Reconstructive techniques for creation of catheterizable channels: tunneled and nipple valve channels
Introduction
Cutaneous catheterizable channels allow for continent bladder emptying when an alternate route is desired. The goals of channel creation in the neurogenic bladder population are successful urine elimination when the native urethra is either compromised or inaccessible, renal preservation, continence and lastly cosmesis (1). Prior to undergoing major surgical reconstruction, most patients will have exhausted all minimally invasive options; these will not be discussed in this review. Operative indications, preoperative evaluation and surgical approaches differ between pediatric and adult patients; this review focuses on the adult patient with neurogenic bladder.
Historically, several retroperitoneal and intraperitoneal structures have been described for the creation of catheterizable channels. Kock et al. first described the use of an intussuscepted ileal nipple valve as a continence mechanism in 1982 (2). In 1980, Mitrofanoff described a method for utilizing the appendix through a fashioned detrusor tunnel (3). While Mitrofanoff is most notable for his use of the appendix, the application of his technique has been described in the use of native ureter, fallopian tube, vas deferens, reconfigured gastric tissue, small intestine, colon, and bladder (4-8). Many of these techniques have not been widely accepted due to high rates of stricture, poor technical reproducibility, and the increased need for additional procedures. When selecting a particular technique, one must keep in mind the published success and failure rates, a given surgeon’s technical familiarity as well as myriad of patient specific factors.
Patient selection—physical and social requirements
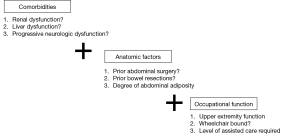
In addition to a particular surgeon’s comfort and experience with a given procedure, individual patient factors should be central to the selection of a surgical approach. A combination of medical comorbidities, anatomic factors, as well as occupational function, all affect which approach is best suited for a given patient (Figure 1).
Comorbidities
Prior to selecting a surgical approach, performing a complete past medical history is critical. Catheterizable channel creation in the neurogenic population is often combined with enterocystoplasty. Further, all well established channel techniques include bowel resection. As a result, one should exercise caution when considering continent diversion in individuals with underlying bowel disease such as Crohn’s or ulcerative colitis, poor nutritional status, and either abdominal or pelvic radiation. Due to reabsorption concerns, patients proceeding with enterocystoplasty should have normal liver function as well as optimal renal function. As many patients with neurogenic bladder have central and peripheral neurologic abnormalities, baseline neurologic function and its expected trajectory should be assessed. While a patient may be able to self-catherize an abdominal stoma at the time of surgery that ability may change if there is progression of their neurologic condition.
Anatomic factors
The surgeon must be flexible on the day of surgery as the surgical plan may evolve given the unique anatomic roadmap of a patient—body habitus, prior bowel surgery, or a short appendix may change what approach would most benefit a given patient. Prior to undertaking a case, one should be familiar with multiple approaches using a range of techniques and bowel segments.
Occupational function
One must also consider the patient’s present and anticipated future physical function and psychosocial situation. Does the patient have impaired upper extremity function? Does the patient have appropriate coordination and strength to perform intermittent catheterization with clean technique? Would the patient be able to perform the tasks independently or would assistance be needed? Is the patient’s neurologic condition stable or progressive? Inability to perform self-catheterization is not necessarily a contraindication to creating a catheterizable channel as long as the patient has appropriate long-term support from a committed set of care assistants. One must feel confident that the patient and caretakers are highly motivated and dedicated to managing continent catheterizable channel. The surgeon should be supported by a team of occupational therapists, social workers and a care coordinator. She/he will partner with them to optimize the patient’s hand function, social support system and peri-operative rehabilitation before embarking on surgery. Should the patient be deemed an appropriate candidate, quality of life has been shown to be improved secondary to elimination of drainage bags, increased freedom as well as improved cosmesis (9).
