Female with bladder cancer: what and why is there a difference?
Introduction
Differences in the incidence, diagnosis, management and survival among women and men have been reported in various malignancies, including colorectal, lung, head and neck cancer, and have contributed to the implementation of gender-specific recommendations in clinical oncology and health care (1). Urothelial carcinoma of the bladder (UCB) is the sixth most common malignancy and the second most common genitourinary cancer, accounting for 130,946 and 74,000 new cases, and 43,080 and 17,852 deaths in the European Union and the US in 2015, respectively (2).
Compared to their female counterparts, male patients are at a three to 4-fold higher risk of developing UCB (3). In addition, the UCB incidence increased 25% faster in men than in women during the past decade (2). Despite this gender disparity in the UCB epidemiology, there is substantial evidence that women present with more advanced disease stages at the primary diagnosis and may face worse outcomes compared to their male counterparts (3-12). In consequence, gender was included as prognosticator for UCB outcomes in various prediction tools (13,14). However, more recently, controversial findings on gender-specific survival in UCB have been reported (7-9,12,13,15,16).
Elucidating the reasons for these gender-specific differences in the UCB incidence and survival may allow optimizing the uro-oncologic treatment and enhancing the quality of care among both genders. However, the specific causes remain currently a matter of debates. In general, gender-specific differences in outcomes of various malignancies seem to be multifactorial, comprising genetic, physiological and anatomic characteristics, heterogeneous exposure and responses to carcinogens, as well as treatment-related particularities (1). In UCB, gender-specific variabilities in the exposure and degradation of carcinogens, the sex-steroid hormone regulation, the anatomy of the bladder and pelvis, the diagnostic work-up, and the management of the disease (17-26), as well as discrepancies in the quality of care have turned into the focus of recent investigations (27-29).
In this non-systematic review we comprehensively summarize the most relevant studies on gender-specific differences on outcomes in non-muscle invasive, muscle-invasive as well as locally advanced and metastatic UCB. In addition, we summarize and discuss the underlying established and suggested biologic, anatomic and treatment-related mechanisms.
Female with bladder cancer: what is the difference?
Gender-specific differences in urothelial carcinoma of the bladder (UCB) biologic characteristics
In UCB, female gender is associated with the presence of aggressive tumor biologic characteristics. Numerous studies consistently demonstrated that women present with more advanced UCB compared to men (4-8,10,12). Since an increasing pathologic tumor stage is a strong predictor for unfavorable outcomes in UCB (7,12,30), the established association of the female gender with more advanced disease is an accepted reason for the worse survival compared to male patients. In addition, female patients suffer more frequently from high-grade UCB (3,7,8,10) and seem to present more frequently with multiple and larger tumors (31) as well as variant UCB histologies (12), which also may unfavorably impact outcomes .
Gender-specific differences in outcomes of non-muscle invasive bladder cancer (NMIBC)
Table 1 summarizes selected studies on gender-specific differences in outcomes of NMIBC. Especially in high-grade UCB, numerous studies indicate an increased risk of disease recurrence or progression in women treated with TURB with or without intravesical immunotherapy or chemotherapy, compared to their male counterparts (32,34-36). However, controversial findings have also been reported. In patients treated with Bacillus Calmette-Guérin (BCG) instillation therapy, some studies found no differences in outcomes between both genders (33,36,37).
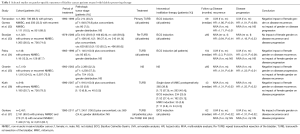
Full table
Gender-specific differences in outcomes of urothelial carcinoma of the bladder (UCB) treated with radical cystectomy
Evidence regarding gender-specific differences in UCB outcomes derives mainly from retrospective RC studies. Table 2 presents an overview of selected studies on gender-specific differences in UCB patients treated with RC. The largest and most recent multi-center study by Kluth et al. comprised 8,102 UCB patients and found that female gender was an independent risk factor for reduced survival (7), which corroborates the findings of several previous reports (9,15,22,38,40,42). Importantly, the most recent analyses included known confounders such as the pathologic tumor stage and the disease stage (7,9). Thus, findings were adjusted for gender-specific discrepancies in the tumor stage at the primary diagnosis. Conversely, other authors found after adjustment for the performance status, comorbidities, pathologic tumor stage or treatment modalities no association between female gender and inferior survival (8,12,16,30,39,41,43). Inconsistent findings across different studies may be due to differences in cohort sizes, treatment modalities prior to RC, variable confounders that the studies have been adjusted for, as well as modifications in the surgical techniques and UCB management over time between older and more contemporary reports.
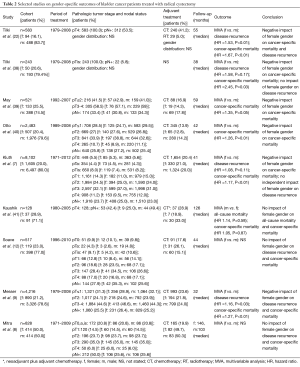
Full table
In summary, numerous retrospective RC series suggest that female gender is associated with unfavorable outcomes, even after adjustment for established confounders. However, these findings are inconsistent across the literature and some studies found no differences in outcomes among both genders. In the future, prospective studies are warranted to add more evidence on gender-specific differences in UCB outcomes.
