Urinary tract infection in the neurogenic bladder
Introduction
Patients with spinal cord injury (SCI) and/or neurological conditions (multiple sclerosis (MS), Parkinson’s disease, spina bifida, etc.) are at risk for neurogenic lower urinary tract dysfunction which is commonly known as neurogenic bladder (NB). The sequelae of NB are vast and include renal failure, incontinence, autonomic dysreflexia, infection and occasionally death. Technological advances and a better understanding of the pathophysiology of the NB have reduced the occurrence of many of these complications. Unfortunately, urinary tract infection (UTI) persists as one of the most difficult complications to diagnose, treat and prevent in patients with a NB.
A large proportion of patients with neuro-urologic disorders are managed with indwelling catheterization (IC) or clean intermittent catheterization (CIC). Bacterial biofilm formation and/or colonization are the norm in these patients and increase the risk of symptomatic recurrent UTI (rUTI). There are other less well understood functional, immunological, cellular and inflammatory mechanisms that contribute to UTI predisposition (1).
The heterogeneity of this patient population and paucity of quality evidence have made establishing guidelines difficult. This has led to significant variation in diagnosis and treatment of NB UTI between centers. It seems that UTI management in this population is largely based on familiarity rather than existing evidence and guidelines (2).
Here we provide a contemporary review of the literature regarding the epidemiology, pathogenesis, diagnosis and management of UTI in patients with a NB.
Epidemiology
The incidence of UTI in patients with a NB is high. It is estimated that the overall rate of UTI in patients with a NB is 2.5 episodes per patient per year (3). In a large retrospective cohort of 46,271 NB patients in the United States, more than one third (36.4%) of patients were diagnosed with a lower UTI at least one-year post NB diagnosis (4). Of the 33.3% of patients that required hospitalization from this large cohort, more than one fifth had a lower UTI as the primary diagnosis (4). This rate of hospitalization was similarly reported in a large, prospective cohort study of SCI patients. UTI once again accounted for the etiology in over one fifth of patients re-hospitalized with an average length of stay of 15.5 days (5). A large cohort study of Canadian veterans with a history of traumatic SCI found a significant number of emergency department (ED) visits within the first year following their injury (110 visits/100 persons). UTI accounted for 51.2% of the visits that were classified as ‘potentially preventable’ (6). The morbidity of a UTI is not unique to the outpatient setting and accounted for 70% of fevers in SCI patients admitted to a rehabilitation unit and was shown to significantly prolong admission (7).
UTI morbidity can have more than just local infectious sequelae, particularly in the MS population. Many of these patients will be admitted to hospital with acute exacerbations of their MS and a number of precipitating factors have been postulated. Prospective studies support viral infections as a likely trigger with a pathogenic mechanism involving T-cells and cytokines, although this remains largely unclear (8). An increasing amount of retrospective data also supports bacterial infections as an important MS exacerbation trigger that significantly impacts management. Patients with documented bacterial infection have shown little response to steroid therapy until appropriate antibiotics are co-administered (9). In a cohort of 100 patients presenting with acute MS exacerbation, 35% of patients were found to be acutely infected with 30% of infections attributed to UTI (9). The often recurrent nature of UTIs in the MS population has been shown to result in neurological progression in multiple cases (8).
In order to characterize NB dysfunction, patients will undergo multichannel urodynamic studies (UDS) or videourodynamic (VUD) studies. The invasive nature of these studies increases the risk of UTI. A prospective study of SCI patients undergoing urodynamic assessment demonstrated the incidence of post-UDS UTI to be 16%. This incidence decreased to 8.6% in patients who were found to have sterile urine pre-UDS, indicating a higher risk in NB patients with pre-UDS asymptomatic bacteriuria (AB) (10). This incidence is significantly increased compared to the non-neurogenic population (11,12).
The high incidence of ED visits and hospitalizations is essentially proportional to the incidence of antibiotic use. 1 in 5 veterans who presented to the ED, and did not require admission, received a course of antibiotics over the 6-year study period. Seventeen percent of these patients received more than one antibiotic per visit and UTI was the most commonly diagnosed infection. Both the number of infections and UTI were the only factors found to be significantly associated with a higher rate of prescribing. Approximately 60% of visits received broad-spectrum antibiotics and fluoroquinolones accounted for more than 40% of antibiotics used (13). Unfortunately, this pattern of practice has contributed to rising multi-drug resistance (MDR). A recent prospective study of SCI patients with AB or a UTI showed that approximately 50% of strains isolated were found to be MDR (14). This high level of resistance was similarly shown in a large study of SCI inpatients and outpatients where greater than 50% of strains were resistant to ampicillin, levofloxacin, cefazolin and clavulin. Additionally, 41.7% of patients were classified as extended spectrum beta lactamase positive (15).
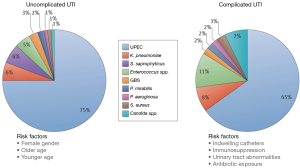
The Enterobacteriaceae family represent the most commonly isolated organisms in the neurogenic population (14-16). Within this family, E. coli and Klebsiella species dominate with E. coli comprising 50% of all strains isolated in two large studies. The frequency of E. coli and Klebsiella infections are lower than reported in the non-neurogenic population and this can be explained by an increase in the incidence of organisms classically seen in hospitalized patients i.e., Pseudomonas, Serratia, Proteus, Acinentobacter, Enterococcus. The incidence of these organisms in the neurogenic population has been: Pseudomonas 8.7–15%, Acinentobacter 6–15% and Enterococcus 6–12% (15,17). In addition to an increased risk of nosocomial organisms, NB patients are also susceptible to fungal infections which have been found to be associated with recent antibiotic use and IC. In a prospective study of SCI patients, the incidence of candiduria was found to be 17% and patients with an IC and suprapubic catheter (SPC) were 10× more likely to develop candiduria compared to patients who used CIC (18). Figure 1 illustrates the epidemiological differences in pathogenic organisms responsible for UTIs in non-neurogenic (uncomplicated) and NB (complicated) patients (19). Although the complicated group also encompasses non-neurogenic, high-risk patients (e.g., pregnant, immunosuppressed) it echoes the above reported shift in bacteriological epidemiology within the NB population.
Pathogenesis
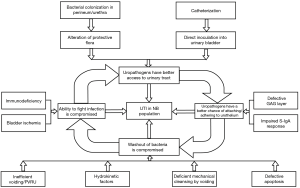
Uropathogenic bacteria have the ability to adhere to, and often internalize into, the apical urothelial cells that line the lower and, parts of, the upper urinary tract. Through pathogen-host interactions such as Fim-H/uroplakin Ia and LPS/toll like receptor binding, a local immune and resultant inflammatory response occurs (19). UTI in NB patients is considered complicated as they possess risk factors that do not exist in non-neurogenic patients. The underlying neurogenic pathology will dictate the structural/physiological and immunological differences as well as the choice of bladder management. Risk factors will therefore be unique for each patient and will also be influenced by their socio-economic situation and caregiver dependence. An appreciation and thorough understanding of risk factors that can exist in NB patients is imperative so that they might be effectively recognized and potentially modified. Figure 2 provides an overview of the discussed risk factors and illustrates their interrelated nature in the pathogenesis of a complicated UTI (1).
Structural and physiological
Bladder ischemia resulting from increased intravesical pressure and overdistention is believed to predispose to infection from tissue hypoperfusion and decreased delivery of inflammatory cells and antibiotics (1). This is supported by studies that show improvements in urodynamic parameters are associated with fewer UTIs in NB patients (20,21). Bladder overdistention or urinary stasis similarly contribute to a risk of infection by eliminating one of the most important natural protective mechanisms: voiding (22). Voiding dysfunction can result from problems with the detrusor muscle (i.e., areflexia) and/or sphincteric dysfunction [i.e., detrusor sphincter dyssynergia (DSD)] (1). In a study of SCI patients, a residual volume <50 cc was associated with a 5% rate of UTI compared to 24% in patients with a residual >251 cc (23).
