Brain-derived neurotrophic factor promotes nerve regeneration by activating the JAK/STAT pathway in Schwann cells
Introduction
Schwann cells are essential for the maintenance of integrity and function of peripheral nerves. Schwann cells create a microenvironment of cell-adhesion molecules, extracellular matrix proteins, and neurotrophins that support neurons (1). Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family. It promotes neuronal survival and neurite outgrowth, prevents neuronal death. BDNF functions through interaction with tropomyosin-related kinase B (TrkB) and pan-neurotrophin 75 (p75) receptors, thereby activating ras/MEK, PI-3, ERK, PKA, and PLCγ/PKC pathways (2).
During radical prostatectomy (RP) injury to the cavernous nerves (CNs) that run along the posterolateral aspect of the prostate may occur. This carries the significant risk of post-operative erectile dysfunction (ED) in 60.8–93% of patients (3). Nerve injury can occur despite the development of the nerve-sparing approach, which is still associated with an approximate 20% risk of ED at 24 months (4). This is likely due to neurapraxia from CN injury secondary to stretching, heating, or direct injury, which result in Wallerian degeneration (5). During neurapraxia, CN nitric oxide (NO)-releasing fibers cease to produce NO, resulting in smooth muscle apoptosis and cavernosal fibrosis (6). Despite this pathophysiologic mechanism for post-prostatectomy ED, phosphodiesterase-5 inhibitors are frequently used for penile rehabilitations after RP but with limited effectiveness as they work downstream of the NO pathway (6).
Therefore, other therapeutic strategies are needed to enhance nerve regeneration after prostatectomy. Although the ability of nerves to regenerate after RP is somewhat limited, evidence suggests that recovery of erectile function after RP depends on re-sprouting of axonal fibers from intact neurons (7). Factors enhancing nerve regeneration include immunophilin ligands, hormones, growth factors, stem cells, and neurotrophins (8,9).
Using an established CN crush injury rat model, we previously demonstrated that BDNF enhances the recovery of erectile function, acts synergistically with the vascular endothelial growth factor (VEGF), and regenerates neuronal oxide synthase (nNOS)-containing nerve fibers (10,11). We also developed a culture system of the dorsal-caudal region of the major pelvic ganglion (DCR-MPG), which is an effective and inexpensive in vitro culture system for studying the effect of growth factors and neurite sprouting of the MPG and proximal CN segment. Through using this culture system; we confirmed that the neurite growth-enhancing effect of BDNF occurs primarily through the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) molecular pathway. In addition, we identified the phosphorylation patterns of JAK2, STAT1, and STAT3, while p75, TrkB, and TrkC receptors co-localized to the DCR-MPG (12,13). More recently, we showed the upregulation of BDNF and activation of the JAK/STAT pathway in MPG following CN injury in rat models (14). However, the cell type response to BNDF treatment for JAK/STAT activation in MPG is not well understood.
In this article, we hypothesize that BDNF activates the JAK/STAT pathway in Schwann cells, not neurons, resulting in secretion of cytokines (such as IL-6 and OSM-M) that facilitate axonal regeneration. IL6 and OSM are known inflammatory cytokines that are secreted by Schwann cells and are involved in nerve regeneration. They are considered markers for nerve regeneration (15-17). Hirota et al. showed an accelerated regeneration of axotomized nerves in mice overexpressing IL6 and IL6 receptor (18).
Materials and methods
Cell lines and reagents
Four cell lines were all purchased from American Type Culture Collection (ATCC, Manassas, VA, USA), including human neuroblastoma BE(2)-C and SH-SY5Y, rat Schwann cell (RSC) RT4-D6P2T, and human Schwann cell (HSC) from ScienCell Research Lab (San Diego, CA, USA). Following the manual, the cell culture medium and related reagents were used, and all were purchased from the cell culture facility at University of California, San Francisco. Recombinant human BDNF was purchased from R&D systems Inc. (Minneapolis, MN, USA) and used for the treatment. S100, p75 antibodies (Chemicon, Inc., Temecula, CA, USA) and secondary antibody FITC-conjugated goat anti-mouse IgG (Chemicon, Inc., Temecula, CA, USA) were used for immunofluorescence staining. Beta-actin, JAK2, phospho-JAK2 (Chemicon, Inc., Temecula, CA, USA), STAT1, phospho-STAT1, STAT3, and phospho-STAT3 (BD Biosciences, CA, USA) antibodies were used for immunoblot. The reagents in ECL kit (Amersham Life Sciences Inc., Arlington Heights, IL, USA) were used as substrate for all the immunoblotting. OSM-M and IL-6 enzyme linked immunosorbent assay (ELISA) kit (R&D systems Inc., Minneapolis, MN, USA) were applied to assay the secretion of cytokine from Schwann cells.