Continent channel options
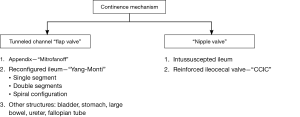
Herein we organize our discussion of channel types first by their continence mechanism and then subcategorize by tissue origin (Figure 2). An ideal channel is one that is short, straight, and well supported by associated blood supply and surrounding adventitia, so as to minimize difficulty with catheterization. The appendix and tapered or reconfigured ileum are the most widely accepted techniques for creating tunneled channels. They utilize a detrusor tunnel flap valve for continence. Tapered ileum can also be used with a nipple valve—as either a reinforced intussuscepted portion of ileum or a reinforced ileocecal valve. The intussuscepted ileal nipple valve was first described by Kock (2). This technique has not been widely adopted due to the large segment of ileum potentially needed, technical difficulty, and suboptimal outcomes due to high rates of dessusception (10,11). Throughout this review we will highlight the most common surgical techniques, as well as their potential success and complication rates.
Tunneled catheterizable channels
The mechanism of continence in tunneled channels is based on cooptation of the channel. As the bladder fills, the intraluminal pressure within the channel increases, by the action of the bladder wall compressing the channel and results in a lack of flow. The channel is anastomosed to the urothelium of the bladder and tunneled through surrounding detrusor muscle.
The Mitrofanoff appendicovesicostomy
First described by Mitrofanoff in 1980, the appendix is repurposed as a catheterizable channel either into the native bladder or into a diverting pouch (3). The benefits of utilizing appendix are first its diameter and length making it a natural structure for catheteriziation; second, given its minimal function in digestion and elimination, relocating it away from the gastrointestinal tract has theoretically fewer metabolic side effects.
Surgical approach
The procedure can be performed via an open, laparoscopic, or robotic-assisted laparoscopic approach with similar success (12,13). If utilizing an open approach, this can be performed through either a midline or Pfannenstiel incision. Once the appendix is identified, it should be measured and deemed an appropriate length for a tension-free anastomosis between the bladder wall and abdominal wall stoma site. Should the appendiceal mesentery appear too superior to reach both the native bladder or pouch and abdominal wall, one may need to mobilize the ascending colon along the line of Toldt. After mobilization, the appendix should be transected with a small cuff of cecum (14). This adds a centimeter or so to the channel length and creates a larger cutaneous stomal circumference. The distal tip of appendix should then be transected and spatulated in preparation for anastomosis to the bladder.
Continence is achieved through submucosal tunneling of the isolated distal appendix. Continence has been demonstrated in tunnel lengths of approximately 2–4 cm (15,16). Location of implantation into the bladder wall should depend of the location of the eventual stoma and length of appendix; lateral, anterior and posterior are all used commonly. Tunneling into the bladder can be done intra- or extravesically, borrowing from the ureteral reimplantation techniques of Politano et al. (17), Glenn et al. (18), Cohen (19) or Lich et al. and Gregoir et al. (20,21). With the intravesical approaches the bladder is opened anteriorly and the appendix will necessarily be tunneled in the posterior wall. A detrusor opening is made to accommodate the appendix at the cephalad end of the planned tunnel. Then, either a trough is made by opening the mucosa and the appendix is laid in this trough before the mucosa is closed over it, or, a clamp is used to make a submucosal tunnel from the upper opening to the planned neohiatus. In either case the spatulated tip of the appendix is then anastomosed to the bladder mucosa. With the extravesical approach, the bladder is filled and detrusor flaps are created until the mucosa bulges. The mucosa is then opened at the distal end of the trough and the spatulated tip of the appendix is sutured to the mucosa. The detrusor flaps are closed over the appendix. With all of the approaches it is common to fix the appendix to the muscle in a few places along the tunnel to prevent loss of tunnel length from an accordion like effect of the detrusor muscle.
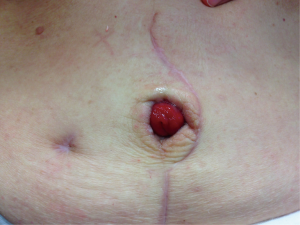
The proximal end of the appendix with cecal stump should then be brought in a relatively straight lie and in a location readily accessible to the patient and or patient’s caregiver. The umbilicus is a common location for stomal creation given its relative absence of adipose tissue making the course to the skin often shorter than other abdominal wall locations. Stomal stenosis rates have not been higher with abdominal wall maturation of appendicovesicostomies (22) in children, but the fat adult abdominal wall can prove more difficult. Additionally, the umbilicus has the benefit of a more cosmetic and discrete appearance. Anastomosis to the skin can be achieve in a simple interrupted fashion, modified skin flap (U, V, or VQZ plasty) (23-26) or with a rose-bud where a portion of distal channel sidewall is captured in the interrupted closure similar to a Brooke ileostomy or ileal conduit (Figure 3). Some preserve the umbilical skin whereas others prefer to excise the umbilical stump. It is unclear in the literature if these techniques affect stomal stenosis rates. Regardless, upon conclusion of surgery, one should ensure ease of catherization with a well-accessible and well-vascularized stoma under a tension free anastomosis.
