Clinical applications of low-intensity pulsed ultrasound and its potential role in urology
Introduction
Ultrasound is traditionally widely used in imaging medicine for the purpose of medical diagnosis. In 1927, ultrasound was first recognized to produce lasting changes in biological systems (1), and this was the start of study on therapeutic ultrasound. Low-intensity pulsed ultrasound (LIPUS) is a form of ultrasound that delivered at a much lower intensity (<3 W/cm2) than traditional ultrasound energy and output in the mode of pulse wave, and it is typically used for therapeutic purpose in rehabilitation medicine. Especially in recent decades of years, LIPUS can be found to have a rage of biological effects on tissues, including promoting bone-fracture healing (2), accelerating soft-tissue regeneration (3,4), inhibiting inflammatory responses (5) and so on.
LIPUS has minimal thermal effects due to its low intensity and pulsed output mode, and its non-thermal effects which is normally claimed to induce therapeutic changes in tissues attract most researchers attentions (6). However, the underlying cellular and molecular mechanisms of biological effects of LIPUS on human body remains obscure and needs to be investigated, which may be mainly associated with the upregulation of cell proliferation and promoting multilineage differentiation of mesenchyme stem/progenitor cell lines through various signaling pathways (3,7). Therefore, LIPUS may become an effective clinical procedure for the treatment of urological diseases, such as chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), erectile dysfunction (ED), and stress urinary incontinence (SUI) in the field of urology.
Here we thus review the current evidence of clinical applications of LIPUS and its potential role of medical treatment for urological diseases, and make a brief summary of its underlying molecular mechanisms in the field of rehabilitation medicine.
The physical characteristics of ultrasound
Ultrasound is a form of mechanical energy with its acoustic pressure wave at frequencies beyond the upper limit of the normal human sound range, which is from 16 Hz to something approaching 15–20,000 Hz (in children and young adults) or is also normally known from 20 to 20,000 Hz. Ultrasound consists important physical characteristics as shown in the following description.
Generation of ultrasound and current equipments
Basically, a sound source vibrating sinusoidally with time and back and forth in space around its initial position leads to the generation of a sound wave. When the frequency of vibrating is above the typical human audible range, this type of sound wave is called ultrasound. In practice, a simple sound source is often a circular ceramic disk that exhibits a piezoelectric effect and has a radius of a finite dimension (8).
A typical ultrasound therapy equipment offers an operating frequency choice of 1 or 3 MHz, while the LIPUS sound wave has been generated almost exclusively at 1.5 MHz (9). Nowadays, almost all devices on the market offer LIPUS at this frequency, though the some device also offers a 0.75 MHz option which would be effective for the more deep seated lesions. Recently, the LIPUS at frequencies of 1.7 MHz has been used for improving erectile function in penile tissue of STZ-induced type I diabetic ED rats. It is currently not known whether other frequencies are effective, not as effective, or possibly more effective.
Basic concept about ultrasound
Ultrasound waves
Ultrasound waves are a kind of sound waves with the frequencies beyond 20,000 Hz. There are usually three characteristics about ultrasound waves: frequency, wavelength, and velocity. Frequency is the number of times a particle experiencing a complete compression and rarefaction cycle in one second. Currently, the therapeutic ultrasound devices typically operate in 1 or 3 MHz, while LIPUS devices usually operate in 1.5 MHz (9). Wavelength is the distance between two equivalent points on the waveform in a particular medium. The wavelength is usually about 1.5 mm at 1 MHz or 0.5 mm at 3 MHz in an average tissue of human body. Velocity is the velocity at which the wave travels through the medium. The velocity of ultrasound is approximately 350 m/s in air and it can travels more rapidly in a denser medium.
Ultrasound beam
As the ultrasound beam emerges from the treatment head of a therapy ultrasound device, the energy within the beam is not equal in space, it has areas of higher and areas of lower intensity (10). The ultrasound beam nearest the treatment head is called the near field (or the Frenzel zone), where the ultrasound energy in this part of field can be many times higher than the average energy of the beam. Beyond the outer boundary of the near field lies the far field (or the Fraunhofer zone), where the ultrasound beam is more uniform and gently divergent. The far field is typically out of use in the field of therapeutic applications. The beam nonuniformity ratio (BNR) is the numerical ratio of the intensity peaks to the mean intensity of the Near Field beam; it indicates the quality of the ultrasound applicators. For most applicators, the BNR is approximately 4–6, and the theoretical best value for BNR is infinitely near the number 1.
Ultrasound intensity
Usually, at intensities of 0.05–0.50 W/cm2, ultrasound is widely used in imaging medicine (11). At intensities of 0.03–1,000 W/cm2, the surgical and therapeutic benefits of ultrasound have been typically used and explored (9). Through the review of literature, depending on the intensity of exposure, the therapeutic ultrasound can be divided into two groups: low-intensity ultrasound (<3 W/cm2) and high-intensity ultrasound (≥3 W/cm2). The dosage of low-intensity ultrasound can be further divided into three groups: low dose (<1 W/cm2), middle dose (1–2 W/cm2), and high dose (2–3 W/cm2). In regular clinical applications, the intensity of ultrasound applied ranges from about 0.03–1.0 W/cm2 (12,13).
Ultrasound frequency
In addition, ultrasound waves can be divided into three ranges depending on the ultrasound frequency: high frequency ultrasound (1–20 MHz) used in medical diagnosis, medium frequency ultrasound (0.7–3.0 MHz) used in therapeutic medicine, and low frequency (LF) ultrasound (20–200 kHz) used in industrial and therapeutic applications (14). LF ultrasound is a specific kind of medical ultrasound in the kHz range of frequencies with its upper limit frequencies below 1 MHz, whose frequency mainly range from 20 kHz to 100 kHz (14). Of course, LF ultrasound can be divided into two groups: low-intensity LF ultrasound and high-intensity LF ultrasound. Typically, low-intensity LF ultrasound is applied in the field of enhancement of drug delivery (15) and high intensity focused ultrasound (HIFU) is applied in the field of cancer ablation and palliative treatment (16-19). The classifications and applications of ultrasound waves depending on ultrasound intensity or frequency are summarized in Table 1.