Gender-specific differences in outcomes of locally advanced and metastatic urothelial carcinoma of the bladder (UCB)
According to the TNM classification of tumors, the pathologic tumor substage 4a is defined by gender-specific anatomic particularities in UCB (44). In women, the tumor is invading the vagina or the uterus, whereas in men, the tumor is extending into the prostate (44). In pT4a UCB, controversial findings have been published regarding gender-specific differences in outcomes. One single-center study found no gender-specific differences in recurrence-free, cancer-specific and overall survival (41). However, according to several multi-center studies and Surveillance, Epidemiology and End Results (SEER) analyses, females seem to experience worse survival compared to men in pT4a UCB (13,22,42). Accordingly, in pT4a UCB patients following RC, female gender has been included as a strong predictor for reduced survival in several nomograms (13,14). In pT4b UCB, the impact of the gender on survival has currently not been sufficiently investigated.
There is a paucity of data on gender and outcomes in metastatic UCB. A pooled analysis of phase II and III trials on first-line cisplatin-based chemotherapy showed similar tolerability, efficacy and outcomes in women and men (45). These findings have recently been supported by a SEER study, showing no gender-specific difference in the survival of 3,110 patients diagnosed with metastatic UCB from 1990 to 2010 (46). Correspondingly, gender was not an independent predictor for poor outcomes in metastatic UCB patients prior to cisplatin-based chemotherapy (47), and has therefore not been included in nomograms for predicting survival in metastatic UCB (47,48).
Regional variations of gender-specific differences in outcomes of urothelial carcinoma of the bladder (UCB)
Results from an open cancer incidence and mortality database including 182 countries indicate that increased UCB-specific mortality in women compared to men is a common finding in the majority of countries (49). In 43% of countries, however, there was no gender-specific difference in the mortality among UCB patients (49). Interestingly, gender-specific disparities diminished over time in certain countries: especially in Germany, female patients experienced inferior outcomes in historical RC cohorts that were treated before the year 2000, whereas more contemporary cohorts show conflicting findings (11). Changes over time and regional variations of gender-specific UCB outcomes may be due to differences in UCB incidence and outcome registries (50), variable awareness of gender-specific differences in UCB behavior, different exposure to carcinogens as well as distinct health care systems with variant delays of diagnosis and treatment (11). However, further studies are needed to define the specific underlying reasons, thus contributing to our understanding of gender-specific differences in UCB outcomes.
Female with bladder cancer: why is there a difference?
Anatomy
UCB in general is a disease of the elderly and almost two-thirds of UCB patients are 65 years of age or older at diagnosis (51). Due to the high prevalence of benign prostatic enlargement with bladder outlet obstruction at this age, men typically have a thicker detrusor muscle compared to women, which may be a reason for a faster extravesical tumor growth in women (52). In addition, the embryonic development of the trigone and the posterior bladder neck from a common origin with the upper part of the vagina is possibly contributing to the more invasive extension pattern of UCB in women (53). The absence of the Fascia Denonvilliers and the anatomic site of the vagina and posterior bladder may represent a minor effective barrier for a continuous or lymphatic tumor spread in women (9,54). Indeed, in men, the prostate and the prostatic urethra may impair the lymphovascular extension of the tumor (55). In women, the tumor extension to the urethra is facilitated by lymphatic vessels, which pass the lateral walls of the vagina and drain the bladder neck to the internal iliac lymph nodes (52,54,55).
Diagnostic work-up
The more aggressive biologic UCB features including the more advanced disease stages among women may be due to the fact that female patients experience a delay of the diagnostic work-up of the typical symptom of UCB, i.e., hematuria, and to be misdiagnosed with urinary tract infection (25). The clinical symptoms of UCB are similarly among both genders (i.e., mainly hematuria and lower urinary tract symptoms) (23,24). According to several analyses, the interval from hematuria to diagnosis of UCB, however, is longer in women than in men, and an adequate work-up including imaging studies is less common in women than in men (25,56). Moreover, females with hematuria are more likely to be diagnosed with urinary tract infection (23-25), and the probability of a referral to a urologist is significantly lower in female patients, compared to their male counterparts (23). As a delay in diagnosis and treatment of UCB is associated with more advanced disease stages at diagnosis, this indirectly also substantially contributes to gender-specific differences in outcomes, since more advanced stages are associated with inferior outcomes. A complete diagnostic work-up, including referral to a urologist, should consistently be encouraged to bypass any delay in diagnosing UCB in both genders. Indeed, the implementation of a protocol based electronic care coordination system decreased the time required for a complete hematuria evaluation, thus enhancing the quality of care (57), as well as potentially eliminating gender-specific UCB differences in the future.
Treatment
In general, treatment strategies do not seem to differ between female and male UCB patients (58). Aggressive therapies (i.e., RC or radiation therapy) are offered equally to both genders according to a SEER database analysis of patients treated from 1992 to 1999 (59). Similarly, a population-based cancer registry study did not find differences in the usage of RC or radiation among both genders (16). Prior to RC, however, women seem to receive more frequently intravesical BCG immunotherapy, compared to their male counterparts (31). An explanation for this finding may be the BCG affiliated risk of prostatitis, presumably leading to a higher reluctance among the general urological practitioners in administering BCG in men. Conversely, other authors found that women were at a decreased probability to receive intravesical treatment before RC (8). However, in this large case-control study the authors did not specifically control for the administration of BCG (8).
At the time of RC, women are often older than men (51). Elderly patients are at an increased risk of cancer-specific mortality and receive less aggressive forms of treatment (51), which contributes to gender-specific differences in UCB outcomes. In addition, female patients are receiving more frequently incontinent urinary diversion compared to men (60), although recently increasing rates of continent diversion have been reported in women (40). Importantly, the type of urinary diversion may influence the postoperative morbidity and mortality (60). Gender-specific differences in the diversion may be due to possible voiding disturbances and concerns regarding the oncological safety of orthotopic urinary diversion in women (5), although a large amount of data provides reliable evidence of feasibility and local control in female UCB patients (22,38).