Cell culture and treatments
Human neuroblastoma, HSCs, and RSCs were cultured in RPMI-1640/F12 medium with 4.5 g/L glucose, 10 mM HEPES, 1 mM sodium pyruvate, supplemented with 10% FBS in a 5% CO2 atmosphere at 37 °C. Human recombinant BDNF was used to treat the cells in order to assess the JAK/STAT pathway. To assay the effects of BDNF on the activation of JAK/STAT pathway, different doses of BDNF were applied to treat BE(2)-C, SH-SY5Y and RT4-D6P2T. Dosage response was accomplished by treating cells with 0, 1, 10, and 100 pM BDNF for 30 minutes. Time response was also accomplished by treating cells with 100 pM BDNF at 0 minute, 10 minutes, 30 minutes, 1 hr, 2 hr and 24 hr for RT4-D6P2T, and at 0 minute, 10 minutes, 30 minutes, 1 hr, 2 hr, 24 hr and 48 hr for HSC. For the cytokine assay experiments, HSC were treated with 100 pM or 25 nM BDNF in serum free RPMI-1640/F12 medium for 0 minute, 10 minutes, 30 minutes, 1 hr, 2 hr, 24 hr, 48 hr and 72 hr.
Western blotting
The BDNF treated BE(2)-C, SH-SY5Y, RT4-D6P2T and HSCs were homogenized in lysis buffer containing 1% IGEPAL, 0.5% sodium deoxycholate, 0.1% SDS, 0.1 mM Na3VO4, aprotinin (10 µg/mL) and leupeptin (10 µg/mL). The homogenate was centrifuged at 14,000 g for 10 minutes, and the supernatant was recovered as protein sample, which was measured for protein concentration by the BCA method (Pierce Chemical Company, Rockford, IL, USA) and analyzed by western blotting as previously described. The primary antibodies used in these experiments were anti-JAK2 (1:500), anti-phospho-JAK2 (1:200) (Chemicon, Inc., Temecula, CA, USA), anti-STAT1 (1:500), anti-phospho-STAT1 (1:500), anti-STAT3 (1:500), and anti-phospho-STAT3 (1:500) (BD Biosciences, CA, USA). The same membrane was probed with an antibody against the phosphorylated form of JAK2, STAT1, or STAT3, then with the antibody against the non-phosphorylated form (e.g., anti-phospho-JAK2, then anti-JAK2). Before re-probing, the membrane was stripped in 62.5 mM Tris-HCl, pH 6.7, 2% SDS, 10 mM 2-mercaptoethanol at 55 °C for 30 min and then washed 4 times in 1× TBS. Detection of reactive proteins on the membrane was performed with the ECL kit (Amersham Life Sciences Inc., Arlington Heights, IL, USA), followed by exposure to X-ray films. The resulting image was analyzed with ChemiImager 4000 to determine the integrated density value (IDV) of each protein band.
Enzyme linked immunosorbent assay (ELISA) for oncostatin M
Oncostatin M ELISAs (R&D Systems, Minneapolis, MN, USA) were done according to the manufacturer’s protocol. The absorbance was recorded using a microplate reader (Bio-Rad, Hercules, CA, USA) at 450-nm wavelength. Briefly, plate preparation was completed by coating the 96-well microplate with 100 µL diluted Capture Antibody per well. After completely washing with the wash buffer, the plate was blocked with 300 µL of Reagent Diluent for each well for 1 hr at room temperature. One hundred micro liter samples or standards were added in each well and incubated for 2 hr at room temperature followed by another 2 hr incubation of 100 µL detection antibody. The signal was developed by adding 100 µL Streptavidin-HRP and a tetramethyl benzidine (TMB) solution served as the substrate for the reaction. Activity of the enzyme was stopped by 1 M phosphoric acid and absorbance was measured at 450 nm on the microplate reader. Three independent experiments were done for each experiment. The SDs were calculated and error bars represent ± SD.