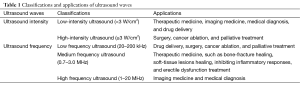
Full table
Pulsed ultrasound
Ultrasound can be output in the mode of pulse wave, and for many clinicians it is a preferable mode for treatment (20). The typical pulse ratios of pulse duration time to pulse rest time are 1:1 and 1:4, though there are other ratios available. The proportion of time that the machine is on compared with off is a relevant factor in ultrasound wave dosage calculations. The pulse frequency is the number of times that the machines offer the ultrasound pulses in one second. Currently, the typical pulsed machine frequencies are 100 and 1,000 Hz, and there is no evidence that which frequency has any clinical advantage over the other. In addition, current pulsed ultrasound machines on available typically deliver ultrasound pulsed at 20% (1:4) and at 1,000 Hz (1 kHz), that is 200 µs ultrasound and 800 µs not ultrasound in 1,000 cycles per second (20).
Low-intensity pulsed ultrasound (LIPUS)
LIPUS is clearly a form of medium frequency ultrasound (0.7–3 MHz) that output in the mode of pulse wave (100 and 1,000 Hz) and delivered at a much lower intensity (<3 W/cm2) than traditional ultrasound energy. As mentioned in the introduction, the power density of LIPUS used for fracture healing is much lower than that with the traditional ultrasound treatments. Almost all of the LIPUS researchers used sound wave of intensity at 0.03 W/cm2 (or known as 30 mW/cm2), pulse ratio 1:4 at 1,000 Hz, and frequency at 1.5 MHz in their studies (10).
Therapeutic mechanisms of LIPUS
There are two types of mechanism which are commonly invoked to explain the effects produced by therapeutic ultrasound: thermal effects and non-thermal effects (21). However, the underlying cellular and molecular mechanisms of biological effects of LIPUS on human body remains obscure and needs to be investigated, which may be mainly associated with the upregulation of cell proliferation through activation of integrin receptors and Rho/ROCK/Src/ERK signaling pathway (3), and with promoting multilineage differentiation of mesenchyme stem/progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway (7). It still needs an intense effort for basic-science and clinical investigators to explore the biomedical applications of ultrasound.
Biophysical effects of therapeutic ultrasound
It is believed that there are two types of mechanism which are commonly invoked to explain the effects produced by therapeutic ultrasound, and the biophysical effects of therapeutic ultrasound are generally divided into two sides: thermal and non-thermal (21). LIPUS has minimal heating effects due to its low intensity and pulsed output mode, and its non-thermal effects which is normally claimed to induce therapeutic changes in tissues attract most researchers attentions (6). However, it is too simplistic to assume that there will either be thermal or non-thermal effects with a particular treatment application, it is almost inevitable that both effects will occur at the same time.
Thermal effects
The energy transported by an ultrasonic beam is attenuated as it passes through tissues, energy scattered out of the beam may be absorbed elsewhere in the tissue. As the energy within the sound wave is passed down into the tissues, it will cause oscillation of the particles (10). The increase in the molecular vibration in the tissue can result in heat generation, and the ultrasound can then produce thermal changes in the tissues.
The rate at which the temperature rises is associated with the intensity attenuation, density of the tissue, and its heat capacity (13). For example, a rate of temperature rise in liver subjected to 3 MHz ultrasound at an intensity of 1 W/cm2 of 0.140 °C/s or to 1 MHz ultrasound at the same intensity of 0.048 °C/s (13). The collagenous tissues, periosteum, and fibrotic muscle are the most preferential heated tissues in the human body, thus these organs give themselves the opportunities of being primarily treated by physiotherapist (22). Usually, the temperatures that higher than 42.0 °C are thought to be toxic to cells, while temperatures that lower than 41.8 °C are thought to be beneficial in pain relief, changes in blood flow, and decrease in muscle spasm (23).
Non-thermal effects
But the current usage of ultrasound therapy does not only focus on the thermal changes. Besides, the vibrations of the tissue molecular particles have effects which are generally considered to be non-thermal in nature. Now, the non-thermal effects of therapeutic ultrasound are typically considered to be a combination of cavitation, acoustic streaming, and micromassage (9,13,21).
Cavitation is the ability of ultrasound to form gas filled voids within the tissues or fluids, which has two types of forms: stable cavitation and unstable cavitation. Stable cavitation occurs at therapeutic doses of ultrasound and the gas bubbles cost 1,000 cycles to reach their maximum size, while unstable cavitation usually occurs at HIFU which has very high intensities (≥1,000 W/cm2) that can produce instantaneous tissue necrosis with its gas bubbles collapse quickly releasing a large amount of energy. Acoustic streaming is the small scale eddying of fluids near a vibrating structure that can affect diffusion rates and membrane permeability, which leads to an alteration in the process of protein synthesis and cellular secretions (10). Micromassage is the effect of molecules vibrating when the sound wave travelling through the medium, which can possibly affect tissue fluid interchange and tissue mobility. In short, the stable cavitation and acoustic streaming contribute mainly to the non-thermal effects in the application of LIPUS.
Biological mechanisms of LIPUS effects
Recent studies showed that effects of LIPUS in healing morbid body tissues may be mainly associated with the upregulation of cell proliferation through activation of integrin receptors and Rho/ROCK/Src/ERK signaling pathway (3), and with promoting multilineage differentiation of mesenchyme stem/progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway (7).
LIPUS promotes cell proliferation
In 2004, Zhou et al. examined the effect of LIPUS on the proliferation of primary human foreskin fibroblasts and the underlying signaling mechanisms, and came out with a conclusion that LIPUS promotes cell proliferation via activation of integrin receptors and a Rho/ROCK/Src/ERK signaling pathway (3). In their study, fibroblast cell was observed to have an increase in the total cell number and an increase of bromodeoxyuridine incorporation after LIPUS stimulation, and LIPUS can induce stress fiber and focal adhesion formation via activation of Rho. Further, they found that LIPUS selectively induced activation of extracellular signal-regulated kinase (ERK) 1/2, and inhibition of Rho-associated coiled-coil-containing protein kinase (ROCK) prevented LIPUS-induced ERK1/2 activation. This indicated that the Rho/ROCK pathway is an upstream regulator of ERK activation in response to LIPUS. Moreover, activation of ROCK and MEK-1 was required for LIPUS-induced DNA synthesis, which can be prevented by an integrin β1 blocking antibody as well as a RGD peptide. In addition, the phosphorylation of Src at Tyr(416) was slightly increased, and Src activity was required for ERK1/2 activation of cell proliferation in response to LIPUS.