DSD predisposes to high intravesical and proximal urethral pressures and can result in chronic dilatation of the posterior urethra and bladder neck—effectively altering the anatomical integrity of the lower urinary tract and consequently the hydrokinetics (1). Alterations in the shape of the hollow tubes of the lower urinary tract results in turbulent flow and stasis which similarly hinders the natural protective mechanisms of voiding (1). Patients with DSD will classically exhibit ballooning of the posterior urethra/bladder neck (1). Elevated intravesical pressure can similarly predispose to vesicoureteral reflux (VUR) which has been shown to significantly increase the risk of UTI in NB patients (1,3). A prospective study of 128 SCI patients demonstrated that patients with VUR had a 23-fold risk for the development of repeat infection (17).
Innate immunity
The normally protective microbiological architecture of the perineum, and in females, vagina, is disrupted in NB patients and studies have demonstrated colonization with many pathogenic and nosocomial species of Enterobacteriaceae, Pseudomonas, Acinetobacter, and Enterococcus (1). Multiple studies have demonstrated a correlation between urethral and perineal flora and the causative organism(s) responsible for a UTI (24,25). About 74.1% of SCI patients with significant bacteriuria had at least one of the bacterial species present in the urine also present in the perineal and/or urethral cultures (25).
It is believed that the glycosaminoglycan (GAG) layer lining the urothelium serves as a protective barrier preventing bacterial binding and invasion and is therefore important in UTI prevention (26). Disruption of the GAG layer has been shown to increase the risk of infection and the GAG layer of NB patients is continually subject to chronic inflammation and infection—leading to disruption and impeding regeneration (1,22,27).
Another important player in the innate immunity of the bladder includes an immunoglobulin that is secreted onto mucosal surfaces: secretory immunoglobulin A (S-IgA) (1). High concentrations exist in the apical cells of the urothelium and it functions primarily by agglutinating bacteria and preventing their adherence to the urothelium (28). This important immunoglobulin appears to be lacking or significantly reduced in NB patients making them more vulnerable to infection. Biopsies taken from non-NB patients demonstrated strong immunostaining for S-IgA in 100% of samples. In the NB group, however, <50% of samples demonstrated strong or moderate immunostaining (29). The apical layer of urothelial cells, known as umbrella cells, play a similarly critical role in the innate immunity of the bladder through exfoliation and excretion of infected cells via a rapid apoptosis-like mechanism (30). The factors implicated in this critical host defense mechanism have been shown to be absent in NB patients, eliminating another first line defense against infection (1,31,32).
Contemporary studies have begun to demonstrate that a dysregulated inflammatory response within the NB may serve as a more important factor in infection than traditionally discussed factors such as post-void residual volume. No correlation was demonstrated between PVR volume and risk of UTI in a recent study of UTI in a SCI rat model (33). The normal innate immune response that is stimulated by bacterial components and results in pro-inflammatory signaling and leukocyte recruitment seems to be altered in the NB. Another SCI-rat model study demonstrated that prior to infection the SCI rats were in a pro-inflammatory state with down regulated antimicrobial peptides. This appears to have placed rats in a vulnerable state and likely accounted for the establishment of infection with an inoculum that was 3 logs below neurologically intact rats (33,34). Twenty-four hours following infection the SCI-rats had decreased expression of several cytokines and chemokines involved in leukocyte recruitment, the adaptive response and inflammation. Antibiotics were administered and successfully cleared infection in all subjects however a prolonged period of inflammation was appreciated in SCI-rats with decreased expression of anti-inflammatory and persistently elevated pro-inflammatory molecules (34). These studies point to a neural mediated vulnerability that is intrinsic to the NB however these pathways are not fully understood and further research is required.
Adaptive immunity
The adaptive response to infection is initiated following the recruitment of macrophages and mast cells (34). A failure to activate this system is likely related to the dysregulated innate response discussed above, however this remains speculative. Bone marrow aspirates in both quadriplegic and paraplegic patients demonstrated that both lymphocytic-mediated nonspecific (NK cell) and adaptive (B and T cell) immunity were significantly impaired and had no correlation with the time since injury (35).
Bladder management
By and large, the most important risk factor for the development of UTI in the NB population is the use of medical devices i.e., catheters. Factors contributing to infection as described by the Infectious Disease Society of America (IDSA) include: inoculation of skin with fecal bacteria, migration of uropathogens from the urethral meatus to the bladder via the catheter-mucosal interface, intraluminal spread of pathogens in the setting of closed drainage violation and contamination, urinary stasis below the catheter bulb and serving as a mode of transmission for pathogens via the hands of health care personnel. Following introduction, catheters provide enhanced microbial adhesion that leads to the development of biofilms (36).
The ascension of bacteria into the bladder occurs either via extraluminal or intraluminal spread with two thirds of uropathogens causing bacteriuria ascending extraluminally (37). As previously discussed, this is closely related to the alteration in microbial flora that is seen in NB patients. The intraluminal mode of bacteriuria is supported by multiple studies that demonstrate that when bacteria are introduced into the urinary collection bag they are later found in the bladder (38-40). Medical devices enhance microbial adhesion and deliver the bacteria directly to bladder epithelial cells with compromised first line defenses (36). For these reasons, it has been shown that fewer virulence factors are required for colonization and infection (41,42).
Once bacteria have successfully colonized the catheter they undergo a phenotypic change that leads to the formation of biofilms. Biofilms are composed of exopolysaccharides with microcolonies of replicating bacteria (43). They are initially unimicrobial however with prolonged catheterization they rapidly become polymicrobial (36). Protective molecules such as Tamm-Horsfall proteins and urinary salts similarly become incorporated into the mature matrix (44). It has been shown that biofilms may be found in the bladder within 1–3 days following insertion and they form both intra and extraluminally (45). Biofilms produced by Proteus species, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Providencia species leads to hydroxyapatite, struvite and encrustations that may obstruct catheters and further prevent urinary drainage (45).
The most problematic microbial advantage conferred by biofilms include their inherent antimicrobial resistance (36). Antimicrobial resistance is more easily established because of enhanced exchange of genetic material within the biofilm (43). Additionally, bacteria are similarly protected from the host immune response and the biofilm continually facilitates seeding of additional sites within the catheter and bladder (36).
Diagnosis
Accurate diagnosis of a symptomatic UTI in patients with NB is clouded by the high rate of lower urinary tract colonization. The different medical devices, whether intermittent or indwelling, inevitably lead to AB thereby decreasing the sensitivity and specificity of diagnostic tests such as urine dipsticks and culture for accurately diagnosing a symptomatic UTI (36). Unfortunately, the literature is difficult to interpret as no gold standard exists and all of the different studies, especially in the NB population, utilize different definitions, collection methods and signs and symptoms for establishing AB or a UTI.