Enzyme linked immunosorbent assay (ELISA) for IL6
IL6 was measured by using a commercially available ELISA (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The kits contained the microtitration plate with adsorbed conjugate of the mouse monoclonal antibody against determined antigens and nonspecific horseradish peroxidase. Every plate contained a doublet of seven standards for construction of the calibration curve and blank, and the pH of the washing phosphate-buffered saline (PBS) solution with 1% Tween 20 was adjusted to 7.4. A TMB solution served as the substrate for the reaction. Activity of the enzyme was stopped by 1 M phosphoric acid and absorbance was measured at 450 nm. Three independent experiments were done. The SDs were calculated and error bars represent ± SD.
Immunofluorescence staining
The HSCs and RSCs RT4-D6P2T were cultured on glass cover slides in 6-well cell culture dishes. Cells were fixed with ice-cold methanol for 8 min, permeabilized with 0.05% Triton X-100 for 5 min, and blocked with 5% normal horse serum in PBS for 1 h at room temperature. The cells were then incubated with the primary antibody anti-S-100 (1:500) and anti-p75 (1:500) for 1 hr at room temperature. After washing with PBS three times, the cells were incubated with the secondary antibody FITC-conjugated goat anti-mouse IgG (1:500, Chemicon, Inc., Temecula, CA, USA) for 1 hr at room temperature. After another three washes with PBS, the cells were further counter stained with 4',6-diamidino-2-phenylindole (DAPI, for nuclear staining) for 5 min and viewed under fluorescence microscope.
Statistical analysis
The results were analyzed using GraphPad Prism (v5) software (GraphPad Software, La Jolla, CA, USA) and expressed as mean ± standard error of the mean. Multiple groups were compared using one-way analysis of variance (ANOVA) followed by the Tukey-Kramer test for post hoc comparisons to analyze the effect of BDNF on the activation of JAK/STAT pathway and secretion of cytokines.
Results
BDNF activates JAK/STAT signal pathway directly in Schwann cell
To validate the Schwann cell, the specific cellular markers S100 and p75 were checked by immunofluorescence staining in HSCs and RSCs RT4-D6P2T cells. The result indicated that both those cells expressed S100 and p75 in the cytoplasm, although the expression level and location are different. The HSCs expressed more S100 and p75, while the RSCs RT4-D6P2T shaped as small spindle-like cells and expressed peri-nucleus S100 (Figure 1A).
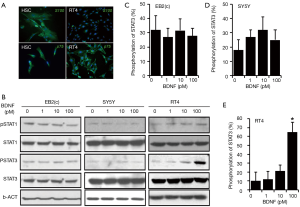
The JAK/STAT pathway, an important cellular signaling transduction pathway in controlling neurite regeneration, is expressed extensively in human neuroblastoma BE(2)-C and SH-SY5Y and RSCs RT4-D6P2T (Figure 1). In our previous experiments, it has been determined that BDNF play a critical role in peripheral nerve regeneration after injury, and acts through the JAK/STAT pathway (12,13). To elicit the mechanism underlying this phenomenon, different doses of BDNF were applied to treat BE(2)-C, SH-SY5Y, and RT4-D6P2T (0, 1, 10, and 100 pM) for 30 minutes. The phosphorylation of STAT3 at Tyr705 and STAT1 at Tyr701 were extensively enhanced by BDNF at a dose of (100 pM) in rat Schwan cells RT4-D6P2T (* P<0.01), while the activation level of JAK/STAT did not change in the same conditions in human neuroblastoma cell lines BE(2)-C and SH-SY5Y. The activation of STAT3 and STAT1 occurred within 30 minutes from the treatment of RT4-D6P2T with BDNF; this implies that BDNF directly induced the phosphorylation of STAT3 at Tyr705 and STAT1 at Tyr701.
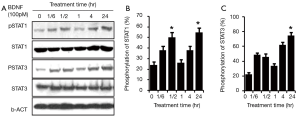
JAK/STAT activation in RT4-D6P2T induced by BDNF
To explore the detailed response of JAK/STAT to BDNF, time response was checked in the RT4-D6P2T cells. One hundred pM BDNF was administered to treat the RSCs at different times, including 0 min, 10 min, 30 min, 60 min, 120 min and 24 hr. The phosphorylation levels of STAT3 at Tyr705 and STAT1 at Tyr701 began to increase 10 min after the treatment with BDNF and peaked at 30 min (*P<0.01). After reaching its peak, the pSTAT3/pSTAT1 returned to near baseline. Surprisingly, STAT3 and STAT1 regained phosphorylation at 2 hr post-treatment by BDNF and reached a second peak at 24 hr, which was even higher than the first one (#P<0.01). The second round activation may relate to mechanisms other than the direct effect of BDNF (Figure 2).