LIPUS on stem/progenitor cells activation and differentiation
In 2013, Lv et al. investigated the role of LIPUS on induced pluripotent stem cells-derived neural crest stem cells (iPSCs-NCSCs) (24). Results showed that LIPUS at 500 mW/cm2 could enhance the viability and proliferation of iPSCs-NCSCs after 2 days, and up-regulated gene and protein expressions of NF-M, Tuj1, S100beta and GFAP in iPSCs-NCSCs after 4 days whereas up-regulated expressions of only NF-M, S100beta and GFAP after 7 days were observed. LIPUS treatment at an appropriate intensity can be an efficient and cost-effective method to enhance cell viability, proliferation and neural differentiation of iPSCs-NCSCs in vitro for peripheral nerve tissue engineering. In 2015, the same research group tested effects of the combination of LIPUS and iPSCs-NCSCs on the regeneration of rat transected sciatic nerve in vivo (25). They found that treatment with 0.3 W/cm2 LIPUS for 2 weeks and 5 min per day could significantly improve the sciatic functional index, static sciatic function index and nerve conduction velocity of rat sciatic nerve, and that there were more regenerative new blood vessels and new neurofilaments, higher expression level of beta-III tubulin (Tuj1) in the experimental group seeded with iPSCs-NCSCs and stimulated with LIPUS. It suggested that combination of LIPUS with iPSCs-NCSCs could promote the regeneration and reconstruction of rat transected sciatic nerve and is an efficient method for peripheral nerve regeneration.
In 2014, Kusuyama et al. found LIPUS can influence the multilineage differentiation of mesenchymal stem and progenitor cell lines via ROCK-Cot/Tpl2-MEK-ERK signaling pathway (7). In their study, LIPUS was applied to adipogenic progenitor cell and mesenchymal stem cell (MSC) lines to analyze how multilineage cell differentiation was affected. Results showed that adipogenic differentiation of both cell types were suppressed by LIPUS and represented by impaired lipid droplet appearance, and peroxisome proliferator-activated receptor gamma2 (Pparg2) and fatty acid-binding protein 4 (Fabp4) gene expression were decreased. On the contrary, LIPUS could promote the MSC lines differentiate into osteogenic cell by inducing the expression of runt-related transcription factor 2 (Runx2) and osteocalcin mRNAs and by increasing cell calcification. LIPUS could also induce the expression of phosphorylation of cancer Osaka thyroid oncogene/tumor progression locus 2 (Cot/Tpl2) kinase, which was essential in the phosphorylation process of mitogen-activated kinase kinase 1 (MEK1) and p44/p42 extracellular signal-regulated kinases (ERKs), while the above process can be prevented by a Cot/Tpl2-specific inhibitor and be attenuated by the inhibition of Rho-associated kinase. In brief, LIPUS suppresses adipogenesis and promotes osteogenesis of MSCs through Rho-associated kinase-Cot/Tpl2-MEK-ERK signaling pathway (7).
Potential genes affected by LIPUS
In a review about the clinical evidence and the associated biological mechanism of LIPUS for fracture healing (26), Pounder and Harrison indicated that the accelerated mineralisation is related with increases in osteocalcin, alkaline phosphatase, VEGF and MMP-13 expression. Integrins and its downstream multiple signaling pathways, including the ERK, NF-kB, and PI3 kinase pathways, are also activated by the ultrasound in the fracture healing process, which are directly linked to the production of key factors that involved in the processes of mineralisation and endochondral ossification (26). In a study performed by Rutten et al. in 2009 (27), osteogenic cells were identified by immunolocalization of RUNX2 protein in patients with a delayed union of the osteotomized fibula, and results showed that LIPUS does not increase osteogenic cell presence but rather likely to affect osteogenic cell differentiation. Kumagai et al. found that LIPUS could induce the homing of circulating osteogenic progenitors to the fracture site by using a parabiotic animal model (28), which were formed by surgically conjoining a green fluorescent protein (GFP) mouse and a syngeneic wild-type mouse. Besides, MAPK and other kinases signaling pathways, gap-junctional intercellular communication, up-regulation and clustering of integrins, involvement of the cyclooxygenase-2 (COX-2)/PGE2, iNOS/NO pathways and activation of ATI mechanoreceptor are also associated with the biological responses of bone fracture healing processes under LIPUS treatment (29).
Current clinical applications of LIPUS
In recent years, LIPUS has been found to have a wide range of biological effects on tissues and have been applicated in many ways in the field of therapeutic medicine, such as promoting bone-fracture healing (2), accelerating soft-tissue regeneration (4), and inhibiting inflammatory responses (5) and so on. Thus, here reviews the current applications of LIPUS in medical activities of rehabilitation medicine. Still, there are many challenges for this relatively new application, and the achievements using it promises to go far beyond the present possibilities.
Therapeutic dosages of LIPUS
The ultrasound intensity of LIPUS is much lower than that of typically used traditional ultrasound energy, the intensity of LIPUS is belonging to the low dose (<1 W/cm2) subgroup within the low-intensity ultrasound (<3 W/cm2) group. The most common application parameters of LIPUS used are: intensity at 0.03 W/cm2 (or known as 30 mW/cm2), pulse ratio 1:4 at 1,000 Hz, and frequency at 1.5 MHz (10). Moreover, for regular therapeutic applications, the intensity of LIPUS applied can range from about 0.03–1.00 W/cm2 (12,13). The energy density (in J/cm2) of LIPUS can be calculated as ultrasound intensity (in W/cm2) × time (in seconds). Thus, as the application time of LIPUS rages from 2–20 min and that range of LIPUS intensity mentioned above, the commonly applied energy density dosage of LIPUS similarly ranges from about 2–150 J/cm2 (30).
Clinical therapeutic procedures
When treatments were performed, the target tissues should be placed in a suitable position with local anesthesia or without anesthesia, and the ultrasound probe head is usually suspended from an articulating arm for flexible movement. Applied locations, treatment duration time, and treatment cycles are chosen according to the kind of equipments and disease applications.
For therapeutic purposes, it is vital to pass the energy into the human tissues completely. So, a number of methods are used to couple the sound into the tissue. Aqueous gel may be used between the source’s head and the tissue skin when the tissue surface is relatively flat and the probe head is plane. Otherwise, water may provide a better coupling medium for awkward tissue geometries. Besides, it is important that the couplant is degassed to prevent the occurrence of cavitation.