Gender-specific discrepancies in the quality of surgical therapy have gained the attention of urologists during the last years. During TURB, female gender represents a risk factor for intraoperative bladder perforation (61), due to a thinner detrusor muscle (52). On the other hand, men are more prone to complications following TURB (62). The oncological quality of the RC is often measured by the soft tissue surgical margin status and the lymph node count, and several authors did not find differences in these variables between both genders (7-9,12). Conversely, others showed a decreased probability of an adequate lymphadenectomy in women (28). Following RC, there seem to be relevant discrepancies in the perioperative quality of care among men and women. The 90-day mortality and perioperative complications seem to be elevated in female patients (28,29,63), which is underlined by a longer operative time and a longer in-hospital stay (27,28) as well as a higher intraoperative blood loss and more frequent perioperative blood transfusions in women (27,28). Importantly, perioperative blood transfusions have a negative impact on survival in UCB patients treated with RC (64). However, a contemporary SEER analysis of more than 5,000 patients showed that women are not at a higher risk of 90-day mortality compared to their male counterparts (65). Correspondingly, a single-center study found that female gender was not an independent predictor for low or high-grade complications after RC according the Clavien-Dindo classification (66).
Risk factors
Cigarette smoking is the most relevant risk factor for the development of UCB accounting for 50% of new UCB cases and increasing the risk of UCB incidence by 2 to 6 fold independent of the gender (67,68). In addition, particularly in female lifelong non-smokers, environmental tobacco smoke exposure may induce UCB development (69). A growing body of evidence suggests that smoking has a dose-dependent negative impact on survival in UCB patients treated with TURB and RC (68,70,71). Moreover, in smoking male patients, hypermethylation of tumor suppressor genes has been described, which is associated with unfavorable outcomes (72). On the other hand, smoking cessation for more than 10 years contributes to a prolonged survival in UCB patients treated with TURB or RC (68,70,71). Currently, the gender-specific effect of smoking on UCB outcomes remains controversial. Particularly in men, smoking may have a detrimental effect on recurrence-free survival following TURB (73). In recurrent NMIBC patients treated with TURB with or without intravesical therapy, women with a history of tobacco use had an increased risk of disease progression (74). In contrast, other studies determined that male patients treated with TURB might have worse overall survival compared to women (75,76). In MIBC patients treated with RC, female smokers are at a higher risk of experiencing unfavorable outcomes, compared to their male counterparts (71). However, other authors found that the gender and smoking did not significantly interact for predicting cancer-specific mortality following RC (77). To date, still more men than women are smokers worldwide, and, in general, smoking prevalence has been constantly decreasing among both genders during the last three decades (78). However, tobacco use in women is rising, with female smoking being predicted to double between 2005 and 2025, while simultaneously declining in men (79), potentially contributing to gender-specific disparities in UCB incidence and survival. However, gender-specific differences in the risk of developing UCB seem to persist after adjustment for tobacco use (80). For example, data from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial and National Lung Cancer Screening Trial cohorts suggest that there remains a gender-specific disparity in the incidence of UCB in subgroups of equal smoking intensity (81). Independent of the gender, urologists should endeavor to counsel patients on smoking cessation, implementing tobacco screening in every day clinical practice, advising patients regarding the deleterious effects of smoking on UCB development and outcomes, as well as the beneficial influence of smoking cessation (70).
Approximately 10% of new UCB cases may be related to occupational risk factors (82). Particularly, workers processing aluminum, metal, aromatic amines, polycyclic aromatic hydrocarbons, oil, leather, dye and paint are at a high risk of development of UCB, leading to specific workplace health and safety regulations in various countries (82). Interestingly, occupational risk factors have mainly not been considered in gender-specific UCB incidence and outcome analyses thus far (11). Therefore, future studies are warranted to define the gender-specific impact on occupational exposure on the risk of UCB incidence and survival. A large population-based case-control study found that women using permanent hair dyes were at an increased risk of developing UCB. Especially women with N-acetyltransferase (NAT)-2 slow acetylation phenotype were at the highest UCB risk (83). In contrast, men using hair dyes are not at an increased risk of developing UCB (83).
Chronic inflammatory reactions seem to influence carcinogenesis, as observed in various malignancies. In bladder cancer, the infection with schistosomas is strongly associated with the risk of squamous carcinoma of the bladder, and there seem to be gender-specific differences in the prevalence of schistosoma infection (84). In addition, other pathogens inducing inflammatory reactions may have an impact on the risk of developing UCB. For example, an elevated risk of UCB development among patients with a history of gonorrhea (85) and Human papillomavirus infection (86) was reported, however, the current evidence is inconsistent (87). In general, women suffer more frequently from urinary tract infections and harbor different urinary pathogens compared to men. Similarly, UCB patients have different microorganisms compared to non-UCB patients. However, it remains unclear, whether variable frequencies of urinary tract infections and different distributions of urinary pathogens among both genders may be associated with a distinct UCB risk (11).
Radiation therapy of pelvic cancers in men and women, including prostate cancer as well as cervical and endometrial cancer, increases the risk of UCB development (88). In addition, cyclophosphamide-containing chemotherapy is associated with an increased UCB risk (89). However, it remains currently unclear, whether women are more susceptible to UCB development after radiation or chemotherapy compared to men.
