Management options for sphincteric deficiency in adults with neurogenic bladder
Introduction
Urinary sphincteric function is variable in patients with neurogenic bladder and depends upon the patient’s underlying etiology of neurologic dysfunction or sometimes coexisting injuries. In general, patients with spinal cord injury or disease involving the supra-sacral spinal cord experience detrusor-sphincter dyssynergia. This refers to the condition when the urethral sphincter (internal and/or external) contracts at the same time as detrusor muscle. During normal voiding, the urethral sphincter relaxes when the detrusor muscle contracts. In patients with dyssynergic sphincters, leakage from intrinsic sphincteric deficiency (ISD) does not occur. However, if a lower motor neuron disorder occurs then de-innervation of the sphincters can result, leading to ISD. Some examples of traumatic injuries, which can produce ISD, are cauda equina from lower spinal column and sacral fractures, laminectomy complications or vertebral disk disease, severe pelvic fractures, and nerve injury from resection of low colorectal cancers.
Some neurologic disease, especially myelomeningocele (spina bifida) can also lead to ISD. This makes anatomic sense since generally the defect in myelomeningocele involve the sacrum or lower lumbar spinal column and therefore disrupt the lowest portion of the spinal cord and nerve roots emerging from spinal cord. Even if the original myelomeningocele lesion does not produce ISD, subsequent procedures to de-tether the nerve roots from associated scarring can produce a lower motor neuron injury leading to loss of sphincteric function.
Direct injury of the urethral sphincter is also an acquired problem in patients with neurogenic bladder. A common scenario is a patient that has a Foley catheter placed for chronic bladder management and the catheter erodes through and damages the bladder neck and/or the external sphincter. In men, the catheter balloon will usually then reside within a large cavity in the prostatic and membraneous urethra; in women, the catheter balloon is commonly expelled, resulting in continual upsizing of the catheter diameter and balloon volume to maintain continence. In these scenarios, there is often little or no sphincteric function remaining. Another potential problem, which can occur in men performing self-catheterization, is membranous urethral stricture that involves the external sphincter. Repair of the urethral stricture with an urethroplasty can damage the external urethral sphincter and result in urinary incontinence if the bladder neck is not intact.
In general the surgical treatment of neurogenic bladder is extremely variable. For instance, virtually every bowel segment has been described for the use of augmentation cystoplasty, with a variety of different techniques. The lack of specific treatment recommendations for neurogenic bladder is even more confusing if treating concomitant ISD. Guidelines in the surgical treatment of patients with neurogenic bladder dysfunction and ISD do not exist. The purpose of this review is to discuss various options for surgical treatment of ISD.
Problems with the literature on treatment of intrinsic sphincter deficiency in neurogenic patients
One major limitation in the literature regarding the management of patients with neurogenic bladder dysfunction and concomitant ISD is the subjective judgment among surgeons regarding who needs treatment of their outlet. A common scenario is a patient with severely overactive but poorly compliant bladder. Urodynamic testing in these patients can be challenging as often the bladder lacks adequate capacity due to the poor compliance thereby creating the impression of stress incontinence—these patients routinely leak throughout the study. If video-urodynamics is available the appearance of the bladder neck and external urethral sphincter may be useful to demonstrate sphincteric competence, however is not always definitive in diagnosing ISD.
Surgeons also have a natural tendency to perform bladder outlet procedures during augmentation cystoplasty if there is a concern for ISD. This strategy helps to avoid the challenge of performing bladder outlet procedures after an augmentation cystoplasty—it is significantly easier to perform both procedures at the same time rather than staged. If surgeons are aggressive in the treatment of ISD at the time of augmentation cystoplasty than bladder outlet procedures will appear to be very successful, as in many cases they were not really needed. This results in a selection bias in the literature where bladder outlet procedures are reported to have a very high success rate.
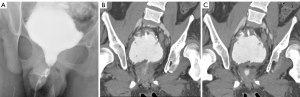
An illustrative example is a case of a 19-year-old male with myelomeningocele. He had very poor bladder dynamics and an equivocal urodynamic study for stress incontinence. He was found to have an open bladder neck on a cystogram (Figure 1) and severe trabeculation. He underwent augmentation cystoplasty with cutaneous catheterizable ileal cecocystoplasty (1) along with Young-Dees bladder neck reconstruction (BNR). In the course of his post-operative care he had a CT cystogram. This showed a persistently open bladder neck despite reconstruction however contrast stopped at the level of the external sphincter. Clinically, he did not have any urinary incontinence per urethra, but in actuality his native tonically active external urethral sphincter likely prevents leakage rather than the bladder neck repair, which has the appearance of a fixed, small fistula (Figure 1). If we had definitive information pre-operatively that showed he would not have ISD-related incontinence after the procedure, his surgery would have been simplified to a small bowel augmentation cystoplasty alone without a catheterizable channel, or BNR.