Clinical applications of LIPUS
Currently, LIPUS is accepted to promote bone-fracture healing (2), accelerating soft-tissue regeneration (4), and inhibit inflammatory responses (5). Besides, it has made it as a tool to be used to enhance regeneration and tissue engineering, for example being used in oral and maxillofacial regions (31).
Bone-fracture healing
Corradi and Cozzolino first reported in 1952 that continuous wave ultrasound could stimulate the formation of bone callus in a radial fracture rabbit model, and it was proved by the same research group in the next year that the ultrasound wave was safe and could produce an increase in periosteal callus in eight patients, and this is the first evidence of application of ultrasound wave on fracture healing (32).
In 1994 and 1997, Heckman and Kristiansen performed two rigorous, prospective, randomized, double-blind, placebo-controlled clinical trials and found that the rate of healing of fresh fractures is accelerated by non-invasive LIPUS (33,34). The first trial tested the efficacy of ultrasound on closed or grade-I open fractures of the tibial shaft, and the second trial tested the efficacy of ultrasound on dorsally angulated fractures (negative volar angulation) of the distal aspect of the radius. The patients in both trials had been imposed ultrasound stimulating device 30 mW/cm2 daily for 20 min at home for 10 weeks as an adjunct to conventional manipulation treatment with a cast. Results showed the specific ultrasound accelerate the healing of fractures and decrease the loss of reduction during fracture-healing, and there were no serious complications related to the use of the ultrasound device. These two clinical trials primarily promoted the U.S. Food and Drug Administration (FDA) approve the use of low-intensity ultrasound for the accelerated healing of fresh fractures in 1994 and for the treatment of established nonunions in 2000 (32).
In a meta-analysis of six randomized controlled trials (RCTs) performed by Busse et al. in 2002 (35), LIPUS was found to have a significant effect on reducing the time to fracture healing for fractures treated nonoperatively, results showed that fracture healing time was significantly shorter in low-intensity ultrasound therapy groups than that in the control groups. In a review of the clinical evidence on LIPUS for fracture healing in 2008 (26), Pounder and Harrison found that typically widely used LIPUS (1.5 MHz ultrasound pulsed at 1 kHz, 20% duty cycle, 30 mW/cm2 intensity) could accelerate the healing time by up to 40% in fresh tibia, radius and scaphoid fractures, and that it was shown to be effective at resolving all types of nonunions of all ages. In a review searching for the evidence of LIPUS for in vitro, animal and human fracture healing in 2011 (36), Martinez de Albornoz et al. agreed that LIPUS can produce significant osteoinductive effects, accelerate the healing process and improve the bone-bending strength in vitro and animal studies. In a cohort study of 4,190 patients treated with LIPUS performed by Zura et al. in 2015, older patients (≥60 y) with fracture risk factors treated with LIPUS were found to exhibit similar heal rates to the population as a whole (37).
But there was still a controversy about the LIPUS effects in fresh, stress fractures and in limb lengthening in human trials. In a systematic review and meta-analysis of seven human clinical trials on fresh fractures in 2012 (20), Bashardoust Tajali et al. found that the time of the third cortical bridging (increase in density or size of initial periosteal reaction) in radiographic healing was statistically earlier following LIPUS therapy in fresh fractures, but there was a paucity of sufficient studies of LIPUS’s beneficial effects on delayed unions and nonunions. In addition, LIPUS may not have a potential beneficial effects for the treatment of acute fractures in adults, and future trials should record functional outcomes and follow-up all trial participants in clinical practice (38).
Soft-tissue regeneration
The targets of LIPUS effects on soft-tissue regeneration cover a wide range of cells and organs, including fibroblasts (3), myoblasts (39), epithelial cells (4), chondrocytes and cartilage (40-45), inter-vertebral discs (IVDs) (46,47), ligaments (48-51), and tendons (52,53).
In 2004, Zhou et al. examined the effects of daily application of LIPUS on the proliferation of primary human foreskin fibroblasts (3). In their study, fibroblast cell was observed to have an increase in the total cell number and an increase of bromodeoxyuridine incorporation after LIPUS (1.5 MHz ultrasound wave, 200 µs pulse modulated at 1 kHz, with an output intensity of 30 mW/cm2) stimulation, and LIPUS can induce stress fiber and focal adhesion formation. They concluded that LIPUS promotes cell proliferation via activation of integrin receptors and a Rho/ROCK/Src/ERK signaling pathway.
Ikeda et al. investigated the effects of LIPUS on the differentiation of C2C12 cell, which is a subclone of C2 myoblasts originally isolated from the thigh muscle of C3H mouse (39). In their study, they found that mRNA expression of Runx2, Msx2, Dlx5, AJ18, and Sox9 was increased by the LIPUS stimulation (1.5 MHz at an intensity of 70 mW/cm2 for 20 min), whereas the expression of MyoD, C/EBP, and PPARγ was decreased. And LIPUS stimulation increased Runx2 protein expression and phosphorylation of ERK1/2 and p38 MAPK. They concluded that LIPUS stimulation converts the differentiation pathway of C2C12 cells into the osteoblast and/or chondroblast lineage via activated phosphorylation of ERK1/2 and p38 MAPK.
Ikai et al. evaluated the effects of LIPUS on wound healing in periodontal tissues after mucoperiosteal flap surgery in beagle dogs (4). After the LIPUS treatment (a 200 µs burst sine wave of 1.5 MHz repeated at a frequency of 1.0 kHz, 30 mW/cm2, daily for 20 min, for a period of 4 weeks), the expression level of heat shock protein 70 (HSP70) was higher in the gingival epithelial cells of the LIPUS-treated tooth, and the regeneration processes of both cementum and mandibular bone were accelerated. They came with a conclusion that ultrasound could accelerate periodontal wound healing and bone repair.
In 2002, Nishikori et al. found that LIPUS exposure (1.5 MHz with a 200 µs tone burst repeated at 1.0 kHz, 30 mW/cm2, 20 min per day) could promote synthesis of chondroitin sulfate, especially chondroitin 6-sulfate, although it did not significantly enhance cell number and stiffness (40). In vitro cell studies, LIPUS was demonstrated to have an effect on stimulating chondrocyte proliferation and matrix production (41-43). The potential mechanisms of LIPUS effects on chondrocytes may be associated with activation of MAPK/Erk pathway and the increase of the anabolic factor (TIMP-1)/catabolic factor (MMP-3) ratio (44,45).