Surgical outcomes
Continence rates with appendiceal channels are high (>95%) in both the pediatric and adult patient populations (27-30). Incontinence can be due to an inadequate continence mechanism and/or elevated bladder pressures. Maintaining a patent bladder neck allows for a safety valve in case of high bladder pressures or inability to catheterize through the channel.
Difficulty with catheterization may be due to stomal or channel stenosis, channel redundancy, or other anatomic variations in the channel caliber leading to challenging catheterization. The most common anatomic complication from appendiceal channels is stomal stenosis. Rates of stomal stenosis and subfascial revision rates are similar about 5–10%, and rates of surgical revision range widely (5–20%) (22,27-30). Should a patient cease to catheterize through the channel, it often eventually obliterates completely. If a concurrent bowel augmentation is not present, mucous and electrolyte abnormalities are generally minimal after an appendiceal continent channel.
Reconfigured ileum (“Yang-Monti”)
As an alternative to the tunneled appendix, Monti et al. and Yang et al. popularized utilizing a short segment of reconfigured ileum in 1997 and 1993 (31,32). The Yang-Monti technique is helpful when the appendix is too short or is surgically absent. When creating simultaneous catheterizable channels for the bladder and for the Malone antegrade continence enema (M-ACE) procedure (33), the appendix is used for the M-ACE and a Yang-Monti tube can be used for the catheterizable bladder channel. Further, when simultaneous ileal enterocystoplasty is being performed, a Yang-Monti tube can be harvested from the bowel adjacent to that which will be used in the augment, allowing for a single small bowel anastomosis.
Surgical technique
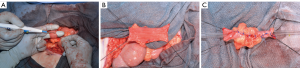
As first described, a 2-cm segment of ileum is isolated, detubularized and then retubularized transversely along the antimesenteric border to create a smaller caliber but longer intestinal tube. When increased channel length is needed, 4 cm of ileum can be removed and two side-by-side Yang-Monti channels can be anastomosed, commonly called a double Monti (16) (Figure 4). To avoid multiple connections within the channel, Casale et al. (34) described a technique in which a 3.5-cm segment of ileum is isolated, transected partially in the center, and two strips of ileum are created by transecting close to the mesentery on opposing sides. The resulting Z-shaped plate of ileum is then retubularized in a similar fashion to the conventional Yang-Monti, but the resulting tube can range from 10–14 cm (Figure 5). This technique has come to be known as the “spiral Monti”. Another approach to small bowel channel creation is tapering the desired segment in a longitudinal fashion using sutures or staples (35). While less labor intensive, this technique requires a longer bowel segment and has high reported catherization difficulty and theoretically increased nutritional deficiencies due to relocation of the distal ileum. In our experience, the broad mesenteric base of the tapered ileum, although at first attractive, can complicate the detrusor tunnel and cause a bow or arc in the trajectory of the channel between the bladder and the stoma, especially in adults where long channels can be required. With all of these ileal channels, tunneling, stomal placement and maturing of the stoma are performed similarly to appendicovesicostomy.
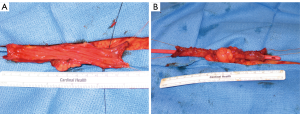
Surgical outcomes
Much like appendicovesicostomy, reports of stomal and channel complications with ileal continent channels vary widely by published series. Reported stomal continence rates are generally >95%, similar to the appendiceal Mitrofanoff. Stomal stenosis rates, however, range from about 5% to 10% (22,36) One series reported that, compared to appendicovesicostomy, revision rates were two times higher for a Monti and four times higher for spiral Monti (37). Whittam et al. found subfascial revision rates to be highest amongst spiral Monti channels with stomal location at the umbilicus (36). Narayanaswamy et al. reported a high rate of diverticular pouch formation in double Monti channels (38) resulting in difficulty with catheterization. For these reasons, when feasible, a single Yang-Monti configuration is the preferred method when utilizing reconfigured ileum.