Fortunately, the Infectious Disease Society of America released clinical practice guidelines in 2010 for the diagnosis of catheter-associated UTI (CA-UTI) in adults and this document can be extrapolated to the NB population because of their disproportionate utilization of different medical devices for bladder management. Appropriate specimen collection is of paramount importance and needs to be standardized for all patients so that the results accurately reflect the microbial environment of the NB. First and foremost, catheter-associated AB (CA-AB) should not be screened for unless there is suspicion of a UTI defined as the ‘presence of significant bacteriuria in a patient with signs or symptoms referable to the urinary tract and no alternate source’ (36). Exceptions include study purposes and pregnant women and these recommendations are based on Grade A, Level III evidence (36). If there is clinical suspicion of a UTI or urosepsis in a NB patient then specimen collection should proceed according to the method of bladder management. Patients with indwelling catheters should have their catheter exchanged (prior to antibiotic therapy) with immediate collection of a specimen following insertion of the new catheter. In cases of short-term catheterization, it is acceptable to obtain a specimen aseptically through the catheter port. Specimens should never be collected from the drainage bag (36). Contamination with periurethral flora is less likely with catheterized urine and therefore even NB patients who are able to spontaneously void a sample should ideally be collected via a catheterized sample (36). Additionally, in patients who wear a condom catheter, a fresh condom catheter should be applied with subsequent urine collection (36).
There is no standard definition for significant bacteriuria in patients who are catheterized or have a NB. The IDSA makes the following recommendation:
CA-UTI in patients with indwelling urethral, indwelling suprapubic, or intermittent catheterization is defined by the presence of symptoms or signs compatible with UTI with no other identified source along with 103 CFU/mL of ≥1 bacterial species in a single catheter urine specimen or in a midstream voided urine specimen from a patient whose urethral, suprapubic or condom catheter has been removed within the previous 48 h (36).
These recommendations are based on the National Institute on Disability and Rehabilitation Research (NIDRR) Consensus Statement published in 1994 on the prevention and management of UTI among people with SCI. Here they defined UTI in IC, CIC and condom-catheter patients as any detectable concentration, ≥102 and ≥104, respectively (46). Utilizing lower colony counts is supported by a single level I study that compared urine cultures from NB patients performing CIC with paired SP aspirates. They demonstrated that the traditional threshold of ≥105 was associated with an unacceptable sensitivity for gram negative (0.65) and gram positive (0.45) organisms. Utilizing a threshold of ≥102 however improved the sensitivity significantly (0.91) (47). In a recent systematic review, a colony count of ≥102 had high sensitivity and reasonable specificity to define significant bacteriuria in patients who perform CIC (48).
Taking all this into consideration the IDSA proposes a cut off of 103 CFU/mL as this is the minimum level of detection used in many microbiological laboratories and does not compromise sensitivity (36). This is supported by an international working group that, in 2013, presented a standardized format for the collection and minimum reporting of information on UTIs in SCI patients (49). It is important to recognize that this cutoff is only a guideline recommendation and any colony count in a catheterized patient may potentially represent significant bacteriuria and should be interpreted along with the patients clinical picture (36).
The IDSA is more stringent in defining CA-AB reporting a cutoff ≥105 with the absence of symptoms compatible with a UTI. A higher cutoff increases the specificity and underscores their motivation to prevent antimicrobial overuse (36).
The IDSA argues against the interpretation of pyuria for defining a CA-UTI, CA-AB, differentiating CA-AB from CA-UTI and serving as a threshold for antimicrobial treatment (36). They do acknowledge however that if there is no evidence of pyuria in a patient with symptoms that another diagnosis should be sought (36). This is supported by the results of a recent systematic review which demonstrated the sensitivity of pyuria among three articles of ranging quality to be 74–83% (48). Similarly, the authors identified three high quality studies in NB patients demonstrating high sensitivity and specificity for positive nitrites and leukocyte esterase to predict a UTI. When used as a criterion for urine culture the sensitivity and specificity improved from 64% to 87% and 52% to 87%, respectively (48). A retrospective study of 89 SCI patients managed with different bladder management methods, evaluated the incidence of AB and correlated this with urinalysis results at routine annual evaluation (49). They found that 45% of patients had positive nitrites and that 100% of patients with positive nitrites had culture positive AB. Of the patients with negative nitrites, 55% had AB. Similarly, 55% of patients presented with ≥6 white blood cells with 95% of them having a positive culture. This correlated with 20-fold increase in the odds of finding a positive culture and the authors recommended against performing a culture if patients had <6 white blood cells on microscopy. Therefore, a positive urine dip stick should be used to prompt urine culture rather than treatment and NB patients who are asymptomatic with a normal urine dip should not undergo a urine culture (48).
Symptoms of a UTI in NB patients are very different from those in patients with an intact central nervous system. Depending on the underlying pathology, level and completeness of injury patients will exhibit very different signs and symptoms. Signs and symptoms that should be taken into consideration when assessing for the presence of a UTI as defined by the International SCI UTI basic data set include fever, urinary incontinence/failure of control or leaking around catheter, spasticity, malaise, lethargy or sense of unease, cloudy urine, malodorous urine, back pain, bladder pain, dysuria and autonomic dysreflexia (AD) (50).
In a prospective study assessing the validity, accuracy and predictive value of the signs and symptoms of UTI in SCI patients using CIC and IC, the authors found that all signs and symptoms, except for spasticity, were more accurate predictors of an infection compared to subjective asking. Patients were more reliably able to predict when they did not have an infection (negative predictive value 82%) than when they did (positive predictive value 32%). Fever and AD had the highest specificities (99% and 99%, respectively) however very low sensitivities (0 and 7%, respectively). Cloudy and malodorous urine has the 2nd highest accuracy scores (83% and 79%, respectively) with reasonable sensitivities (66% and 48%, respectively) (51).
Another prospective study evaluated diagnostic criteria for UTI in male SCI patients performing CIC. Patients with a symptomatic UTI defined as ≥102 CFU/mL with at least 1 sign or symptom as defined by the American Paraplegia Society criterion were assessed. A total of 381 episodes of symptomatic UTI were recorded. The most prevalent clinical signs, alone or in combination, were cloudy and/malodorous urine (51.4%), onset of urinary incontinence (51.2%), and fatigue or sense of unease (41.7%), then fever (30.7%) and increased spasticity (30.2%). The different signs occurred in many different permutations however one third of patients experienced an isolated sign, one third experienced 2 signs and one third experienced 3 signs. Increased spasticity, AD and fatigue or sense of unease occurred very rarely in isolation. Patients with 3 or more signs had significantly higher white blood cell counts compared to the AB group. In patients presenting with an isolated sign there was no significant difference in white blood cell count and colony forming units per milliliter compared to the asymptomatic group (52).
These studies illustrate that signs and symptoms demonstrate mixed results for being able to predict UTI in the NB population. The IDSA recommends that unique neurogenic symptoms i.e., AD, increased spasticity or sense of unease may be suggestive of a CA-UTI (36). Therefore, an awareness of these signs and symptoms is important to prompt the physician of a possible UTI.
NB patients may require more invasive diagnostic assessment with flexible or rigid cystoscopy in the setting of recurrent infections, findings on imaging or recurrent catheter blockage. A retrospective review of 262 traumatic SCI patients managed with either an IC or a SPC underwent a total of 419 cystoscopies and were separated into a symptomatic group and asymptomatic group. The most common indications in both the IC and SPC groups were UTI and recurrent blockage although 31% of patients in the IC group also underwent cystoscopy for the insertion of a SPC. No significant difference was seen in the incidence of findings between the two groups. However, 69% of SPC and 36% of IC patients in the symptomatic group were identified to have either significant proteinaceous debris or bladder calculi on cystoscopy. When broken up by indication, 88% of the SPC and 39% of the IC group were identified to have either bladder calculi or significant proteinaceous debris in the setting of recurrent UTI. With regards to catheter blockage, 89% of the SPC and 56% of the IC group were identified to have bladder calculi or significant proteinaceous debris (53). Although retrospective, well established findings that predispose to recurrent infection and catheter blockage were identified in the bladders of symptomatic SCI patients with a higher incidence in patients with a SPC. Therefore, cystoscopy should be considered as part of the work up in NB patients with recurrent infections or catheter blockage especially if they are managed with a SPC.
