] [PubMed]
- Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res 2011;71:6503-13. [Crossref] [PubMed]
- Hou Z, Huang S, Li Z. Androgens in prostate cancer: A tale that never ends. Cancer Lett 2021;516:1-12. [Crossref] [PubMed]
- Barrie SE, Potter GA, Goddard PM, et al. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase). J Steroid Biochem Mol Biol 1994;50:267-73. [Crossref] [PubMed]
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-2005. [Crossref] [PubMed]
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017;377:352-60. [Crossref] [PubMed]
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138-48. [Crossref] [PubMed]
- Sun Y, Zou Q, Sun Z, et al. Abiraterone acetate for metastatic castration-resistant prostate cancer after docetaxel failure: A randomized, double-blind, placebo-controlled phase 3 bridging study. Int J Urol 2016;23:404-11. [Crossref] [PubMed]
- Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 2008;26:4563-71. [Crossref] [PubMed]
- Li Z, Alyamani M, Li J, et al. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature 2016;533:547-51. [Crossref] [PubMed]
- Li Z, Bishop AC, Alyamani M, et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature 2015;523:347-51. [Crossref] [PubMed]
- Alyamani M, Li Z, Berk M, et al. Steroidogenic Metabolism of Galeterone Reveals a Diversity of Biochemical Activities. Cell Chem Biol 2017;24:825-832.e6. [Crossref] [PubMed]
- Mei Z, Yang T, Liu Y, et al. Management of prostate cancer by targeting 3betaHSD1 after enzalutamide and abiraterone treatment. Cell Rep Med 2022;3:100608. [Crossref] [PubMed]
- Friedlander TW, Graff JN, Zejnullahu K, et al. High-Dose Abiraterone Acetate in Men With Castration Resistant Prostate Cancer. Clin Genitourin Cancer 2017;15:733-741.e1. [Crossref] [PubMed]
- O'Leary MP, Roehrborn C, Andriole G, et al. Improvements in benign prostatic hyperplasia-specific quality of life with dutasteride, the novel dual 5alpha-reductase inhibitor. BJU Int 2003;92:262-6. [Crossref] [PubMed]
- Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010;362:1192-202. [Crossref] [PubMed]
- Lian JP, Gao YY, Tang JJ, et al. Response of prostate cancer to addition of dutasteride after progression on abiraterone. Asian J Androl 2021;23:222-3. [Crossref] [PubMed]
- Hou Z, Huang S, Mei Z, et al. Inhibiting 3betaHSD1 to eliminate the oncogenic effects of progesterone in prostate cancer. Cell Rep Med 2022;3:100561. [Crossref] [PubMed]
- Han Y, Zhuang Q, Sun B, et al. Crystal structure of steroid reductase SRD5A reveals conserved steroid reduction mechanism. Nat Commun 2021;12:449. [Crossref] [PubMed]
- Xiao Q, Wang L, Supekar S, et al. Structure of human steroid 5alpha-reductase 2 with the anti-androgen drug finasteride. Nat Commun 2020;11:5430. [Crossref] [PubMed]
- Cao Y, Li L, Xu M, et al. The ChinaMAP analytics of deep whole genome sequences in 10,588 individuals. Cell Res 2020;30:717-31. [Crossref] [PubMed]
- Alyamani M, Emamekhoo H, Park S, et al. HSD3B1(1245A>C) variant regulates dueling abiraterone metabolite effects in prostate cancer. J Clin Invest 2018;128:3333-40. [Crossref] [PubMed]
- Almassi N, Reichard C, Li J, et al. HSD3B1 and Response to a Nonsteroidal CYP17A1 Inhibitor in Castration-Resistant Prostate Cancer. JAMA Oncol 2018;4:554-7. [Crossref] [PubMed]
- Hearn JWD, Xie W, Nakabayashi M, et al. Association of HSD3B1 Genotype With Response to Androgen-Deprivation Therapy for Biochemical Recurrence After Radiotherapy for Localized Prostate Cancer. JAMA Oncol 2018;4:558-62. [Crossref] [PubMed]
- Hearn JWD, AbuAli G, Reichard CA, et al. HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol 2016;17:1435-44. [Crossref] [PubMed]
- Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371:1028-38. [Crossref] [PubMed]
- Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 2011;1:487-95. [Crossref] [PubMed]
- Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med 2016;22:298-305. [Crossref] [PubMed]
(English Language Editor: C. Gourlay)