LIPUS may also have effects on treating intervertebral disc herniation and delaying the progression of disc degeneration. In 2008, Omi et al. found that LIPUS stimulation could significantly activate TIMP-1 and monocyte chemoattractant protein-1 (MCP-1) in nucleus pulposus cells and macrophages at both the protein and gene levels (46). And in 2009, Kobayashi et al. found that LIPUS could upregulate the cell proliferation and proteoglycan sythesis in human nucleus pulposus cells via enhancement of several matrix-related genes (47).
Takakura et al. found that LIPUS (30 mW/cm2, 20 min daily) is effective for enhancing the early healing of medial collateral ligament injuries in rats in 2002 (48). And Warden et al. found that LIPUS could accelerate ligament healing in a controlled laboratory study in adult rats (49). In a recently published paper, Hu et al. found that LIPUS can facilitate osteogenic differentiation in human periodontal ligament cells, the underlying mechanism may be associated with upregulation of Runx2 and integrin beta1 (50), and so involved p38 MAPK pathway signaling (51).
In addition, bone-tendon healing can also be accelerated under the LIPUS treatment, both in the partial patellectomy model in rabbits (52) and the transosseous-equivalent sheep rotator cuff model (53).
Inhibiting inflammatory responses
During injury or in the forming of the rheumatoid arthritis (RA) and osteoarthritis (OA), inflammatory plays an essential role in these progresses (54). Recent studies have demonstrated that LIPUS could inhibit inflammatory responses both in vitro and in vivo.
In 2014, Nakao et al. reported that LIPUS could inhibit LPS-induced inflammatory responses of osteoblasts through TLR4-MyD88 dissociation (5). In their study, LPS induced mRNA expression of several chemokines including CCL2, CXCL1, and CXCL10 in both mouse osteoblast cell line (MC3T3-E1) and calvaria-derived osteoblasts (from newborn C57BL/6 mouse). After the LIPUS (1.5-MHz, 200 µs burst sine waves at 1.0 kHz, 30 mW/cm2) treatment, CXCL1 and CXCL10 mRNA induction were significantly inhibited, and LPS-induced phosphorylation of ERKs, p38 kinases, MEK1/2, MKK3/6, IKKs, TBK1, and Akt was decreased. LIPUS inhibited the transcriptional activation of NF-kB responsive element and interferon-sensitive response element (ISRE) by LPS, and LIPUS significantly inhibited TLR4-MyD88 complex formation in a transient transfection experiment. And Nakamura et al. investigated the effects of LIPUS on inhibiting inflammatory responses in vitro in the rabbit knee synovial membrane cell line (HIG-82), which was cultured in medium with or without IL-1β or TNF-α (55). The parameters of LIPUS they used in their study were: 15 min of single LIPUS exposure, 3 MHz with a spatial-average intensity of 30 mW/cm2 and pulsed 1:4 (2 ms on and 8 ms off). The proinflammatory cytokines significantly up-regulated cell proliferation, and LIPUS could significantly down-regulated this action.
Nakamura et al. also investigated the effects of LIPUS on inhibiting inflammatory responses in vivo in the knee joints of animal models for RA using MRL/lpr mice (55). The LIPUS parameters were as same as that in the above mentioned in vitro study. In MRL/lpr mice, treated with LIPUS for 3 weeks, histological damage of knee joints and lesions were significantly reduced compared to the control, and COX-2-positive cells were markedly decreased in the knee joints treated with LIPUS compared to the control joints. In 2012, Engelmann et al. evaluated the effect of LIPUS and dimethylsulfoxide (DMSO) gel treatment on the expression of pro-inflammatory molecules in an animal model of traumatic muscle injury (56). Results showed that LIPUS associated with DMSO gel can attenuate TNFα, IL-1β, NF-kB protein levels and JNK phosphorylation in traumatic muscle injury.
Ptential applications of LIPUS for urological diseases
In the modern biopsychosocial model of medicine, biological, psychological, and social factors all play a significant role in human functioning in the context of disease or illness. Combined therapeutic treatments and personal treatments will play an important role in physical medicine and rehabilitation medicine. Since the therapeutic and biological effects and wide clinical applications of LIPUS on various human tissues, LIPUS may become an effective clinical procedure for the treatment of urological diseases.
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS)
CP/CPPS is defined as chronic pelvic pain symptoms last for at least 3 to 6 months, in the absence of a urinary tract infection or another well identifiable cause. It is classified into the third category of prostatitis (category III) and it takes up about 90–95% of men with prostatitis. Although many proposed etiologies and mechanisms exist to explain the pathogenesis of CP/CPPS, neither cause of disease has been exactly known nor effective treatments have been identified. Therefore, effective therapeutic approaches for CP/CPPS are far from well satisfactory by both physicians and patients. A novel therapeutic approach is eagerly needed (57).
Ultrasound therapy was firstly used for chronic prostatitis (CP) by Karpukhin et al. in 1977 (58). In a randomized, double-blind, multi-centered clinical trial conducted by Li et al. in 2013 (59), the clinical efficacy and safety of transperineal ultrasonic therapy for CP was analyzed using the scores of NIH-CPSI and the results of prostate fluid routine examination. The patients were divided into groups A (trial) and B (control), the former were treated by transperineal ultrasound, while the latter with the same machine but no ultrasound waves. The ultrasound therapy lasted 10 min a time every other day for 2 weeks. Then the scores of NIH-CPSI and counts of white blood cells (WBC) and lecithin corpuscles (LC) in the prostate fluid between the two groups before and after treatment were evaluated. Resuts turned out that transperineal ultrasonic therapy with advantages of safety, easy operation and high acceptability is highly effective for CP, especially in relieving prostate pain.
Very recently, several biomarkers have been proved as strongly correlated with CP/CPPS, including interleukin-8 (IL-8), MCP-1, and macrophage inflammatory protein-1α (MIP-1α) (46,60). The patients with higher levels of IL-8 reported the worst symptoms, and CPPS subtypes of prostatitis had statistically higher levels of MCP-1 and MIP-1α than the control group and patients with benign prostatic hyperplasia. LIPUS has been demonstrated to have effects on regulating secretions of these cytokines (46). Moreover, LIPUS exerts anti-inflammatory effects on LPS-stimulated osteoblasts by inhibiting TLR4 signal transduction (5), and LIPUS exposure could inhibit IL-1beta-induced COX-2 expression through the integrin beta1 receptor followed by the phosphorylation of ERK 1/2 (61), since that COX-2 is response for pain. So, LIPUS is regarded as an effective clinical procedure for the treatment of CP/CPPS due to the abovementioned factors, and the mechanism of LIPUS for its biological effects will be further clarified and optional clinical energy dosage and therapeutic protocol will be established in the future.