“Nipple valve”
The continence mechanism in a nipple valve is via circumferential coaptation, created through reinforcing a native valve such as the ileocecal valve or by intussusception of an isolated segment of bowel—generally ileum.
Intussuscepted ileum
The intusscepted ileal flap valve was first described for use in the efferent limb of the Kock pouch (2). Later this was modified for use as an isolated segment of intussuscepted ileum (39). There have been several modifications designed to minimize the complications of this approach; however, this technique has not been widely adopted due to poor technical reproducibility, long-term durability and a propensity for dessusception of the continence mechanism. This technique will not be described further.
Ileocecal valve
The transition of ileum into cecum contains a muscular valve that separates the small and large bowel. It functions to impair backflow of stool contents into the small bowel. When used in urinary diversion, the valve itself functions as the continence mechanism while the associated ileal limb is made smaller in caliber usually by tapering and occasionally by intussuscepting.
Relocating this valve and repurposing it in the urinary tract to achieve continence was first described in 1950 by Gilchrist et al. (40). In 1982, Kock et al. then described its use in a continent ileal reservoir (2). This concept further evolved with Rowland et al. who described the Indiana pouch. This pouch was designed as a bladder substitute after cystectomy and involves a reservoir created from detubularized cecum, a catheterizable channel from tapered terminal ileum, and continence from a reinforced ileocecal valve (41). In 1992, Sarosdy et al. first described a modification of the Indiana pouch for use as a bladder augmentation with an integrated catheterizable channel (42). This type of channel has been reported in the literature under several different names including: hemi-Indiana pouch, continent ileal cecal cystoplasty, and continent cutaneous ileal cecocystoplasty or “continent catheterizable ileal cecocystoplasty (CCIC)”.
There are several benefits of utilizing the ileocecal valve for continence in the neurogenic population. First, compared to small bowel, a significant large bowel augment can be achieved with a short bowel resection. Second, the ileocecal pedicle supplies both the augment and the channel, eliminating concern that one will reach the bladder and the other will not. This robust pedicle may contribute to lower rates of stenosis (43). Third, there is a single bowel resection making for technical ease. Fourth, the length of the channel can be easily modified for the habitus of the adult neurogenic bladder patient. In contrast, when we have used Monti channels in obese adult patients we have had to universally use a double Monti or spiral Monti of 14 cm and find these to be associated with stenosis or difficulty catheterizing (43). Fifth the larger channel (16F) one can achieve with tapered ileum allows for faster emptying and better evacuation of mucous than the smaller channels typical of a Mitrofanoff or a Yang-Monti. Sixth, and perhaps most important, is that one does not need to create a detrusor tunnel. This is particularly helpful in patients with bladders that are small and thick-walled with inflamed mucosa, a situation that is hostile to the creation of a submucosal tunnel.
Surgical technique
The procedure can be performed through a midline laparotomy for adequate mobilization of ascending colon (42,44-46). Often, mobilization must extend around the hepatic flexure and this necessitates extension of the incision to about the midpoint between the umbilicus and the xiphoid. We have described a minimally invasive modification that we have now used in 20 patients (47,48). Mobilization of the ascending colon is performed laparoscopically using a hand assistant port through a Pfannenstiel incision, a camera port at the umbilicus (which will later be the stoma site) and a subxiphoid port for a dissecting instrument. After adequate mobilization of the ascending colon, the remainder of the procedure is performed in an open fashion through the small Pfannenstiel incision. A Pfannenstiel incision with transverse opening of the rectus fascia has been shown to have lower ventral hernia rates than a midline laparotomy in hand-assisted laparoscopic colorectal surgery (49); ventral hernia is a significant concern in the neurogenic patient who frequently has a weak abdominal wall.