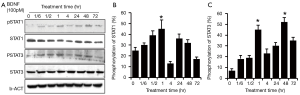
JAK/STAT activation in HSC induced by BDNF
Since HSCs share the same biological properties with RSCs, we repeated the experiment in HSCs. To observe the sustained time frame of the second activation of JAK/STAT pathway, another time point (72 hr) was added. Similar to RSCs RT4-D6P2T, the initial activation time for STAT3/STAT1 was 10 min after the treatment of BDNF. However, in HSCs, STAT3/STAT1 phosphorylation was sustained for a relatively longer response period, and the phosphorylation level peaks appeared later, at 60 min. Two hours later, the pSTAT1 returned to near baseline, while pSTAT3 remained at higher than baseline level. Interestingly, STAT3/STAT1 phosphorylation also peaked again at 24–48 hr. The activation of STAT1 was totally quenched at 72 hr post-BDNF treatment, while pSTAT3 remained at a higher level than baseline (Figure 3).
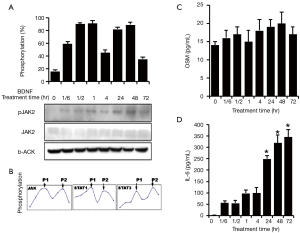
At the same time, activation of JAK2 was also confirmed (Figure 4A). JAK2 was activated by BDNF treatment and consisted of two phosphorylation peaks, which was similar to the response of STAT3/STAT1. All those three major molecules in the JAK/STAT pathway, JAK2, STAT1 and STAT3, possessed the same phosphorylation pattern when treated by BDNF in HSCs—an early phosphorylation peak (P1) and a later phosphorylation peak (P2) (Figure 4B).
BDNF promotes cytokine release from Schwann cells with associated JAK/STAT pathway activation
In order to quantify the amount of cytokine release related to nerve regeneration, OSM-M and IL6 were selected for measurement by ELISA. In addition, JAK2, the upstream molecule in JAK/STAT pathway that is activated by OSM-M and IL6, was also assessed by the Western blotting, as shown above.
The baseline measurements of OSM-M and IL6 secretion were 14.4±1.4 and 2.93±0.15 pg/mL in Schwann cells, respectively. One hour after BDNF treatment, HSCs began to secrete OSM-M at levels reaching ~20±0.8 pg/mL at 24 hr (Figure 4C). Also, BDNF significantly increased the secretion of IL6 from HSCs after 1 hr of treatment and reached ~360.9±74 pg/mL at 72 hr (*P<0.01) (Figure 4C).
Discussion
Schwann cells play an important role in nerve regeneration. In the first few days following nerve injury, Schwann cells undergo marked dedifferentiation, reaching peak activation at day 3. During this phase, they help clear debris along with macrophages. A second important phase involving Schwann cell proliferation occurs thereafter when they proliferate in the basement membrane of endoneurial tubes to aid in nerve regeneration. Schwann cells form bands of Büngner, which form pathways for the regenerating axons. The loss of contact between Schwann cells and axolemma results in the overexpression of receptors for neurotrophic factors on Schwann cells and the overexpression of several neurotrophic factors, including BDNF. The close apposition of Schwann cells to axons permits the inter-cellular transfer of cytokines and neurotrophic factors. Once contact is re-established between Schwann cells and adjacent axons, the receptors and neurotrophic factors in Schwann cells return to baseline (19).