UDS and VUDS similarly play important roles in the workup of a NB patient with recurrent UTIs. It is well established that elevated bladder pressures predispose to infection as well as VUR and upper tract deterioration (1,3,54). Therefore, recognition can allow for targeted therapy with anticholinergic medications or intravesical Botox (54). A systematic review assessing urological follow up in NB patients identified good evidence for the utilization of UDS because of the additional information that is provided compared with clinical symptoms and ultrasound (US) results alone (48). An optimal interval could not be provided as this has not been previously assessed. A baseline UDS/VUDS is required at a minimum following a diagnosis of NB or SCI and serial studies should be performed to monitor treatment or progression, especially in children (48). The use of US is also recommended by the results of the systematic review because of its cost-effective and noninvasive nature. It has good sensitivity for the detection of urinary tract stones and hydronephrosis which can significantly impact treatment or prompt UDS (48).
Treatment
Acute bacterial UTI
NB patients diagnosed with a UTI require antimicrobial therapy in addition to basic primary care and/or sepsis management principles. As previously discussed, and in keeping with IDSA recommendations, any catheter than has been in place for >2 weeks should be immediately removed and replaced and the urine specimen should be obtained from the new catheter before the initiation of antimicrobial therapy. Cultures in general should always be obtained prior to antimicrobial therapy because of increased risk of nosocomial organisms, history of antibiotic use and resistance (36). Following identification of the organism and susceptibility pattern, the site and extent of infection must be assessed in the context of host resistance and specific risk factors (3). UTI often represents an overarching term for many complicated infections including prostatitis, pyelonephritis, bacteremia and simple cystitis. Therefore, patients should be carefully clinically assessed to determine the optimal route, spectrum of coverage and duration of antibiotics.
Antibiotic stewardship is of paramount importance in NB UTI. Patients should be treated with narrow spectrum antibiotics, when possible, for the shortest duration that is clinically safe (3). The IDSA recommends a 7-day course of antibiotics for patients with prompt clinical response. Patients with significant infection or a delayed response should have the duration of treatment extended to 10–14 days. These recommendations are based on Grade A, Level III evidence (3).
A recent noninferiority trial randomized 55 catheter-dependent SCI patients with a CA-UTI to receive 5 days of antibiotics with a catheter change (experimental group) or 10-days of antibiotics with catheter retention (control group). The experimental group was noninferior with regards to clinical cure, however, microbiologic and pyuria resolution were inferior when compared to the control. Additionally, the experimental group was shown to have significantly higher CA-UTI recurrence rates when compared to the control group (hazard ration =0.76; 95% CI, 0.59–0.99; P=0.043) (55). Similar results were shown in another randomized, double-blind, placebo-controlled trial comparing 3-day and 14-day regimens of ciprofloxacin (250 mg twice daily) for CA-UTI in 60 SCI patients. No difference in clinical outcome was detected between the two treatment arms at long-term follow-up however microbiological cure was significantly better in the 14-day regimen arm (56). There is no ideal or optimal duration of antibiotic therapy in NB patients. Instead, antibiotic duration should be made based on current guidelines as well as the patient’s severity of clinical presentation, host risk factors and response to therapy.
Antibiotic selection following culture collection should be based on local resistance patterns and antibiograms should always be consulted, when available, for determining the most appropriate empiric therapy. Therapy should be adjusted following culture results if deemed necessary. The IDSA recommends a 5-day regimen of levofloxacin as suitable therapy in patients with a CA-UTI that are not significantly ill. These recommendations are based on Grade B, Level III evidence and they report that insufficient data exists regarding other fluoroquinolones (36). If there is a suspicion of methicillin-resistant S aureus, as is commonly seen in chronically hospitalized and instrumented NB patients, then vancomycin should be used for serious infections. For patients with an MRSA UTI who are suitable for outpatient management, then trimethoprim-sulfamethoxazole may be considered as suitable oral management. In vitro studies have demonstrated up to 97% sensitivity to trimethoprim-sulfamethoxazole when different strains of MRSA are tested via the disk diffusion method (57). Although not formally assessed, this data is extrapolated from an increasing body of retrospective and prospective data demonstrating that trimethoprim-sulfamethoxazole can be used to treat serious MRSA infections from different sources, although vancomycin remains first line therapy (58,59). This is supported by a recent non-inferiority trial that randomized patients to receive trimethoprim-sulfamethoxazole or vancomycin for serious MRSA infections (59). No significant difference in treatment failure was demonstrated between groups and adverse events were similar. Trimethoprim-sulfamethoxazole however did not meet the non-inferiority criterion and this difference was more significant in patients with bacteremia i.e., more serious infections.
In cases of mild UTIs with no evidence of systemic involvement (e.g., fever) nitrofurantoin is acceptable and ideal because it does not alter bowel or vaginal flora. Caution should be used in patients with a history or suspicion of Pseudomonas and Proteus because they tend to be highly resistant. Trimethoprim/sulfamethoxazole should be considered in patients with more severe infections or history of fever although it does not provide Pseudomonas coverage. The quinolone family represent an excellent option in the NB population because of good bioavailability, decreased bacterial adherence by biofilms, and coverage of nosocomial organisms, i.e., Pseudomonas (3). Again, because of emerging resistance patterns, antibiotics should be prescribed in accordance with local antibiograms.
Fosfomycin was discovered in 1969 and has remerged as a powerful urinary tract antibiotic (60). Currently, the US Food and Drug administration has only approved its use for the treatment of uncomplicated UTIs in females; however it holds significant promise for the NB population. This unique antibiotic is bactericidal and interferes with cell wall synthesis by inhibiting the formation of peptidoglycan (61). Of particular importance to the NB population, fosfomycin has been shown to have good activity against biofilms (60). It works synergistically with other antibiotics (aminoglycosides and fluoroquinolones) to break up the biofilm and improve permeability (62,63). The current oral formulation is fosfomycin tromethamine and it is administered as a single 3 g dose (60). The bioavailability of this antibiotic is excellent and a single dose will achieve a therapeutic concentration in the urine for 1–3 days (60). Single dose therapy has been shown to be as clinically effective as a 7–10 days course of standard treatment regimens (60). Its spectrum of coverage is ideal and includes MRSA, Extended Spectrum Beta-Lactamase (ESBL), as well as the typical urinary gram-negative organisms (60). Unfortunately, Pseudomonas and Acinetobacter are typically resistant (64). The safety profile of this medication is excellent and it is well tolerated and safe during pregnancy. The oral dosage does not need to be adjusted in the setting of hepatic or renal failure (60). Although considered off-label use in the US and Canada, a complicated UTI may be treated with a total of 3 doses of 3 g fosfomycin administered 2–3 days apart (60). Unfortunately, no current literature assessing the use of fosfomycin in the NB population is available. In the face of increasing MDR, drug sensitivities and interactions, its use should be strongly considered as second-line therapy for NB patients and prospective, randomized studies in this special population should be prompted.
AB in the NB patient
Numerous studies and guidelines very clearly outline that AB should not be treated in NB patients. Treatment does not impact subsequent AB in catheterized patients and leads to increased bacterial resistance and early recurrence of infection (3,36,65). There is excellent Grade A, Level I and II evidence recommending against the screening and treatment of CA-AB in patients with IC and CIC, respectively. However, the IDSA, does advocate for the screening and treatment of CA-AB in pregnant patients and patients undergoing urological procedures where mucosal bleeding is likely to be encountered and this is based on Grade A, Level III evidence (36).