We routinely harvest 10 cm of cecum and 10 cm of ileum; but the dimensions should be tailored specially to the patient’s anatomy and habitus. For instance, if a large augment is needed then up to 20–25 cm of the ascending colon can be used. The cecal segment is then detubularized longitudinally along the anti-mesenteric line, between the tenia. If the appendix is present, it is removed. The ileal channel is tapered with a single pass of the GIA-100 stapler; avoiding overlapping staple lines that would occur with shorter staplers minimizes catheterization issues. The staple-taper is performed over a 14-F catheter, which then generally allows for easy passage of a 16-F catheter afterwards. We then reinforce the continence mechanism at the ileocecal valve with interrupted imbricating slow-dissolving sutures. The ileocolonic anastomosis is performed with the GIA-100 in a side-to-side fashion. The native bladder is then incised in a sagittal clamshell fashion anteriorly from the bladder neck to the posterior bladder wall adjacent to the trigone. The detubularized cecal segment is then anastomosed to the native bladder. The efferent limb of the ileal segment can then be brought out through the abdominal wall in a similar fashion to the appendix and Yang-Monti stomas (Figures 3,6).
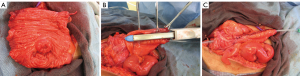
Outcomes
Continence rates with this type of channel are comparable to the tunneled channels, >95% (44-46), excluding one study with an 87% continence rate (50). Stomal stenosis rates have been reported between 3–9% (43,45). Perioperative complications are similar to patients undergoing a channel plus an augmentation, but more than those solely undergoing a tunneled channel (45). Concerns for fecal incontinence given the relocation of the ileocecal valve have been unsubstantiated, specifically in the neurogenic population (45). Husmann et al. (50) found all patients had fecal control, and in fact 23% had improved bowel function following the procedure. This is likely due to the severe concurrent constipation present in many neurogenic bladder patients. Regarding revision rates, Redshaw et al. found that significantly fewer revision procedures were needed in patients with a CCIC vs. tunneled channels (43). Khavari et al. (45) found that 93% of patients at 6 years had no change in renal function, and none had abnormal Vitamin B12 levels after 6 years. Given the concurrent cecal segment, used for augmentation, patients are at similar risk for bladder stones as patients undergoing bladder augmentation (45,50).
Given the increased peri-operative morbidity, careful consideration should be given to first trying a simple tunneled channel (45). Contraindications to a simple tunneled channel are varied but may include absent appendix, need for concurrent bladder augmentation, moderate obesity resulting in a long bladder to cutaneous ostomy site distance and a hostile bladder.
Conclusions
When performed without augmentation, Mitrofanoff appendicovesicostomy is an excellent minimally invasive option in the non-obese patient with a hospitable bladder. When the appendix is not available, the Monti channel provides an alternative means to create a channel without an augment. Revision rates appear greater with this technique. The CCIC provides another alternative to the appendix that is highly adaptable to a given patient’s habitus and bladder characteristics.
Continent channel creation in adults can improve quality of life and minimize morbidity associated with neurogenic bladder. However, the decision to proceed with creation of a catheterizable channel should be made only after careful consideration of the patient’s medical comorbidities, physical abilities and social support. A given patient’s success is contingent upon their surgeon’s technical ability, intraoperative decision-making, in addition to the support of a comprehensive multi-disciplinary team. The surgeon undertaking this patient population should be facile with the full range of reconstructive techniques as well as skilled in a shared decision making approach to treatment selection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Horowitz M, Kuhr CS, Mitchell ME. The Mitrofanoff catheterizable channel: patient acceptance. J Urol 1995;153:771-2. [PubMed]