Degradation of carcinogens
Although, to date, it remains mainly speculative, there might be gender-specific differences in the degradation of carcinogens at the molecular level (90), subsequently influencing gender-specific discrepancies in the UCB incidence and mortality. Different hydroxylation, acetylation and glucuronidation pathways, which include various enzymes such as uridine-diphosphoglucuronosyltransferase (UGT) and NAT-2, play an essential role in the degradation of aromatic amines (11,90,91). In the urothelium, androgen receptor (AR)-mediated signaling influences UGT expression (11,90). Thus, urothelial UGT expression may substantially vary among both genders. Although, in general, a slow acetylation status of NAT-2 is correlated with an elevated UCB risk, a recent meta-analysis did not find associations between the combined effect of NAT-2 slow phenotypes and gender (91). Glutathione-S-transferase M1 (GSTM1) is an enzyme that degrades various substances including certain carcinogens by conjugation to glutathione (90). Providing further evidence that a variable GSTM1 expression among both genders contributes to gender-specific differences in the UCB susceptibility, a population-based case-control study determined that smoking women with a non-functioning GSTM1 are at an elevated bladder cancer risk, compared to men (11).
Taken together, there are findings suggesting disparities in the carcinogen degradation between women and men. However, future investigations are warranted to verify these potential gender-specific differences.
Sex-steroids
Since postmenopausal women are at a higher risk for developing UCB compared to premenopausal women (92), UCB is suggested being a sex-hormone-dependent disease. In addition, women with an older age at the menarche, parity, as well as combined hormone replacement therapy with estrogen plus progestin seem to be at a lower risk for UCB development (11,17,93). Conversely, the supposed protective effect of postmenopausal hormone replacement therapy of estrogen and progesterone was shown to forfeit significance in treatment-periods lasting ≥10 years (17), whereas other authors did not find any impact on the development of UCB (93). To date, numerous studies have indicated a potential role of androgens and estrogens as well as the associated receptors in influencing UCB development and the course of the disease (18-21,90).
The AR is a steroid hormone receptor, which is activated by the androgens testosterone and dihydrotesterone (DHT) (94). Following the binding of androgens, the AR translocates from the cytoplasm to the nucleus and controls the transcription of various genes. In the absence of androgens, signaling initiated by other receptors, e.g., epidermal growth factor receptor (EGFR), may facilitate the activation of AR (94). In UCB, the AR expression is decreasing with increasing pathologic stage, with 88.9% of pTa UCB and 0% of pT3 UCB expressing the AR, respectively (20). In addition, co-regulators of the AR enabling the formation of the AR transcriptional complex are expressed in 85% to 100% of UCB specimens (95). Moreover, high-risk UCB may lose the expression of 5-α-reductase, leading to an impaired conversion of testosterone to the more potent DHT (11). Increased androgen-dependent susceptibility of the urothelium to carcinogens, impaired degradation of carcinogens by androgen-dependent pathways or direct oncogenic effects of androgens presumably represent underlying molecular mechanisms, by which androgens promote UCB development and influence the course of disease (19). These hypotheses are mainly supported by animal studies. For example, the castration of transgenic bladder cancer-developing mice subsequently decreased the tumor size, compared to non-castrated mice and castrated mice treated with DHT, whereas AR knock-out hampered nitrosamine-induced bladder cancer in mice (11). In addition, AR signaling may promote UCB development by down-regulation of the expression of UGT in the urothelium (96). Importantly, the AR may influence various other signaling pathways to promote carcinogenesis (94), including the interaction with β-catenin, cyclin-d and EGFR, which have been shown to be associated with an aggressive UCB biologic behavior (97-99). Still, anti-androgenic therapies usually are not applied in the treatment of UCB patients.
The expression of the estrogen receptor (ER)-β is increasing with advancing pathologic tumor stage and higher grading (21), with 53% of pTa UCB and 75% of pT4 tumors, as well as 58% of WHO Grade 1 and 2 tumors and 70% of Grade 3 tumors expressing the isoform ER-β, respectively (100). In contrast, the ER-α is rarely expressed in the urothelium and not associated with the UCB behavior (18,21). The role of the progesterone receptor A in UCB, which is expressed in the squamous epithelium of the urethra, is currently not completely understood (18). Data of in vitro and animal experiments suggest that anti-estrogen treatment (e.g., tamoxifen) may result in a reduction of UCB incidence following carcinogen exposure (101). However, anti-estrogens have thus far not been regularly included in studies on therapies of UCB in women and men.
On the genetic level, it has been shown that various single-nucleotide polymorphisms on chromosome 8q24, especially the PSCA gene, are associated with an increased UCB risk. In its promoter region, the PSCA gene contains an androgen response element. Similarly to prostate cancer, the loss of AR reactivity may induce an androgen-independent status facilitating the metastatic spread. Speculatively, the lower androgen levels in women may cause an earlier loss of AR reactivity, subsequently leading to the more aggressive tumor biology in female UCB patients (11,19).
In summary, the sex-steroids and their corresponding receptors may influence the carcinogenesis of UCB at various levels. Although, to date, targeting of the sex-steroid signaling, has not been routinely included in the treatment of UCB, the gender-specific differences in the circulating sex-hormones and their related receptors among women and men may represent an opportunity for the emerging targeted therapies in UCB, presumably allowing a more tailored treatment among both genders in the future.