Erectile dysfunction (ED)
Recently, low-energy shock wave therapy (LESWT), another important form of therapeutic sound waves used in rehabilitation medicine, was shown to markedly improve erectile function in patients with organic ED (62), as well as in diabetic rats (63,64). LESWT has been recommended as a first-line therapy for ED by European Association of Urology (EAU) and International Society for Sexual Medicine (ISSM). The mechanisms of LESWT on ED may involve down-regulating receptor for advanced glycation end products (63) and recruiting endogenous MSCs (64). ED shares common risk factors with cardiovascular disease (CVD), and ED could be an early sign of symptomatic CVD (65). As was stated above, LIPUS has been demonstrated to have benefits on diverse pathological processes of human body, moreover, novel angiogenesis-promoting effect of LIPUS was found in studies of the human cardiovascular system (66,67). Therefore, the question of whether LIPUS has the effects of improving erectile function in the field of ED is badly in need of validation.
In a preliminary study by Lei et al. in 2015, LIPUS therapy was found to have effects on improving erectile function and reversing pathologic changes in penile tissue of STZ-induced type I diabetic ED rats (68). Results showed that LIPUS (100, 200, and 300 mW/cm2 intensity, 20% duty cycle at 1.0 kHz, 1.7 MHz) therapy lasted for 3 min per time, 3 times per week for 2 weeks could increase endothelial and smooth muscle content, collagen I/collagen III ratio, quantity of elastic fibers, and eNOS and nNOS expression in diabetic penis, as well as downregulate of the TGF-beta1/Smad/CTGF signaling pathway in penile tissue. Whether LIPUS has the effects of recruiting endogenous MSCs in erectile tissues need to be further evaluated. However, before the future potential application of LIPUS therapy for ED in the clinic, safety profiles, clinical trials, and repeated treatments are needed to be investigated in further studies.
Stress urinary incontinence (SUI)
The International Continence Society defines SUI as an involuntary leakage of urine on exertion effort, coughing or sneezing. About 35% of women had experienced incontinence, and SUI is the most prevalent type of incontinence with 50% of women presenting with urinary incontinence exhibiting pure SUI (69). Normally, urinary continence is maintained through co-ordination between the bladder, urethra, pelvic floor muscles, and the nervous system. The development of SUI is attributable to two recognized mechanisms: hypermobility and intrinsic sphincter deficiency (ISD), which can coexist. Hypermobility can lead to uneven pressure transmission and opening of the bladder neck, which results in urine leakage during exertion; while during ISD the sphincter is unable to maintain resting urethral closing pressure (70). Currently, the treatment of SUI can be stratified into conservative, pharmacological and surgical methods, and the treatment of patients with SUI should be tailored to the individual to optimize care.
Recently, stem cell based therapy has been utilized for the deficient urinary sphincter and nerve regeneration to treat SUI in many studies (71). The application of MSCs as part of a therapeutic strategy for functional regeneration of the urinary sphincter complex is due to two ways of mechanisms: the MSCs are capable to differentiate and functionally replace degenerated smooth muscular cells of the urethral sphincter, and growth factors released by the MSCs could regenerate the sphincter complex. Since LIPUS has effects of activation and differentiation of stem/progenitor cells in vitro and in vivo, LIPUS treatment at an appropriate intensity used alone or combined could help for muscle and nerve regeneration in urinary sphincter complex. With the potential application of LIPUS treatment, the pathological and functional recoveries in SUI patients would be in a safety and efficacy way.
Conclusions
To date, ultrasound waves are not only used in imaging medicine for diagnosis, but also are performed in rehabilitation medicine for the purpose of preventing and curing disease due to its thermal and non-thermal effects. LIPUS takes up most part of above-mentioned ultrasound applications that including promoting bone-fracture healing, accelerating soft-tissue regeneration, inhibiting inflammatory responses and so on. LIPUS may become an effective clinical procedure for the treatment of CP/CPPS, ED, and SUI in the field of urology. However, the underlying mechanisms of therapeutic ultrasound biological effects on human body remains to be investigated, and best designed rigorous basic and clinical studies are needed to explore its further applications in rehabilitation medicine. Ultrasound waves, perhaps especially of LIPUS, might be a more comprehensively utilized method than we could foresee for personal treatments in the modern biopsychosocial model of medicine.
Acknowledgements
Funding: This work is supported by the National Natural Science Foundation of China: No. 81470921 and No. 81272531.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wood RW, Loomis AL. XXXVIII. The physical and biological effects of high-frequency sound-waves of great intensity. London, Edinburgh, Dublin Philosophical Magazine J Sci 1927;4:417-36.
- Urita A, Iwasaki N, Kondo M, et al. Effect of low-intensity pulsed ultrasound on bone healing at osteotomy sites after forearm bone shortening. J Hand Surg Am 2013;38:498-503. [Crossref] [PubMed]
- Zhou S, Schmelz A, Seufferlein T, et al. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J Biol Chem 2004;279:54463-9. [Crossref] [PubMed]
- Ikai H, Tamura T, Watanabe T, et al. Low-intensity pulsed ultrasound accelerates periodontal wound healing after flap surgery. J Periodontal Res 2008;43:212-6. [Crossref] [PubMed]
- Nakao J, Fujii Y, Kusuyama J, et al. Low-intensity pulsed ultrasound (LIPUS) inhibits LPS-induced inflammatory responses of osteoblasts through TLR4-MyD88 dissociation. Bone 2014;58:17-25. [Crossref] [PubMed]
- Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol 2007;93:384-98. [Crossref] [PubMed]
- Kusuyama J, Bandow K, Shamoto M, et al. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem 2014;289:10330-44. [Crossref] [PubMed]
- Wu J, Nyborg WL. Ultrasound, cavitation bubbles and their interaction with cells. Adv Drug Deliv Rev 2008;60:1103-16. [Crossref] [PubMed]
- ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol 2007;93:111-29. [Crossref] [PubMed]
- Watson T. Ultrasound in contemporary physiotherapy practice. Ultrasonics 2008;48:321-9. [Crossref] [PubMed]
- Warden SJ. A new direction for ultrasound therapy in sports medicine. Sports Med 2003;33:95-107. [Crossref] [PubMed]
- Khanna A, Nelmes RT, Gougoulias N, et al. The effects of LIPUS on soft-tissue healing: a review of literature. Br Med Bull 2009;89:169-82. [Crossref] [PubMed]
- ter Haar G. Therapeutic ultrasound. Eur J Ultrasound 1999;9:3-9. [Crossref] [PubMed]
- Ahmadi F, McLoughlin IV, Chauhan S, et al. Bio-effects and safety of low-intensity, low-frequency ultrasonic exposure. Prog Biophys Mol Biol 2012;108:119-38. [Crossref] [PubMed]
- Azagury A, Khoury L, Enden G, et al. Ultrasound mediated transdermal drug delivery. Adv Drug Deliv Rev 2014;72:127-43. [Crossref] [PubMed]
- Zhou Y. High-intensity focused ultrasound treatment for advanced pancreatic cancer. Gastroenterol Res Pract 2014;2014:205325.