- Kock NG, Nilson AE, Nilsson LO, et al. Urinary diversion via a continent ileal reservoir: clinical results in 12 patients. J Urol 1982;128:469-75. [PubMed]
- Mitrofanoff P. Trans-appendicular continent cystostomy in the management of the neurogenic bladder. Chir Pediatr 1980;21:297-305. [PubMed]
- Veeratterapillay R, Morton H, Thorpe AC, et al. Reconstructing the lower urinary tract: The Mitrofanoff principle. Indian J Urol 2013;29:316-21. [PubMed]
- Cain MP, Rink RC, Yerkes EB, et al. Long-term followup and outcome of continent catheterizable vesicocstomy using the Rink modification. J Urol 2002;168:2583-5. [PubMed]
- Hensle TW, Connor JP, Burbige KA. Continent urinary diversion in childhood. J Urol 1990;143:981-3. [PubMed]
- Roth S, Weining C, Hertle L. Continent cutaneous urinary diversion using the full-thickness bowel flap tube as continence mechanism: a simplified tunneling technique. J Urol 1996;156:1922-5. [PubMed]
- Van Savage JG, Khoury AE, McLorie GA, et al. Outcome analysis of Mitrofanoff principle applications using appendix and ureter to umbilical and lower quadrant stomal sites. J Urol 1996;156:1794-7. [PubMed]
- Zommick JN, Simoneau AR, Skinner DG, et al. Continent lower urinary tract reconstruction in the cervical spinal cord injured population. J Urol 2003;169:2184-7. [PubMed]
- deKernion JB, DenBesten L, Kaufman JJ, et al. The Kock pouch as a urinary reservoir. Pitfalls and perspectives. Am J Surg 1985;150:83-9. [PubMed]
- Skinner DG. Intussuscepted ileal nipple valve--development and present status. Scand J Urol Nephrol Suppl 1992;142:63-5. [PubMed]
- Cadeddu JA, Docimo SG. Laparoscopic-assisted continent stoma procedures: our new standard. Urology 1999;54:909-12. [PubMed]
- Famakinwa OJ, Rosen AM, Gundeti MS. Robot-assisted laparoscopic Mitrofanoff appendicovesicostomy technique and outcomes of extravesical and intravesical approaches. Eur Urol 2013;64:831-6. [PubMed]
- Cromie WJ, Barada JH, Weingarten JL. Cecal tubularization: lengthening technique for creation of catheterizable conduit. Urology 1991;37:41-2. [PubMed]
- Duckett JW, Snyder HM 3rd. Use of the Mitrofanoff principle in urinary reconstruction. Urol Clin North Am 1986;13:271-4. [PubMed]
- Kaefer M, Retik AB. The Mitrofanoff principle in continent urinary reconstruction. Urol Clin North Am 1997;24:795-811. [PubMed]
- Politano VA, Leadbetter WF. An operative technique for the correction of vesicoureteral reflux. J Urol 1958;79:932-41. [PubMed]
- Glenn JF, Anderson EE. Distal tunnel ureteral reimplantation. J Urol 1967;97:623-6. [PubMed]
- Cohen SJ. Ureterozystoneostomie. Eine neue antirefluxtechnik. Akt Urol 1975;6:1-8.
- Lich RJ, Howerton LW, Davis LA. Reucrrent urosepsis in children. J Urol 1961;86:554-8.
- Gregoir W, Vanregemorter G. Congenital vesico-ureteral reflux. Urol Int 1964;18:122-36. [PubMed]
- Szymanski KM, Whittam B, Misseri R, et al. Long-term outcomes of catheterizable continent urinary channels: What do you use, where you put it, and does it matter? J Pediatr Urol 2015;11:210.e1-7.
- Glassman DT, Docimo SG. Concealed umbilical stoma: long-term evaluation of stomal stenosis. J Urol 2001;166:1028-30. [PubMed]
- Khoury AE, Van Savage JG, McLorie GA, et al. Minimizing stomal stenosis in appendicovesicostomy using the modified umbilical stoma. J Urol 1996;155:2050-1. [PubMed]
- Franc-Guimond J, González R. Simplified technique to create a concealed catheterizable stoma: the VR flap. J Urol 2006;175:1088-91. [PubMed]
- Landau EH, Gofrit ON, Cipele H, et al. Superiority of the VQZ over the tubularized skin flap and the umbilicus for continent abdominal stoma in children. J Urol 2008;180:1761-5; discussion 1765-6.
- Welk BK, Afshar K, Rapoport D, et al. Complications of the catheterizable channel following continent urinary diversion: their nature and timing. J Urol 2008;180:1856-60. [PubMed]
- Thomas JC, Dietrich MS, Trusler L, et al. Continent catheterizable channels and the timing of their complications. J Urol 2006;176:1816-20; discussion 1820.