Prevention
Catheter-related measures
Closed catheter drainage
In NB patients with IC or SPC, closed catheter drainage system remains one of the most important preventative measures against infection and is strongly recommended by the IDSA for the prevention of CA-UTI and CA-AB in patients with chronic IC and SPC (36). Many historical reports support its role in the prevention of infection with a 95% incidence of CA-bacteriuria after 96 hours in patients without closed drainage (66). Frequent violation of the closed drainage junction has been shown to significantly increase the risk of CA-AB (36,67,68). Multiple studies demonstrate that patients who undergo catheter placement with a preconnected junction are significantly less likely to develop CA-AB (36,68,69). Additionally, the location of the urinary drainage bag is of equal importance and CA-bacteriuria follows contamination of the urinary bag (38,39). Therefore, the drainage bag and tubing should always be situated below the level of the bladder and the IDSA recommends hospital policies to minimize closed drainage violation and to ensure appropriate location of the drainage bag and tubing in all catheterized patients (36).
Routine catheter changes
NB patients with chronic IC and SPC typically will undergo regular catheter changes occurring at intervals anywhere from every 2 to 6 weeks, with monthly being the most common time interval. These practices are not evidence-based and insufficient evidence exists for guideline recommendations. Because of the tendency for catheters to develop biofilms and encrustation, it seems plausible that routine changes with fresh catheters maintains the microbial burden manageable by grossly removing established intra- and extraluminal biofilms. Comparison of urine culture results in patients with long-term catheters and those immediately following catheter replacement demonstrates both a quantitative and qualitative reduction in bacterial species (36,70,71).
Method of bladder management
Multiple factors need to be taken into consideration when deciding on the most appropriate method of bladder management for NB patients including status of the lower urinary tract, duration of catheterization, degree of mobility, sensation and dexterity, access to health care personnel and patient preferences. Intermittent catheterization is associated with fewer infections and complications compared to the other commonly used methods (3,36). A commonly cited prospective study comparing the different methods of bladder management in patients with acute SCI reported an incidence of CA-UTI per 100 person-days of 2.72 in the IC group, 0.41 in the CIC group, 0.36 in the condom catheter group, 0.34 in the SPC group and 0.06 in the normal voiding group (17). Although the condom catheter and SPC group had lower incidences of CA-UTI, their sample sizes were significantly smaller than the IC and CIC groups. Additionally, only female patients comprised the SPC group.
In the non-neurogenic population, contemporary studies comparing SPC use to IC and CIC do not demonstrate a difference in the incidence of CA-UTI and CA-ASB, respectively (72,73). Data in the NB population is lacking, especially randomized controlled studies. A comparative study in quadriplegic patients comparing SPC and CIC did not show a significant difference in the incidence of UTI. There was, however, a significantly increased incidence of bladder calculi in the SPC group compared to the CIC group (74). Therefore, SPC is comparable to CIC with regards to infection and may be suitable for some patients (females or those with significant stricture disease) however it requires invasive insertion and increases the risk of stone formation (75).
Condom catheter drainage provides a less painful and more comfortable alternative in appropriately selected patients (36). Although non-invasive, bacteriuria is still a problem and studies have shown that condom catheters are significantly associated with Pseudomonas and Klebsiella bacteriuria (76,77). However, the incidence of condom catheter associated bacteriuria is less than that of IC and the incidence of CA-UTI appears to be comparable to that of CIC (3,17,36). Penile skin breakdown and scarring can also occur with long-term condom catheter use. It should be noted that safe and effective condom catheter drainage is dependent upon reasonable bladder storage pressures and bladder emptying. In the NB population this should be determined by UDS in order to avoid silent upper tract deterioration (36). In summary, condom catheters can provide an effective and safe alternative to catheterization in NB patients with low PVRs and no evidence of DSD (36).
Another prospective study of 67 SCI patients evaluating the incidence of bacteriuria between the different methods of bladder management (CIC, IC, credé/reflex voiding, normal voiding) showed that the lowest incidence of AB existed in the normal voiding group followed closely by the CIC group. A significantly increased incidence of UTIs was seen in the Credé/reflex voiding and IC groups compared with the normal voiding group. This difference was not appreciated between the normal voiding and CIC group (78). Good, randomized, long-term data is lacking. Current evidence supports intermittent catheterization as the optimal bladder management method in candidate NB patients to help minimize CA-AB and CA-UTI. This position is supported by the IDSA (36).
Different technical considerations for intermittent catheterization include clean versus sterile intermittent catheterization and hydrophilic versus standard uncoated catheters. There is no significant difference in the incidence of CA-UTI and CA-AB between the clean versus sterile techniques for intermittent catheterization in both NB and non-neurogenic patients (36,79). A recent Cochrane review does not provide evidence to support hydrophilic coated catheter use over uncoated catheter use; a position previously recommended by the IDSA in 2009 (36,79).
Impregnated catheters
Both antibiotic and silver-coated catheters have been studied for the prevention of UTIs. Both catheter types have been shown to have an impact on bacteriuria and infection but only in the very short term (54,79). Additionally, concern exists regarding antibiotic resistance and silver toxicity with long-term use (54,69).
Other measures
Strong evidence exists against the use of antimicrobials or antiseptics in the urinary drainage bag (80-84), enhanced meatal care (85,86), and catheter irrigation with antimicrobials or normal saline for reducing or eradicating CA-AB and CA-UTI (67,87,88).
Medical measures
Antibiotic prophylaxis
A meta-analysis of 15 RCTs did not support the use of antibiotic prophylaxis for the prevention of UTI in NB patients (89). Patient groups were divided into acute (<90 days post SCI) and non-acute. Antibiotic prophylaxis was only shown to significantly decrease the incidence of AB in the acute patient group and one patient would need to be treated with 3.7 weeks of treatment in order to prevent one episode of AB. Five of the included studies reported on the incidence of antimicrobial resistance. Three of the five studies reported an approximately twofold increase in antimicrobial resistant bacteria with antibiotic prophylaxis. A recent update of a Cochrane review assessing antibiotic prophylaxis in patients with long-term catheterization was unable to provide recommendations for clinical practice with regards to prophylaxis in IC or CIC performing patients (90). The overall quality of the trials was poor and they were fraught with multiple biases and small sample sizes. Weak evidence was found for the reduction in the episodes of bacteriuria in CIC patients however this has been previously well established in all catheterized patients (36,38,90,91). The IDSA similarly recommends against the use of prophylaxis for the reduction of CA-UTI and CA-AB primarily because of increased concerns regarding antimicrobial resistance. Therefore, antibiotic prophylaxis is not recommended for the prevention of UTI in NB patients. With regards to antibiotic prophylaxis at the time of catheter replacement, no studies currently exist and the IDSA recommends against this as well as pre-replacement bladder irrigation to prevent CA-AB and CA-UTI (36).
Cranberry prophylaxis
The literature does not support the use of cranberry for the prevention of UTIs, CA-UTIs and CA-AB in the NB population (36,92). Only a single study has demonstrated a significant reduction in the incidence of UTI with cranberry prophylaxis in the NB population. Patients were randomized to receive 6 months of cranberry extract tablet or placebo. The frequency of UTI was reduced to 0.3 UTI per year vs. 1.0 UTI per year while receiving placebo. However, this study has been criticized for its small sample size and for the fact that 74% of patients were managed with a condom catheter (93).
Methenamine salt prophylaxis
In urine, methenamine salts hydrolyze into ammonia and formaldehyde. The antimicrobial activity of these agents are correlated with the urinary concentration of formaldehyde which is dependent on urinary methenamine concentration, pH and dwell time (36). For these reasons, its use has been limited in the NB population that is largely catheterized, thereby shortening the dwell time significantly. A meta-analysis failed to show a significant effect for methenamine for preventing UTI in NB patients when the results for specific agents were pooled (89). Therefore, at this time, methenamine salts are not recommended for the prevention of CA-UTI and CA-AB (36).