- Uchida T, Tomonaga T, Kim H, et al. Improved outcomes with advancements in high intensity focused ultrasound devices for the treatment of localized prostate cancer. J Urol 2015;193:103-10. [Crossref] [PubMed]
- Fornage BD, Hwang RF. Current status of imaging-guided percutaneous ablation of breast cancer. AJR Am J Roentgenol 2014;203:442-8. [Crossref] [PubMed]
- Khokhlova TD, Hwang JH. HIFU for palliative treatment of pancreatic cancer. J Gastrointest Oncol 2011;2:175-84. [PubMed]
- Bashardoust Tajali S, Houghton P, MacDermid JC, et al. Effects of low-intensity pulsed ultrasound therapy on fracture healing: a systematic review and meta-analysis. Am J Phys Med Rehabil 2012;91:349-67. [Crossref] [PubMed]
- Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: biophysical effects. Phys Ther 2001;81:1351-8. [PubMed]
- Dyson M, Suckling J. Stimulation of tissue repair by ultrasound: a survey of the mechanisms involved. Physiotherapy 1978;64:105-8. [PubMed]
- Draper DO, Mahaffey C, Kaiser D, et al. Thermal ultrasound decreases tissue stiffness of trigger points in upper trapezius muscles. Physiother Theory Pract 2010;26:167-72. [Crossref] [PubMed]
- Lv Y, Zhao P, Chen G, et al. Effects of low-intensity pulsed ultrasound on cell viability, proliferation and neural differentiation of induced pluripotent stem cells-derived neural crest stem cells. Biotechnol Lett 2013;35:2201-12. [Crossref] [PubMed]
- Lv Y, Nan P, Chen G, et al. In vivo repair of rat transected sciatic nerve by low-intensity pulsed ultrasound and induced pluripotent stem cells-derived neural crest stem cells. Biotechnol Lett 2015;37:2497-506. [Crossref] [PubMed]
- Pounder NM, Harrison AJ. Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action. Ultrasonics 2008;48:330-8. [Crossref] [PubMed]
- Rutten S, Nolte PA, Korstjens CM, et al. Low-intensity pulsed ultrasound affects RUNX2 immunopositive osteogenic cells in delayed clinical fracture healing. Bone 2009;45:862-9. [Crossref] [PubMed]
- Kumagai K, Takeuchi R, Ishikawa H, et al. Low-intensity pulsed ultrasound accelerates fracture healing by stimulation of recruitment of both local and circulating osteogenic progenitors. J Orthop Res 2012;30:1516-21. [Crossref] [PubMed]
- Padilla F, Puts R, Vico L, et al. Stimulation of bone repair with ultrasound: a review of the possible mechanic effects. Ultrasonics 2014;54:1125-45. [Crossref] [PubMed]
- Robertson VJ, Baker KG. A review of therapeutic ultrasound: effectiveness studies. Phys Ther 2001;81:1339-50. [PubMed]
- Tanaka E, Kuroda S, Horiuchi S, et al. Low-intensity pulsed ultrasound in dentofacial tissue engineering. Ann Biomed Eng 2015;43:871-86. [Crossref] [PubMed]
- Rubin C, Bolander M, Ryaby JP, et al. The use of low-intensity ultrasound to accelerate the healing of fractures. J Bone Joint Surg Am 2001;83-A:259-70. [PubMed]
- Heckman JD, Ryaby JP, McCabe J, et al. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am 1994;76:26-34. [PubMed]
- Kristiansen TK, Ryaby JP, McCabe J, et al. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am 1997;79:961-73. [PubMed]
- Busse JW, Bhandari M, Kulkarni AV, et al. The effect of low-intensity pulsed ultrasound therapy on time to fracture healing: a meta-analysis. CMAJ 2002;166:437-41. [PubMed]
- Martinez de Albornoz P, Khanna A, Longo UG, et al. The evidence of low-intensity pulsed ultrasound for in vitro, animal and human fracture healing. Br Med Bull 2011;100:39-57. [Crossref] [PubMed]
- Zura R, Mehta S, Della Rocca GJ, et al. A cohort study of 4,190 patients treated with low-intensity pulsed ultrasound (LIPUS): findings in the elderly versus all patients. BMC Musculoskelet Disord 2015;16:45. [Crossref] [PubMed]
- Griffin XL, Smith N, Parsons N, et al. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Database Syst Rev 2012;2:CD008579. [PubMed]
- Ikeda K, Takayama T, Suzuki N, et al. Effects of low-intensity pulsed ultrasound on the differentiation of C2C12 cells. Life Sci 2006;79:1936-43. [Crossref] [PubMed]
- Nishikori T, Ochi M, Uchio Y, et al. Effects of low-intensity pulsed ultrasound on proliferation and chondroitin sulfate synthesis of cultured chondrocytes embedded in Atelocollagen gel. J Biomed Mater Res 2002;59:201-6. [Crossref] [PubMed]
- Korstjens CM, van der Rijt RH, Albers GH, et al. Low-intensity pulsed ultrasound affects human articular chondrocytes in vitro. Med Biol Eng Comput 2008;46:1263-70. [Crossref] [PubMed]
- Takeuchi R, Ryo A, Komitsu N, et al. Low-intensity pulsed ultrasound activates the phosphatidylinositol 3 kinase/Akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: a basic science study. Arthritis Res Ther 2008;10:R77. [Crossref] [PubMed]
- Vaughan NM, Grainger J, Bader DL, et al. The potential of pulsed low intensity ultrasound to stimulate chondrocytes matrix synthesis in agarose and monolayer cultures. Med Biol Eng Comput 2010;48:1215-22. [Crossref] [PubMed]
- Whitney NP, Lamb AC, Louw TM, et al. Integrin-mediated mechanotransduction pathway of low-intensity continuous ultrasound in human chondrocytes. Ultrasound Med Biol 2012;38:1734-43. [Crossref] [PubMed]