We previously determined that BDNF enhances the recovery of erectile function post-CN injury through neurite re-sprouting, and demonstrated its action through the activation of the JAK/STAT pathway. Stimulating Schwann cells to produce cytokines in regenerating the axonal nerve fibers is likely the principle neurotrophic effect of BDNF, rather than direct action on nerve fibers themselves. BDNF enhances myelination in Schwann cells and promotes neuronal survival and neurite outgrowth through its interaction with p75 and TrkB receptors (20). In order to validate Schwann cells, we checked the specific cellular markers p75 and S100. Our results indicated that both HSCs and RSCs were abundant of the two cellular markers. HSCs generally displayed higher expression level of S100 and p75. S100 was mainly located in the peri-nuclear region in RSCs, which corresponds to our previous observation whereby the unphosphorylated form of STAT3 was located in the cytoplasm, and later in the nucleus upon its activation (phosphorylation) following injury and release of BDNF (14). It is also in line with the function of JAK/STAT pathway, being involved directly in the transduction signal molecules from the cellular membrane to the nucleus, thereby mediating central and peripheral nerve regeneration (21,22).
Transected peripheral nerves demonstrate elevated levels of JAK2, STAT1, and most importantly STAT3 (23). BDNF activation of the JAK/STAT pathway involves the phosphorylation of these molecules (14). In this article we demonstrate the ability of BDNF to increase the phosphorylated forms of STAT1 and STAT3 in RSCs but not in the neuroblastoma cell lines. This is a novel result that helps understand elucidate the indirect action of BDNF through Schwann cells; this was achieved using the100 pM (3 ng/mL) dose. We previously showed that the optimal BDNF dosage to promote MPG neurite growth was between 25 and 50 ng/mL (13). We utilized a lower dose in this study for the purpose of working with cells rather than ganglia.
We confirm here the previous observation that JAK/STAT activation occurs in a delayed fashion (around 24 hours later) (24). After peaking initially for a brief period at 1 hour, the phosphorylated forms of STAT1/3 and JAK2 peaked again at 24 hours in rat and HSCs. This is likely the time needed by Schwann cells to produce cytokines and growth factors as a response to injury in order to facilitate nerve regeneration. This is evidenced by the gradual increase in cytokines release (OSM and IL6) by human Schwan cells in response to BDNF administration, which has particularly reached statistical significance in IL-6. In this article, although we showed the increase in OSM and IL6 in Schwan cells upon exposure to BDNF, we do not examine directly their effect on nerve regeneration. We only utilize them here as markers for the overall cytokine release from Schwann cells.
Although our previous work looked primarily at nerve injury post-RP, we believe that the work in this article applies to nerve regeneration in the peripheral nervous system as a whole. Therefore BDNF would represent an attractive treatment modality for both post-RP ED and other nerve injuries in addition to peripheral neuropathic disorders, such as diabetes mellitus. BDNF recently was found to enhance survival and neuronal differentiation of human neural precursor cells in rat models of auditory neuronal damage (25). BDNF from bone marrow-derived cells promoted post-injury repair of sciatic nerve in mice (26). Allografts of acellular sciatic nerve and BDNF were found to promote axonal regeneration and functional recovery after spinal cord injury in rats (27). Continuous local release from sciatic nerve post-injury promotes a more rapid nerve regeneration and reduction of neuropathic pain in rats (28).
Outside the nervous system, BDNF through Trk receptors was found to promote the growth of human embryonic stem cells and enhance clonal survival by 36 folds (29). Our future directions would include examining other neurotrophic factors that would act in synergism with BDNF in post-RP ED and in other nerve injury models.
Conclusions
We demonstrated for the first time the indirect mechanism of BDNF enhancement of CN regeneration through the activation of JAK/STAT pathway in Schwann cells, rather than directly on nerve fibers. As a result, Schwann cells produce cytokines that promote CN regeneration.
Acknowledgements
Funding: Research reported in this publication was supported by NIDDK of the National Institutes of Health under award number R37 DK045370.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study needs no approval of the Institutional Review Board (IRB) due to containing no samples from patients.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Ogata T, Yamamoto S, Nakamura K, et al. Signaling axis in schwann cell proliferation and differentiation. Mol Neurobiol 2006;33:51-62. [Crossref] [PubMed]
- Lin G, Chen KC, Hsieh PS, et al. Neurotrophic effects of vascular endothelial growth factor and neurotrophins on cultured major pelvic ganglia. BJU Int 2003;92:631-5. [Crossref] [PubMed]
- Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med 2013;368:436-45. [Crossref] [PubMed]
- Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol 2009;55:1037-63. [Crossref] [PubMed]
- Albersen M, Kendirci M, Van der Aa F, et al. Multipotent stromal cell therapy for cavernous nerve injury-induced erectile dysfunction. J Sex Med 2012;9:385-403. [Crossref] [PubMed]
- Fode M, Ohl DA, Ralph D, et al. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int 2013;112:998-1008. [PubMed]
- Burnett AL. Neuroprotection and nerve grafts in the treatment of neurogenic erectile dysfunction. J Urol 2003;170:S31-4; discussion S34.