Conclusions
Men and women have distinct differences in UCB incidence, behavior and outcomes. While men are at a higher risk of UCB development, there is evidence indicating that women present with more aggressive tumor biologic features and experience worse outcomes. The disparity between women and men is proposed to be a multifactorial result of differential exposures to environmental factors, such as carcinogens (i.e., tobacco and chemicals), as well as genetic, anatomic, hormonal, and societal factors, as well as quality of care and regional variations. Finally, the complexity of distinguished gender-specific variations and their coherence influencing UCB outcomes are yet not entirely understood. Nevertheless, it is important that urologists and general medical practitioners are already aware of these gender-specific disparities in UCB outcomes today, to improve the diagnostic workup and optimize treatment and outcomes, especially in women.
Acknowledgements
None.
Footnote
Conflicts of Interest: SF Shariat owns or co-owns the following patents: Methods to determine prognosis after therapy for prostate cancer. Granted 2002-09-06. Methods to determine prognosis after therapy for bladder cancer. Granted 2003-06-19. Prognostic methods for patients with prostatic disease. Granted 2004-08-05. Soluble Fas: urinary marker for the detection of bladder transitional cell carcinoma. Granted 2010-07-20. He is advisory board member of Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanofi, Wolff. He is speaker for Astellas, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, Wolff. R Mathieu—Consultant: Astellas, Ipsen, Janssen; Speaker: Janssen, Sanofi, Novartis, Takeda. The other authors have no conflicts of interest to declare.
References
- Najari BB, Rink M, Li PS, et al. Sex disparities in cancer mortality: the risks of being a man in the United States. J Urol 2013;189:1470-4. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Shariat SF, Sfakianos JP, Droller MJ, et al. The effect of age and gender on bladder cancer: a critical review of the literature. BJU Int 2010;105:300-8. [Crossref] [PubMed]
- Mungan NA, Kiemeney LA, van Dijck JA, et al. Gender differences in stage distribution of bladder cancer. Urology 2000;55:368-71. [Crossref] [PubMed]
- Scosyrev E, Noyes K, Feng C, et al. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68-74. [Crossref] [PubMed]
- Mallin K, David KA, Carroll PR, et al. Transitional cell carcinoma of the bladder: racial and gender disparities in survival (1993 to 2002), stage and grade (1993 to 2007). J Urol 2011;185:1631-6. [Crossref] [PubMed]
- Kluth LA, Rieken M, Xylinas E, et al. Gender-specific differences in clinicopathologic outcomes following radical cystectomy: an international multi-institutional study of more than 8000 patients. Eur Urol 2014;66:913-9. [Crossref] [PubMed]
- Mitra AP, Skinner EC, Schuckman AK, et al. Effect of gender on outcomes following radical cystectomy for urothelial carcinoma of the bladder: a critical analysis of 1,994 patients. Urol Oncol 2014;32:52.e1-9. [Crossref] [PubMed]
- Messer JC, Shariat SF, Dinney CP, et al. Female gender is associated with a worse survival after radical cystectomy for urothelial carcinoma of the bladder: a competing risk analysis. Urology 2014;83:863-7. [Crossref] [PubMed]
- Fajkovic H, Halpern JA, Cha EK, et al. Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol 2011;29:457-63. [Crossref] [PubMed]
- Dobruch J, Daneshmand S, Fisch M, et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol 2016;69:300-10. [Crossref] [PubMed]
- Soave A, Dahlem R, Hansen J, et al. Gender-specific outcomes of bladder cancer patients: a stage-specific analysis in a contemporary, homogenous radical cystectomy cohort. Eur J Surg Oncol 2015;41:368-77. [Crossref] [PubMed]
- May M, Bastian PJ, Brookman-May S, et al. Gender-specific differences in cancer-specific survival after radical cystectomy for patients with urothelial carcinoma of the urinary bladder in pathologic tumor stage T4a. Urol Oncol 2013;31:1141-7. [Crossref] [PubMed]
- Aziz A, Shariat SF, Roghmann F, et al. Prediction of cancer-specific survival after radical cystectomy in pT4a urothelial carcinoma of the bladder: development of a tool for clinical decision-making. BJU Int 2016;117:272-9. [Crossref] [PubMed]
- Mungan NA, Aben KK, Schoenberg MP, et al. Gender differences in stage-adjusted bladder cancer survival. Urology 2000;55:876-80. [Crossref] [PubMed]
- Patafio FM, Robert Siemens D, et al. Is there a gender effect in bladder cancer? A population-based study of practice and outcomes. Can Urol Assoc J 2015;9:269-74. [Crossref] [PubMed]
- Daugherty SE, Lacey JV Jr, Pfeiffer RM, et al. Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer 2013;133:462-72. [Crossref] [PubMed]
- Bolenz C, Lotan Y, Ashfaq R, et al. Estrogen and progesterone hormonal receptor expression in urothelial carcinoma of the bladder. Eur Urol 2009;56:1093-5. [Crossref] [PubMed]
- Gakis G, Stenzl A. Gender-specific differences in muscle-invasive bladder cancer: the concept of sex steroid sensitivity. World J Urol 2013;31:1059-64. [Crossref] [PubMed]
- Boorjian S, Ugras S, Mongan NP, et al. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology 2004;64:383-8. [Crossref] [PubMed]
- Miyamoto H, Yao JL, Chaux A, et al. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int 2012;109:1716-26. [Crossref] [PubMed]