Procedural interventions
Intravesical botulinum toxin A
Botox has become an indispensable tool for the treatment of neurogenic detrusor overactivity (NDO) caused by SCI or MS. It provides significant improvement in UDS parameters, continence and quality of life (94). Evidence is beginning to mount that it may additionally impart protection against infection in a subset of NB patients (54). Two prospective, non-randomized trials have demonstrated a significant reduction in the incidence of UTI following 300 U of intravesical Botox in patients with NDO (20,21). All patients in both studies underwent UDS, voiding cystourethrography, urine culture and maintained voiding diaries before and after Botox administration. The mean number of symptomatic UTIs 6 months before injection was 1.39±1.36 and 1.75±1.87 in each trial. Six months following injection the mean number of symptomatic UTIs decreased to 0.78±0.96 and 0.20±0.41, respectively. These differences were found to be significant in both trials. All patients experienced improvement in their urodynamic parameters however the degree of improvement was less in some patients with ongoing detrusor overactivity. Interestingly, both studies demonstrated that patients without urodynamic improvement, primarily in bladder pressure, were significantly associated with a risk of UTI post injection (20,21). It is plausible that elevated bladder pressures predisposes to VUR, bladder ischemia and stasis, all of which are significant risk factors for UTIs in NB patients (1). Intradetrusor botox for the prevention of NB UTI is an attractive option but does require further study.
Bacterial interference
Bacterial interference is characterized by intentional bladder colonization with a bacterial strain of low virulence in an attempt to deter uropathogenic bacterial binding, internalization and subsequent infection. A randomized, multicenter, double-blind placebo controlled trial assessed the anti-infective potential of HU2117, a non-pathogenic strain of E. coli, following inoculation into the bladders of NB patients (95). Patients were managed with IC, CIC, or external collection devices and had a history of recurrent UTIs (>2 episode/year). Successful colonization increased with repeated inoculations however patient participation inversely decreased. This experiment was carried out safely and no patients developed a symptomatic E. coli HU2117 infection. Kaplan-Meier estimates of the risk of UTI by the interval elapsed since bladder inoculation illustrates that successful bladder colonization is protective against infection. Additionally, the average number of episodes of UTI/patient-year was significantly lower in the experimental group (0.50) compared to the control group (1.68) (95). Only 27 patients completed this trial which was related to low patient compliance. Given the promising results and absence of adverse events, it is imperative that future trials with increased patient enrollment and compliance (alternative methods of inoculation) are developed.
Sacral neuromodulation
Implantation of a neural sacral modulator has been used for the treatment of detrusor overactivity, NDO, fecal incontinence and erectile function. It has been shown to improve urodynamic parameters and evidence is beginning to emerge that it may help to prevent UTIs in NB patients (54). A cross-sectional study of SCI patients with NDO who previously underwent implantation of a Brindley sacral simulator and dorsal rhizotomy demonstrated that patients who continued to use their stimulator (67%), reported improved quality of life, continence and infrequent UTIs compared to the control group of NDO patients managed by alternative methods (96). A small observational study compared the incidence of UTI in 10 NB patients post implantation to that of 6 patients managed traditionally. Patients underwent implantation soon after their traumatic SCI, during the acute bladder-areflexia phase. Following implantation, patients had a mean of 0.5 UTIs/year compared to 3.8 in the control group (97). Like intradetrusor botox, the mechanism of action is probably related to improved bladder capacity and storage pressures.
Conclusions
UTIs in NB patients represent a significant problem for patients, healthcare providers and the healthcare system. They are difficult to prevent, diagnose accurately and treat. The importance of understanding the unique risk factors and pathogenesis of a NB UTI cannot be overstated. NB patients with frequent recurrent UTI should be investigated with UDS/VUDS, US and cystoscopy to search for modifiable causes such as calculi, bladder debris, poor bladder compliance and/or DSD. Lower urinary tract immune system alterations subsequent to neurologic injury or disease are likely important in the pathogenesis of UTI in the NB population. This requires more research as it could lead to novel prevention strategies and therapeutics. At the present time, the most effective means of UTI prevention in the NB patient remain simple and time-tested: closed catheter drainage and CIC. There is a need for more high-quality randomized, controlled trials however, the heterogeneous nature of the NB population makes this a difficult task. Bacterial interference, intravesical Botox and sacral neuromodulation hold significant promise however more evidence is required before widespread recommendations can be made.
Acknowledgements
The authors thank Chad Lorentz for editing and recreating the figures into a publishable format.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vasudeva P, Madersbacher H. Factors implicated in pathogenesis of urinary tract infections in neurogenic bladders: some revered, few forgotten, others ignored. Neurourol Urodyn 2014;33:95-100. [PubMed]
- Pannek J. Treatment of urinary tract infection in persons with spinal cord injury: guidelines, evidence, and clinical practice. J Spinal Cord Med 2011;34:11-5. [PubMed]
- Siroky MB. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med 2002;113:67S-79S. [PubMed]
- Manack A, Motsko SP, Haag-Molkenteller C, et al. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol Urodyn 2011;30:395-401. [PubMed]
- DeJong G, Tian W, Hsieh CH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil 2013;94:S87-97. [PubMed]
- Guilcher SJ, Craven BC, Calzavara A, et al. Is the emergency department an appropriate substitute for primary care for persons with traumatic spinal cord injury? Spinal Cord 2013;51:202-8. [PubMed]
- Unsal-Delialioglu S, Kaya K, Sahin-Onat S, et al. Fever during rehabilitation in patients with traumatic spinal cord injury: analysis of 392 cases from a national rehabilitation hospital in Turkey. J Spinal Cord Med 2010;33:243-8. [PubMed]
- Metz LM, McGuinness SD, Harris C. Urinary tract infections may trigger relapse in multiple sclerosis. Axone 1998;19:67-70. [PubMed]
- Rapp NS, Gilroy J, Lerner AM. Role of bacterial infection in exacerbation of multiple sclerosis. Am J Phys Med Rehabil 1995;74:415-8. [PubMed]
- Böthig R, Fiebag K, Thietje R, et al. Morbidity of urinary tract infection after urodynamic examination of hospitalized SCI patients: the impact of bladder management. Spinal Cord 2013;51:70-3. [PubMed]
- Quek P, Tay LH. Morbidity and significant bacteriuria after urodynamic studies. Ann Acad Med Singapore 2004;33:754-7. [PubMed]