- Carrier S, Zvara P, Nunes L, et al. Regeneration of nitric oxide synthase-containing nerves after cavernous nerve neurotomy in the rat. J Urol 1995;153:1722-7. [Crossref] [PubMed]
- Khan Z, Ferrari G, Kasper M, et al. The non-immunosuppressive immunophilin ligand GPI-1046 potently stimulates regenerating axon growth from adult mouse dorsal root ganglia cultured in Matrigel. Neuroscience 2002;114:601-9. [Crossref] [PubMed]
- Porowski T, Kirejczyk JK, Zoch-Zwierz W, et al. Assessment of lithogenic risk in children based on a morning spot urine sample. J Urol 2010;184:2103-8. [Crossref] [PubMed]
- Hsieh PS, Bochinski DJ, Lin GT, et al. The effect of vascular endothelial growth factor and brain-derived neurotrophic factor on cavernosal nerve regeneration in a nerve-crush rat model. BJU Int 2003;92:470-5. [Crossref] [PubMed]
- Bella AJ, Lin G, Tantiwongse K, et al. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part I. J Sex Med 2006;3:815-20. [Crossref] [PubMed]
- Lin G, Bella AJ, Lue TF, et al. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part 2. J Sex Med 2006;3:821-7; discussion 828-9. [Crossref] [PubMed]
- Bella AJ, Lin G, Garcia MM, et al. Upregulation of penile brain-derived neurotrophic factor (BDNF) and activation of the JAK/STAT signalling pathway in the major pelvic ganglion of the rat after cavernous nerve transection. Eur Urol 2007;52:574-80. [Crossref] [PubMed]
- Nakashima K, Wiese S, Yanagisawa M, et al. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci 1999;19:5429-34. [PubMed]
- Leibinger M, Müller A, Gobrecht P, et al. Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis 2013;4:e609. [Crossref] [PubMed]
- Gadient RA, Otten UH. Interleukin-6 (IL-6)--a molecule with both beneficial and destructive potentials. Prog Neurobiol 1997;52:379-90. [Crossref] [PubMed]
- Hirota H, Kiyama H, Kishimoto T, et al. Accelerated Nerve Regeneration in Mice by upregulated expression of interleukin (IL) 6 and IL-6 receptor after trauma. J Exp Med 1996;183:2627-34. [Crossref] [PubMed]
- Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol 2012;98:16-37. [Crossref] [PubMed]
- Bella AJ, Lin G, Cagiannos I, et al. Emerging neuromodulatory molecules for the treatment of neurogenic erectile dysfunction caused by cavernous nerve injury. Asian J Androl 2008;10:54-9. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Chin YE, Kitagawa M, Su WC, et al. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science 1996;272:719-22. [Crossref] [PubMed]
- Darnell JE Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994;264:1415-21. [Crossref] [PubMed]
- Liu KD, Gaffen SL, Goldsmith MA. JAK/STAT signaling by cytokine receptors. Curr Opin Immunol 1998;10:271-8. [Crossref] [PubMed]
- Jiao Y, Palmgren B, Novozhilova E, et al. BDNF increases survival and neuronal differentiation of human neural precursor cells cotransplanted with a nanofiber gel to the auditory nerve in a rat model of neuronal damage. Biomed Res Int 2014;2014:356415.
- Takemura Y, Imai S, Kojima H, et al. Brain-derived neurotrophic factor from bone marrow-derived cells promotes post-injury repair of peripheral nerve. PLoS One 2012;7:e44592. [Crossref] [PubMed]
- Li C, Zhang X, Cao R, et al. Allografts of the acellular sciatic nerve and brain-derived neurotrophic factor repair spinal cord injury in adult rats. PLoS One 2012;7:e42813. [Crossref] [PubMed]
- Vögelin E, Baker JM, Gates J, et al. Effects of local continuous release of brain derived neurotrophic factor (BDNF) on peripheral nerve regeneration in a rat model. Exp Neurol 2006;199:348-53. [Crossref] [PubMed]
- Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol 2006;24:344-50. [Crossref] [PubMed]