- Tilki D, Svatek RS, Karakiewicz PI, et al. Characteristics and outcomes of patients with pT4 urothelial carcinoma at radical cystectomy: a retrospective international study of 583 patients. J Urol 2010;183:87-93. [Crossref] [PubMed]
- Henning A, Wehrberger M, Madersbacher S, et al. Do differences in clinical symptoms and referral patterns contribute to the gender gap in bladder cancer? BJU Int 2013;112:68-73. [Crossref] [PubMed]
- Aziz A, Madersbacher S, Otto W, et al. Comparative analysis of gender-related differences in symptoms and referral patterns prior to initial diagnosis of urothelial carcinoma of the bladder: a prospective cohort study. Urol Int 2015;94:37-44. [Crossref] [PubMed]
- Cohn JA, Vekhter B, Lyttle C, et al. Sex disparities in diagnosis of bladder cancer after initial presentation with hematuria: a nationwide claims-based investigation. Cancer 2014;120:555-61. [Crossref] [PubMed]
- Hollenbeck BK, Dunn RL, Ye Z, et al. Delays in diagnosis and bladder cancer mortality. Cancer 2010;116:5235-42. [Crossref] [PubMed]
- Cárdenas-Turanzas M, Cooksley C, Kamat AM, et al. Gender and age differences in blood utilization and length of stay in radical cystectomy: a population-based study. Int Urol Nephrol 2008;40:893-9. [Crossref] [PubMed]
- Siegrist T, Savage C, Shabsigh A, et al. Analysis of gender differences in early perioperative complications following radical cystectomy at a tertiary cancer center using a standardized reporting methodology. Urol Oncol 2010;28:112-7. [Crossref] [PubMed]
- Liberman D, Lughezzani G, Sun M, et al. Perioperative mortality is significantly greater in septuagenarian and octogenarian patients treated with radical cystectomy for urothelial carcinoma of the bladder. Urology 2011;77:660-6. [Crossref] [PubMed]
- Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol 2006;176:2414-22; discussion 22. [Crossref] [PubMed]
- Puente D, Malats N, Cecchini L, et al. Gender-related differences in clinical and pathological characteristics and therapy of bladder cancer. Eur Urol 2003;43:53-62. [Crossref] [PubMed]
- Fernandez-Gomez J, Solsona E, Unda M, et al. Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guerin: multivariate analysis of data from four randomized CUETO trials. Eur Urol 2008;53:992-1001. [Crossref] [PubMed]
- Boorjian SA, Zhu F, Herr HW. The effect of gender on response to bacillus Calmette-Guerin therapy for patients with non-muscle-invasive urothelial carcinoma of the bladder. BJU Int 2010;106:357-61. [Crossref] [PubMed]
- Palou J, Sylvester RJ, Faba OR, et al. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease-specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette-Guerin. Eur Urol 2012;62:118-25. [Crossref] [PubMed]
- Chamie K, Litwin MS, Bassett JC, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer 2013;119:3219-27. [Crossref] [PubMed]
- Kluth LA, Fajkovic H, Xylinas E, et al. Female gender is associated with higher risk of disease recurrence in patients with primary T1 high-grade urothelial carcinoma of the bladder. World J Urol 2013;31:1029-36. [Crossref] [PubMed]
- Gontero P, Sylvester R, Pisano F, et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guerin: results of a retrospective multicenter study of 2451 patients. Eur Urol 2015;67:74-82. [Crossref] [PubMed]
- Tilki D, Reich O, Svatek RS, et al. Characteristics and outcomes of patients with clinical carcinoma in situ only treated with radical cystectomy: an international study of 243 patients. J Urol 2010;183:1757-63. [Crossref] [PubMed]
- May M, Stief C, Brookman-May S, et al. Gender-dependent cancer-specific survival following radical cystectomy. World J Urol 2012;30:707-13. [Crossref] [PubMed]
- Otto W, May M, Fritsche HM, et al. Analysis of sex differences in cancer-specific survival and perioperative mortality following radical cystectomy: results of a large German multicenter study of nearly 2500 patients with urothelial carcinoma of the bladder. Gend Med 2012;9:481-9. [Crossref] [PubMed]
- Kaushik D, Frank I, Eisenberg MS, et al. Gender-specific survival following radical cystectomy for pT4 bladder cancer. World J Urol 2014;32:1433-9. [Crossref] [PubMed]
- Liberman D, Alasker A, Sun M, et al. Radical cystectomy for patients with pT4 urothelial carcinoma in a large population-based study. BJU Int 2011;107:905-11. [Crossref] [PubMed]
- Patel MI, Bang A, Gillett D, et al. Poor survival of females with bladder cancer is limited to those aged 70 years or over: a population-wide linkage study, New South Wales, Australia. Cancer Med 2015;4:1145-52. [Crossref] [PubMed]
- Witjes JA, Comperat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014;65:778-92. [Crossref] [PubMed]
- Haines L, Bamias A, Krege S, et al. The impact of gender on outcomes in patients with metastatic urothelial carcinoma. Clin Genitourin Cancer 2013;11:346-52. [Crossref] [PubMed]
- Pal SK, Lin YI, Yuh B, et al. Conditional Survival in de novo Metastatic Urothelial Carcinoma. PLoS One 2015;10:e0136622. [Crossref] [PubMed]
- Apolo AB, Ostrovnaya I, Halabi S, et al. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst 2013;105:499-503. [Crossref] [PubMed]
- Galsky MD, Moshier E, Krege S, et al. Posttreatment prognostic nomogram for patients with metastatic urothelial cancer completing first-line cisplatin-based chemotherapy. Urol Oncol 2014;32:48 e1-8.