- Almallah YZ, Rennie CD, Stone J, et al. Urinary tract infection and patient satisfaction after flexible cystoscopy and urodynamic evaluation. Urology 2000;56:37-9. [PubMed]
- Evans CT, Rogers TJ, Chin A, et al. Antibiotic prescribing trends in the emergency department for veterans with spinal cord injury and disorder 2002-2007. J Spinal Cord Med 2013;36:492-8. [PubMed]
- Togan T, Azap OK, Durukan E, et al. The prevalence, etiologic agents and risk factors for urinary tract infection among spinal cord injury patients. Jundishapur J Microbiol 2014;7:e8905. [PubMed]
- Yoon SB, Lee BS, Lee KD, et al. Comparison of bacterial strains and antibiotic susceptibilities in urinary isolates of spinal cord injury patients from the community and hospital. Spinal Cord 2014;52:298-301. [PubMed]
- Martins CF, Bronzatto E, Neto JM, et al. Urinary tract infection analysis in a spinal cord injured population undergoing rehabilitation--how to treat? Spinal Cord 2013;51:193-5. [PubMed]
- Esclarín De Ruz A, García Leoni E, Herruzo Cabrera R. Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. J Urol 2000;164:1285-9. [PubMed]
- Goetz LL, Howard M, Cipher D, et al. Occurrence of candiduria in a population of chronically catheterized patients with spinal cord injury. Spinal Cord 2010;48:51-4. [PubMed]
- Flores-Mireles AL, Walker JN, Caparon M, et al. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015;13:269-84. [PubMed]
- Gamé X, Castel-Lacanal E, Bentaleb Y, et al. Botulinum toxin A detrusor injections in patients with neurogenic detrusor overactivity significantly decrease the incidence of symptomatic urinary tract infections. Eur Urol 2008;53:613-8. [PubMed]
- Jia C, Liao LM, Chen G, et al. Detrusor botulinum toxin A injection significantly decreased urinary tract infection in patients with traumatic spinal cord injury. Spinal Cord 2013;51:487-90. [PubMed]
- Neal DE. Host defense mechanisms in urinary tract infections. Urol Clin North Am 1999;26:677-86. [PubMed]
- Merritt JL. Residual urine volume: correlate of urinary tract infection in patients with spinal cord injury. Arch Phys Med Rehabil 1981;62:558-61. [PubMed]
- Taylor TA, Waites KB. A quantitative study of genital skin flora in male spinal cord-injured outpatients. Am J Phys Med Rehabil 1993;72:117-21. [PubMed]
- Waites KB, Canupp KC, DeVivo MJ. Microbiology of the urethra and perineum and its relationship to bacteriuria in community-residing men with spinal cord injury. J Spinal Cord Med 2004;27:448-52. [PubMed]
- Parsons CL, Greenspan C, Moore SW, et al. Role of surface mucin in primary antibacterial defense of bladder. Urology 1977;9:48-52. [PubMed]
- Parsons CL, Shrom SH, Hanno PM, et al. Bladder surface mucin. Examination of possible mechanisms for its antibacterial effect. Invest Urol 1978;16:196-200. [PubMed]
- Wold AE, Mestecky J, Tomana M, et al. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun 1990;58:3073-7. [PubMed]
- Vaidyanathan S, McDicken IW, Soni BM, et al. Secretory immunoglobulin A in the vesical urothelium of patients with neuropathic bladder--an immunohistochemical study. Spinal Cord 2000;38:378-81. [PubMed]
- Mulvey MA, Lopez-Boado YS, Wilson CL, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 1998;282:1494-7. [PubMed]
- Schlager TA, Grady R, Mills SE, et al. Bladder epithelium is abnormal in patients with neurogenic bladder due to myelomeningocele. Spinal Cord 2004;42:163-8. [PubMed]
- Vaidyanathan S, McDicken IW, Ikin AJ, et al. A study of cytokeratin 20 immunostaining in the urothelium of neuropathic bladder of patients with spinal cord injury. BMC Urol 2002;2:7. [PubMed]
- Balsara ZR, Ross SS, Dolber PC, et al. Enhanced Susceptibility to Urinary Tract Infection in the Spinal Cord-Injured Host with Neurogenic Bladder. Infect Immun 2013;81:3018-26. [PubMed]
- Chaudhry R, Madden-Fuentes RJ, Ortiz TK, et al. Inflammatory response to Escherichia coli urinary tract infection in the neurogenic bladder of the spinal cord injured host. J Urol 2014;191:1454-61. [PubMed]
- Iversen PO, Hjeltnes N, Holm B, et al. Depressed immunity and impaired proliferation of hematopoietic progenitor cells in patients with complete spinal cord injury. Blood 2000;96:2081-3. [PubMed]
- Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50:625-63. [PubMed]
- Tambyah PA, Halvorson KT, Maki DG. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc 1999;74:131-6. [PubMed]
- Garibaldi RA, Burke JP, Dickman ML, et al. Factors predisposing to bacteriuria during indwelling urethral catheterization. N Engl J Med 1974;291:215-9. [PubMed]
- Hartstein AI, Garber SB, Ward TT, et al. Nosocomial urinary tract infection: a prospective evaluation of 108 catheterized patients. Infect Control 1981;2:380-6. [PubMed]
- Nickel JC, Grant SK, Costerton JW. Catheter-associated bacteriuria. An experimental study. Urology 1985;26:369-75. [PubMed]
- Ikäheimo R, Siitonen A, Kärkkäinen U, et al. Virulence characteristics of Escherichia coli in nosocomial urinary tract infection. Clin Infect Dis 1993;16:785-91. [PubMed]
- Johnson JR. Microbial virulence determinants and the pathogenesis of urinary tract infection. Infect Dis Clin North Am 2003;17:261-78. viii. [PubMed]
- Jacobsen SM, Stickler DJ, Mobley HL, et al. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev 2008;21:26-59. [PubMed]
- Stamm WE. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am J Med 1991;91:65S-71S. [PubMed]
- Saint S, Chenoweth CE. Biofilms and catheter-associated urinary tract infections. Infect Dis Clin North Am 2003;17:411-32. [PubMed]
- The prevention and management of urinary tract infections among people with spinal cord injuries. National Institute on Disability and Rehabilitation Research Consensus Statement. January 27-29, 1992. J Am Paraplegia Soc 1992;15:194-204. [PubMed]
- Gribble MJ, McCallum NM, Schechter MT. Evaluation of diagnostic criteria for bacteriuria in acutely spinal cord injured patients undergoing intermittent catheterization. Diagn Microbiol Infect Dis 1988;9:197-206. [PubMed]
- Cameron AP, Rodriguez GM, Schomer KG. Systematic review of urological followup after spinal cord injury. J Urol 2012;187:391-7. [PubMed]
- Jayawardena V, Midha M. Significance of bacteriuria in neurogenic bladder. J Spinal Cord Med 2004;27:102-5. [PubMed]
- Goetz LL, Cardenas DD, Kennelly M, et al. International Spinal Cord Injury Urinary Tract Infection Basic Data Set. Spinal Cord 2013;51:700-4. [PubMed]
- Massa LM, Hoffman JM, Cardenas DD. Validity, accuracy, and predictive value of urinary tract infection signs and symptoms in individuals with spinal cord injury on intermittent catheterization. J Spinal Cord Med 2009;32:568-73. [PubMed]
- Ronco E, Denys P, Bernede-Bauduin C, et al. Diagnostic Criteria of Urinary Tract Infection in Male Patients With Spinal Cord Injury. Neurorehabil Neural Repair 2011;25:351-8. [PubMed]
- El Masri y WS, Patil S, Prasanna KV, et al. To cystoscope or not to cystoscope patients with traumatic spinal cord injuries managed with indwelling urethral or suprapubic catheters? That is the question! Spinal Cord 2014;52:49-53. [PubMed]
- Salameh A, Mohajer Al M, Darouiche RO. Prevention of urinary tract infections in patients with spinal cord injury. CMAJ 2015;187:807-11. [PubMed]
- Darouiche RO, Al Mohajer M, Siddiq DM, et al. Short versus long course of antibiotics for catheter-associated urinary tract infections in patients with spinal cord injury: a randomized controlled noninferiority trial. Arch Phys Med Rehabil 2014;95:290-6. [PubMed]