- Cain MP, Casale AJ, King SJ, et al. Appendicovesicostomy and newer alternatives for the Mitrofanoff procedure: results in the last 100 patients at Riley Children's Hospital. J Urol 1999;162:1749-52. [PubMed]
- Harris CF, Cooper CS, Hutcheson JC, et al. Appendicovesicostomy: the mitrofanoff procedure-a 15-year perspective. J Urol 2000;163:1922-6. [PubMed]
- Monti PR, Lara RC, Dutra MA, et al. New techniques for construction of efferent conduits based on the Mitrofanoff principle. Urology 1997;49:112-5. [PubMed]
- Yang WH. Yang needle tunneling technique in creating antireflux and continent mechanisms. J Urol 1993;150:830-4. [PubMed]
- Malone PS, Curry JI, Osborne A. The antegrade continence enema procedure why, when and how? World J Urol 1998;16:274-8. [PubMed]
- Casale AJ. A long continent ileovesicostomy using a single piece of bowel. J Urol 1999;162:1743-5. [PubMed]
- Adams MC, Bihrle R, Foster RS, et al. Conversion of an ileal conduit to a continent catheterizable stoma. J Urol 1992;147:126-8. [PubMed]
- Whittam BM, Szymanski KM, Flack C, et al. A comparison of the Monti and spiral Monti procedures: A long-term analysis. J Pediatr Urol 2015;11:134.e1-6.
- Leslie JA, Cain MP, Kaefer M, et al. A comparison of the Monti and Casale (spiral Monti) procedures. J Urol 2007;178:1623-7; discussion 1627. [PubMed]
- Narayanaswamy B, Wilcox DT, Cuckow PM, et al. The Yang-Monti ileovesicostomy: a problematic channel? BJU Int 2001;87:861-5. [PubMed]
- Thüroff JW, Gillitzer R, Franzaring L, et al. Intussuscepted ileal flap valve for revisional surgery. BJU Int 2005;96:1425-37. [PubMed]
- Gilchrist RK, Merricks JW, Hamlin HH, et al. Construction of a substitute bladder and urethra. Surg Gynecol Obstet 1950;90:752-60. [PubMed]
- Rowland RG, Mitchell ME, Bihrle R, et al. Indiana continent urinary reservoir. J Urol 1987;137:1136-9. [PubMed]
- Sarosdy MF. Continent urinary diversion using cutaneous ileocecocystoplasty. Urology 1992;40:102-6. [PubMed]
- Redshaw JD, Elliott SP, Rosenstein DI, et al. Procedures needed to maintain functionality of adult continent catheterizable channels: a comparison of continent cutaneous ileal cecocystoplasty with tunneled catheterizable channels. J Urol 2014;192:821-6. [PubMed]
- Sutton MA, Hinson JL, Nickell KG, et al. Continent ileocecal augmentation cystoplasty. Spinal Cord 1998;36:246-51. [PubMed]
- Khavari R, Fletcher SG, Liu J, et al. A modification to augmentation cystoplasty with catheterizable stoma for neurogenic patients: technique and long-term results. Urology 2012;80:460-4. [PubMed]
- King DH, Hlavinka TC, Sarosdy MF. Additional experience with continent urinary diversion using cutaneous ileocecocystoplasty. Urology 1996;47:471-5. [PubMed]
- Pagliara T, Liberman D, Elliott SP. Hand-Assisted Laparoscopic Right Colon Mobilization for Continent Cutaneous Ileal Cecocystoplasty”, a video presentation at the Annual Meeting of American Urologic Association, New Orleans, LA. Available online: https://www.youtube.com/watch?v=8GMvuasLECI
- Elliott SP. Cutaneous Catheterizable Ileal Cecocystoplasty Bladder Augmentation. Available online: https://www.youtube.com/watch?v=8GMvuasLECI
- DeSouza A, Domajnko B, Park J, et al. Incisional hernia, midline versus low transverse incision: what is the ideal incision for specimen extraction and hand-assisted laparoscopy? Surg Endosc 2011;25:1031-6. [PubMed]
- Husmann OA, Cain MP. Fecal and urinary continence after ileal cecal cystoplasty for the neurogenic bladder. J Urol 2001;165:922-5. [PubMed]