- Donsky H, Coyle S, Scosyrev E, et al. Sex differences in incidence and mortality of bladder and kidney cancers: national estimates from 49 countries. Urol Oncol 2014;32:40.e23-31. [Crossref] [PubMed]
- Schmid M, Shariat SF, Soave A, et al. Contemporary gender-specific outcomes in Germany after radical cystectomy for bladder cancer. Curr Urol Rep 2014;15:409. [Crossref] [PubMed]
- Rink M, Chun FK, Chromecki TF, et al. Advanced bladder cancer in elderly patients. Prognostic outcomes and therapeutic strategies. Urologe A 2012;51:820-8. [Crossref] [PubMed]
- Madeb R, Messing EM. Gender, racial and age differences in bladder cancer incidence and mortality. Urol Oncol 2004;22:86-92. [Crossref] [PubMed]
- Saez S, Martin PM. Evidence of estrogen receptors in the trigone area of human urinary bladder. J Steroid Biochem 1981;15:317-20. [Crossref] [PubMed]
- Schilling D, Horstmann M, Nagele U, et al. Cystectomy in women. BJU Int 2008;102:1289-95. [Crossref] [PubMed]
- Maralani S, Wood DP Jr, Grignon D, et al. Incidence of urethral involvement in female bladder cancer: an anatomic pathologic study. Urology 1997;50:537-41. [Crossref] [PubMed]
- Bassett JC, Alvarez J, Koyama T, et al. Gender, race, and variation in the evaluation of microscopic hematuria among Medicare beneficiaries. J Gen Intern Med 2015;30:440-7. [Crossref] [PubMed]
- Casey JT, Berkowitz LL, Cashy J, et al. A protocol based, electronic medical record enabled care coordination system improves the timeliness and efficiency of care for patients with hematuria. J Urol 2013;190:212-7. [Crossref] [PubMed]
- Snyder C, Harlan L, Knopf K, et al. Patterns of care for the treatment of bladder cancer. J Urol 2003;169:1697-701. [Crossref] [PubMed]
- Konety BR, Joslyn SA. Factors influencing aggressive therapy for bladder cancer: an analysis of data from the SEER program. J Urol 2003;170:1765-71. [Crossref] [PubMed]
- Schmid M, Rink M, Traumann M, et al. Evidence from the 'PROspective MulticEnTer RadIcal Cystectomy Series 2011 (PROMETRICS 2011)' study: how are preoperative patient characteristics associated with urinary diversion type after radical cystectomy for bladder cancer? Ann Surg Oncol 2015;22:1032-42. [Crossref] [PubMed]
- Herkommer K, Hofer C, Gschwend JE, et al. Gender and body mass index as risk factors for bladder perforation during primary transurethral resection of bladder tumors. J Urol 2012;187:1566-70. [Crossref] [PubMed]
- Valerio M, Cerantola Y, Fritschi U, et al. Comorbidity and nutritional indices as predictors of morbidity after transurethral procedures: A prospective cohort study. Can Urol Assoc J 2014;8:E600-4. [Crossref] [PubMed]
- Lavallée LT, Schramm D, Witiuk K, et al. Peri-operative morbidity associated with radical cystectomy in a multicenter database of community and academic hospitals. PLoS One 2014;9:e111281. [Crossref] [PubMed]
- Gierth M, Mayr R, Aziz A, et al. Preoperative anemia is associated with adverse outcome in patients with urothelial carcinoma of the bladder following radical cystectomy. J Cancer Res Clin Oncol 2015;141:1819-26. [Crossref] [PubMed]
- Schiffmann J, Gandaglia G, Larcher A, et al. Contemporary 90-day mortality rates after radical cystectomy in the elderly. Eur J Surg Oncol 2014;40:1738-45. [Crossref] [PubMed]
- Roghmann F, Trinh QD, Braun K, et al. Standardized assessment of complications in a contemporary series of European patients undergoing radical cystectomy. Int J Urol 2014;21:143-9. [Crossref] [PubMed]
- Crivelli JJ, Xylinas E, Kluth LA, et al. Effect of smoking on outcomes of urothelial carcinoma: a systematic review of the literature. Eur Urol 2014;65:742-54. [Crossref] [PubMed]
- Rink M, Crivelli JJ, Shariat SF, et al. Smoking and bladder cancer: a systematic review of risk and outcomes. Eur Urol 2015;1:17-27. [Crossref]
- Jiang X, Yuan JM, Skipper PL, et al. Environmental tobacco smoke and bladder cancer risk in never smokers of Los Angeles County. Cancer Res 2007;67:7540-5. [Crossref] [PubMed]
- Simonis K, Shariat SF, Rink M. Smoking and smoking cessation effects on oncological outcomes in nonmuscle invasive bladder cancer. Curr Opin Urol 2014;24:492-9. [Crossref] [PubMed]
- Rink M, Zabor EC, Furberg H, et al. Impact of smoking and smoking cessation on outcomes in bladder cancer patients treated with radical cystectomy. Eur Urol 2013;64:456-64. [Crossref] [PubMed]
- Marsit CJ, Houseman EA, Schned AR, et al. Promoter hypermethylation is associated with current smoking, age, gender and survival in bladder cancer. Carcinogenesis 2007;28:1745-51. [Crossref] [PubMed]
- Rink M, Furberg H, Zabor EC, et al. Impact of smoking and smoking cessation on oncologic outcomes in primary non-muscle-invasive bladder cancer. Eur Urol 2013;63:724-32. [Crossref] [PubMed]
- Rink M, Xylinas E, Babjuk M, et al. Impact of smoking on outcomes of patients with a history of recurrent nonmuscle invasive bladder cancer. J Urol 2012;188:2120-7. [Crossref] [PubMed]