- Dow G, Rao P, Harding G, et al. A prospective, randomized trial of 3 or 14 days of ciprofloxacin treatment for acute urinary tract infection in patients with spinal cord injury. Clin Infect Dis 2004;39:658-64. [PubMed]
- Memikoğlu KO, Bayar B, Kurt O, Cokça F. In vitro sensitivity of methicillin-resistant Staphylococcus aureus to fusidic acid and trimethoprim-sulfamethoxazole. Mikrobiyol Bul 2002;36:141-5. [PubMed]
- Grim SA, Rapp RP, Martin CA, et al. Trimethoprim-sulfamethoxazole as a viable treatment option for infections caused by methicillin-resistant Staphylococcus aureus. Pharmacotherapy 2005;25:253-64. [PubMed]
- Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ 2015;350:h2219. [PubMed]
- Michalopoulos AS, Livaditis IG, Gougoutas V. The revival of fosfomycin. Int J Infect Dis 2011;15:e732-9. [PubMed]
- Kahan FM, Kahan JS, Cassidy PJ, et al. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci 1974;235:364-86. [PubMed]
- Cai Y, Fan Y, Wang R, et al. Synergistic effects of aminoglycosides and fosfomycin on Pseudomonas aeruginosa in vitro and biofilm infections in a rat model. J Antimicrob Chemother 2009;64:563-6. [PubMed]
- Rodríguez-Martínez JM, Ballesta S, Pascual A. Activity and penetration of fosfomycin, ciprofloxacin, amoxicillin/clavulanic acid and co-trimoxazole in Escherichia coli and Pseudomonas aeruginosa biofilms. Int J Antimicrob Agents 2007;30:366-8. [PubMed]
- Falagas ME, Kastoris AC, Karageorgopoulos DE, et al. Fosfomycin for the treatment of infections caused by multidrug-resistant non-fermenting Gram-negative bacilli: a systematic review of microbiological, animal and and clinical studies. Int J Antimicrob Agents 2009;34:111-20. [PubMed]
- D’Hondt F, Everaert K. Urinary tract infections in patients with spinal cord injuries. Curr Infect Dis Rep 2011;13:544-51. [PubMed]
- Kass EH. Asymptomatic infections of the urinary tract. Trans Assoc Am Physicians 1956;69:56-64. [PubMed]
- Warren JW, Platt R, Thomas RJ, et al. Antibiotic irrigation and catheter-associated urinary-tract infections. N Engl J Med 1978;299:570-3. [PubMed]
- Platt R, Polk BF, Murdock B, et al. Reduction of mortality associated with nosocomial urinary tract infection. Lancet 1983;1:893-7. [PubMed]
- Siddiq DM, Darouiche RO. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urol 2012;9:305-14. [PubMed]
- Tenney JH, Warren JW. Bacteriuria in women with long-term catheters: paired comparison of indwelling and replacement catheters. J Infect Dis 1988;157:199-202. [PubMed]
- Grahn D, Norman DC, White ML, et al. Validity of urinary catheter specimen for diagnosis of urinary tract infection in the elderly. Arch Intern Med 1985;145:1858-60. [PubMed]
- Niël-Weise BS, , van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev 2005.CD004203. [PubMed]
- Jannelli ML, Wu JM, Plunkett LW, et al. A randomized controlled trial of clean intermittent self-catheterization versus suprapubic catheterization after urogynecologic surgery. Am J Obstet Gynecol 2007;197:72.e1-4.
- Mitsui T, Minami K, Morita H, et al. Is suprapubic cystostomy an optimal urinary management in high quadriplegics? A comparative study of suprapubic cystostomy and clean intermittent catheterization. Eur Urol 2000;38:434-8. [PubMed]
- Sheriff MK, Foley S, McFarlane J, et al. Long-term suprapubic catheterisation: clinical outcome and satisfaction survey. Spinal Cord 1998;36:171-6. [PubMed]
- Montgomerie JZ, Morrow JW. Long-term Pseudomonas colonization in spinal cord injury patients. Am J Epidemiol 1980;112:508-17. [PubMed]
- Montgomerie JZ , Gilmore DS, Graham IE, et al. Klebsiella pneumoniae colonization in patients with spinal cord injury. Diagn Microbiol Infect Dis 1987;7:229-35. [PubMed]
- Shen L, Zheng X, Zhang C, et al. Influence of different urination methods on the urinary systems of patients with spinal cord injury. J Int Med Res 2012;40:1949-57. [PubMed]
- Prieto J, Murphy CL, Moore KN, et al. Intermittent catheterisation for long-term bladder management. Cochrane Database Syst Rev 2014;9:CD006008. [PubMed]
- Reiche T, Lisby G, Jørgensen S, et al. A prospective, controlled, randomized study of the effect of a slow-release silver device on the frequency of urinary tract infection in newly catheterized patients. BJU Int 2000;85:54-9. [PubMed]
- Thompson RL, Haley CE, Searcy MA, et al. Catheter-associated bacteriuria. Failure to reduce attack rates using periodic instillations of a disinfectant into urinary drainage systems. JAMA 1984;251:747-51. [PubMed]
- Gillespie WA, Simpson RA, Jones JE, et al. Does the addition of disinfectant to urine drainage bags prevent infection in catheterised patients? Lancet 1983;1:1037-9. [PubMed]
- Sweet DE, Goodpasture HC, Holi K, et al. Evaluation of H2O2 prophylaxis of bacteriuria in patients with long-term indwelling Foley catheters: a randomized controlled study. Infect Control 1985;6:263-6. [PubMed]
- Classen DC, Larsen RA, Burke JP, et al. Prevention of catheter-associated bacteriuria: clinical trial of methods to block three known pathways of infection. Am J Infect Control 1991;19:136-42. [PubMed]
- Burke JP, Jacobson JA, Garibaldi RA. Evaluation of daily meatal care with poly-antibiotic ointment in prevention of urinary catheter-associated bacteriuria. J Urol 1983;129:331-4. [PubMed]
- Burke JP, Garibaldi RA, Britt MR, et al. Prevention of catheter-associated urinary tract infections: efficacy of daily meatal care regimens. Am J Med 1981;70:655-8. [PubMed]
- Davies AJ, Desai HN, Turton S, et al. Does instillation of chlorhexidine into the bladder of catheterized geriatric patients help reduce bacteriuria? J Hosp Infect 1987;9:72-5. [PubMed]
- Dudley MN, Barriere SL. Antimicrobial irrigations in the prevention and treatment of catheter-related urinary tract infections. Am J Hosp Pharm 1981;38:59-65. [PubMed]
- Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil 2002;83:129-38. [PubMed]
- Niël-Weise BS, van den Broek PJ, da Silva E, et al. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev 2012;8:CD004201. [PubMed]
- Hustinx WN, Mintjes-de Groot AJ, Verkooyen RP, et al. Impact of concurrent antimicrobial therapy on catheter-associated urinary tract infection. J Hosp Infect 1991;18:45-56. [PubMed]
- Opperman EA. Cranberry is not effective for the prevention or treatment of urinary tract infections in individuals with spinal cord injury. Spinal Cord 2010;48:451-6. [PubMed]
- Hess MJ, Hess PE, Sullivan MR, et al. Evaluation of cranberry tablets for the prevention of urinary tract infections in spinal cord injured patients with neurogenic bladder. Spinal Cord 2008;46:622-6. [PubMed]
- Cruz F, Nitti V. Chapter 5: Clinical data in neurogenic detrusor overactivity (NDO) and overactive bladder (OAB). Neurourol Urodyn 2014;33 Suppl 3:S26-31. [PubMed]
- Darouiche RO, Green BG, Donovan WH, et al. Multicenter randomized controlled trial of bacterial interference for prevention of urinary tract infection in patients with neurogenic bladder. Urology 2011;78:341-6. [PubMed]
- Martens FM, den Hollander PP, Snoek GJ . Quality of life in complete spinal cord injury patients with a Brindley bladder stimulator compared to a matched control group. Neurourol Urodyn 2011;30:551-5. [PubMed]
- Sievert KD, Amend B, Gakis G, et al. Quality of life in complete spinal cord injury patients with a Brindley bladder stimulator compared to a matched control group. Ann Neurol 2010;67:74-84. [PubMed]