- Yuan LJ, Niu CC, Lin SS, et al. Effects of low-intensity pulsed ultrasound and hyperbaric oxygen on human osteoarthritic chondrocytes. J Orthop Surg Res 2014;9:5. [PubMed]
- Omi H, Mochida J, Iwashina T, et al. Low-intensity pulsed ultrasound stimulation enhances TIMP-1 in nucleus pulposus cells and MCP-1 in macrophages in the rat. J Orthop Res 2008;26:865-71. [Crossref] [PubMed]
- Kobayashi Y, Sakai D, Iwashina T, et al. Low-intensity pulsed ultrasound stimulates cell proliferation, proteoglycan synthesis and expression of growth factor-related genes in human nucleus pulposus cell line. Eur Cell Mater 2009;17:15-22. [PubMed]
- Takakura Y, Matsui N, Yoshiya S, et al. Low-intensity pulsed ultrasound enhances early healing of medial collateral ligament injuries in rats. J Ultrasound Med 2002;21:283-8. [PubMed]
- Warden SJ, Avin KG, Beck EM, et al. Low-intensity pulsed ultrasound accelerates and a nonsteroidal anti-inflammatory drug delays knee ligament healing. Am J Sports Med 2006;34:1094-102. [PubMed]
- Hu B, Zhang Y, Zhou J, et al. Low-intensity pulsed ultrasound stimulation facilitates osteogenic differentiation of human periodontal ligament cells. PLoS One 2014;9:e95168. [Crossref] [PubMed]
- Ren L, Yang Z, Song J, et al. Involvement of p38 MAPK pathway in low intensity pulsed ultrasound induced osteogenic differentiation of human periodontal ligament cells. Ultrasonics 2013;53:686-90. [Crossref] [PubMed]
- Hu J, Qu J, Xu D, et al. Combined application of low-intensity pulsed ultrasound and functional electrical stimulation accelerates bone-tendon junction healing in a rabbit model. J Orthop Res 2014;32:204-9. [Crossref] [PubMed]
- Lovric V, Ledger M, Goldberg J, et al. The effects of low-intensity pulsed ultrasound on tendon-bone healing in a transosseous-equivalent sheep rotator cuff model. Knee Surg Sports Traumatol Arthrosc 2013;21:466-75. [Crossref] [PubMed]
- Benito MJ, Veale DJ, FitzGerald O, et al. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 2005;64:1263-7. [Crossref] [PubMed]
- Nakamura T, Fujihara S, Yamamoto-Nagata K, et al. Low-intensity pulsed ultrasound reduces the inflammatory activity of synovitis. Ann Biomed Eng 2011;39:2964-71. [Crossref] [PubMed]
- Engelmann J, Vitto MF, Cesconetto PA, et al. Pulsed ultrasound and dimethylsulfoxide gel treatment reduces the expression of pro-inflammatory molecules in an animal model of muscle injury. Ultrasound Med Biol 2012;38:1470-5. [Crossref] [PubMed]
- Cohen JM, Fagin AP, Hariton E, et al. Therapeutic intervention for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a systematic review and meta-analysis. PLoS One 2012;7:e41941. [Crossref] [PubMed]
- Karpukhin VT, Nesterov NI, Roman DL. Ultrasonic therapy of chronic prostatitis. Vopr Kurortol Fizioter Lech Fiz Kult 1977.75-7. [PubMed]
- Li HS, Wang B, Han L, et al. Transperineal ultrasonic therapy for chronic prostatitis. Zhonghua Nan Ke Xue 2013;19:49-53. [PubMed]
- Mazzoli S, Cai T, Rupealta V, et al. Interleukin 8 and anti-chlamydia trachomatis mucosal IgA as urogenital immunologic markers in patients with C. trachomatis prostatic infection. Eur Urol 2007;51:1385-93. [Crossref] [PubMed]
- Iwabuchi Y, Tanimoto K, Tanne Y, et al. Effects of low-intensity pulsed ultrasound on the expression of cyclooxygenase-2 in mandibular condylar chondrocytes. J Oral Facial Pain Headache 2014;28:261-8. [Crossref] [PubMed]
- Vardi Y, Appel B, Jacob G, et al. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol 2010;58:243-8. [Crossref] [PubMed]
- Liu J, Zhou F, Li GY, et al. Evaluation of the effect of different doses of low energy shock wave therapy on the erectile function of streptozotocin (STZ)-induced diabetic rats. Int J Mol Sci 2013;14:10661-73. [Crossref] [PubMed]
- Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med 2013;10:738-46. [Crossref] [PubMed]
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014;65:968-78. [Crossref] [PubMed]
- Hanawa K, Ito K, Aizawa K, et al. Low-intensity pulsed ultrasound induces angiogenesis and ameliorates left ventricular dysfunction in a porcine model of chronic myocardial ischemia. PLoS One 2014;9:e104863. [Crossref] [PubMed]
- Toyama Y, Sasaki K, Tachibana K, et al. Ultrasound stimulation restores impaired neovascularization-related capacities of human circulating angiogenic cells. Cardiovasc Res 2012;95:448-59. [Crossref] [PubMed]
- Lei H, Xin H, Guan R, et al. Low-intensity Pulsed Ultrasound Improves Erectile Function in Streptozotocin-induced Type I Diabetic Rats. Urology 2015;86:1241.e11-8.
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003;61:37-49. [Crossref] [PubMed]
- Verghese T, Latthe P. Recent status of the treatment of stress urinary incontinence. Int J Urol 2014;21:25-31. [Crossref] [PubMed]
- Klein G, Hart ML, Brinchmann JE, et al. Mesenchymal stromal cells for sphincter regeneration. Adv Drug Deliv Rev 2015;82-83:123-36. [Crossref] [PubMed]