- Rieken M, Xylinas E, Kluth L, et al. Long-term cancer-specific outcomes of TaG1 urothelial carcinoma of the bladder. Eur Urol 2014;65:201-9. [Crossref] [PubMed]
- Martin-Doyle W, Leow JJ, Orsola A, et al. Improving selection criteria for early cystectomy in high-grade t1 bladder cancer: a meta-analysis of 15,215 patients. J Clin Oncol 2015;33:643-50. [Crossref] [PubMed]
- Boström PJ, Alkhateeb S, Trottier G, et al. Sex differences in bladder cancer outcomes among smokers with advanced bladder cancer. BJU Int 2012;109:70-6. [Crossref] [PubMed]
- Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA 2014;311:183-92. [Crossref] [PubMed]
- Amos A, Greaves L, Nichter M, et al. Women and tobacco: a call for including gender in tobacco control research, policy and practice. Tob Control 2012;21:236-43. [Crossref] [PubMed]
- Hemelt M, Yamamoto H, Cheng KK, et al. The effect of smoking on the male excess of bladder cancer: a meta-analysis and geographical analyses. Int J Cancer 2009;124:412-9. [Crossref] [PubMed]
- Krabbe LM, Svatek RS, Shariat SF, et al. Bladder cancer risk: Use of the PLCO and NLST to identify a suitable screening cohort. Urol Oncol 2015;33:65.e19-25. [Crossref] [PubMed]
- Cumberbatch MG, Cox A, Teare D, et al. Contemporary Occupational Carcinogen Exposure and Bladder Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2015;1:1282-90. [Crossref] [PubMed]
- Koutros S, Silverman DT, Baris D, et al. Hair dye use and risk of bladder cancer in the New England bladder cancer study. Int J Cancer 2011;129:2894-904. [Crossref] [PubMed]
- Botelho MC, Figueiredo J, Alves H. Bladder cancer and urinary Schistosomiasis in Angola. J Nephrol Res 2015;1:22-4. [Crossref] [PubMed]
- Michaud DS, Platz EA, Giovannucci E. Gonorrhoea and male bladder cancer in a prospective study. Br J Cancer 2007;96:169-71. [Crossref] [PubMed]
- Gutiérrez J, Jiménez A, de Dios Luna J, et al. Meta-analysis of studies analyzing the relationship between bladder cancer and infection by human papillomavirus. J Urol 2006;176:2474-81; discussion 81. [Crossref] [PubMed]
- Shigehara K, Sasagawa T, Namiki M. Human papillomavirus infection and pathogenesis in urothelial cells: a mini-review. J Infect Chemother 2014;20:741-7. [Crossref] [PubMed]
- Tubiana M. Can we reduce the incidence of second primary malignancies occurring after radiotherapy? A critical review. Radiother Oncol 2009;91:4-15; discussion 1-3. [Crossref] [PubMed]
- Nilsson S, Ullén A. Chemotherapy-induced bladder cancer. Scand J Urol Nephrol Suppl 2008.89-92. [Crossref] [PubMed]
- Zhang Y. Understanding the gender disparity in bladder cancer risk: the impact of sex hormones and liver on bladder susceptibility to carcinogens. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2013;31:287-304. [Crossref] [PubMed]
- Wu H, Wang X, Zhang L, et al. Association between N-acetyltransferase 2 polymorphism and bladder cancer risk: results from studies of the past decade and a meta-analysis. Clin Genitourin Cancer 2016;14:122-9. [Crossref] [PubMed]
- McGrath M, Michaud DS, De Vivo I. Hormonal and reproductive factors and the risk of bladder cancer in women. Am J Epidemiol 2006;163:236-44. [Crossref] [PubMed]
- Cantwell MM, Lacey JV Jr, Schairer C, et al. Reproductive factors, exogenous hormone use and bladder cancer risk in a prospective study. Int J Cancer 2006;119:2398-401. [Crossref] [PubMed]
- Lombard AP, Mudryj M. The emerging role of the androgen receptor in bladder cancer. Endocr Relat Cancer 2015;22:R265-77. [Crossref] [PubMed]
- Boorjian SA, Heemers HV, Frank I, et al. Expression and significance of androgen receptor coactivators in urothelial carcinoma of the bladder. Endocr Relat Cancer 2009;16:123-37. [Crossref] [PubMed]
- Izumi K, Zheng Y, Hsu JW, et al. Androgen receptor signals regulate UDP-glucuronosyltransferases in the urinary bladder: a potential mechanism of androgen-induced bladder carcinogenesis. Mol Carcinog 2013;52:94-102. [Crossref] [PubMed]
- Ren B, Li W, Yang Y, et al. The impact of cyclin D1 overexpression on the prognosis of bladder cancer: a meta-analysis. World J Surg Oncol 2014;12:55. [Crossref] [PubMed]
- Li Y, Zheng Y, Izumi K, et al. Androgen activates beta-catenin signaling in bladder cancer cells. Endocr Relat Cancer 2013;20:293-304. [Crossref] [PubMed]
- Zhao J, Xu W, Zhang Z, et al. Prognostic role of HER2 expression in bladder cancer: a systematic review and meta-analysis. Int Urol Nephrol 2015;47:87-94. [Crossref] [PubMed]
- Shen SS, Smith CL, Hsieh JT, et al. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer 2006;106:2610-6. [Crossref] [PubMed]
- George SK, Tovar-Sepulveda V, Shen SS, et al. Chemoprevention of BBN-induced bladder carcinogenesis by the selective estrogen receptor modulator tamoxifen. Transl Oncol 2013;6:244-55. [Crossref] [PubMed]