The problem illustrated in this case is not novel and several papers have tried to identify reliable markers for patients that need bladder outlet procedures for ISD at the time of augmentation cystoplasty versus those that do not. Authors in one study looked at extensive urodynamic parameters, including bladder capacity, detrusor leak point pressures, filling pressures, and video-urodynamic appearance of the bladder neck (2). In this study, the authors found that only an open bladder neck through the external sphincter predicted failure of augmentation cystoplasty alone. Using this criterion, the patient illustrated in Figure 1 would warrant a bladder outlet procedure at the time of augmentation cystoplasty, just as we performed. In a second study, the only factor that significantly predicted patients that did not need bladder outlet procedures was the presence of severe trabeculation on a pre-operative cystogram (2). Using this criterion, our patient would not have warranted a bladder outlet procedure due to the severe trabeculation on the pre-op cystogram. A notable finding in these two studies is that in patients that did not undergo bladder outlet procedures, continence was achieved in 79% and 91% respectively with augmentation cystoplasty alone (2,3). Taken together, we would recommend a conservative approach to treatment of the bladder outlet at the time of augmentation cystoplasty, unless ISD is obviously present.
Treatments options for intrinsic sphincteric deficiency (ISD) in neurogenic bladder patients
- Artificial urinary sphincter (AUS);
- Urethral and bladder neck slings;
- BNR;
- Injection of bulking agents.
Artificial urinary sphincter (AUS) use in neurogenic bladder
The AUS was first described in 1972. The AUS has undergone some modifications over the years, but its current form, the AMS 800 (American Medical Systems, Minnesota, USA), essentially functions the same as the original design, by cycling fluid between a reservoir and a cuff surrounding the urethra. The majority of patients that have an AUS placed have moderate to severe post-prostatectomy incontinence. In this patient population outcomes have been well described and patients have high satisfaction and usually excellent continence with the device (4,5).
While post-prostatectomy incontinence is the main reason for AUS placement, other indications include neurologic disease resulting in stress incontinence due to ISD. Most AUS data in patients with neurogenic bladder dysfunction has been in children with myelomeningocele. One potential advantage of AUS placement in this population, in contrast to a sling or reconstruction of the bladder neck which both create a fixed outlet resistance, is that patients may be able to void spontaneously. In one study, 49% of patients that had an AUS voided adequately and did not need to perform intermittent catheterization (6). The disadvantage of the AUS in this clinical scenario is a high revision, replacement, and removal rate in up to 60% of cases. Despite the high surgical revision rate, at a median follow-up of 5 years, 73% of patients remained continent after AUS revision or using the original AUS (6). Another very important aspect of this study’s findings is that 34% of children without augmentation cystoplasty prior to AUS placement required augmentation due to deterioration of bladder dynamics after AUS placement.
As mentioned above, the downside of using an AUS for treatment of patients with neurologic disease is the inherent high rate of revision surgery. In post-prostatectomy patients, approximately 50% of patients require revision at 5 years due to problems like infection, urethral atrophy or erosion, and mechanical failure (4,7). Unfortunately, for reasons that are not clear, this revision rate is even higher in patients with neurogenic bladder. In one comparison of patients with neurogenic versus non-neurogenic ISD, 85% in the neurogenic group underwent revision at 6 years follow-up compared to 59% in the non-neurogenic group (8). Another problem is the relative age of patients with neurogenic bladder; neurogenic bladder patients are often much younger compared to men after prostatectomy and implanting a device that has a published average lifespan of about 10 years into a 75-year-old man is much different than placing the same device in a 20-year-old. Because each revision requires moving the AUS cuff to a different location along the urethra, there is no way that the device can undergo enough revisions to last a lifetime in a younger patient.
Bladder neck placement of artificial urinary sphincter (AUS)
There is limited data on the use of AUS in adult patients with neurogenic bladder, but within that small body of literature, the majority of studies look at placement at the bladder neck rather than bulbar urethra. There are several reasons for placement of a bladder neck AUS. One reason is that a bladder neck AUS is much larger than those placed in the perineum and will omit a large bore rigid cystoscope with less chance of damage to the AUS. This maneuver is often needed in cases of bladder or ureteral stones, which are common complications in patients with neurogenic bladder. Another indication for a bladder neck AUS is the frequent need for intermittent catheterization in patients with neurogenic bladder, which theoretically may cause less damage if the AUS is located at the bladder neck. In addition, there is minimal pressure put upon a bladder neck AUS from extended sitting in a wheelchair in comparison to an AUS located at the bulbar urethra. For this reason, there is less potential erosion of the device due to decubitus ulcers. The largest study of adult patients undergoing AUS at the bladder neck in the setting of neurogenic bladder is a retrospective review of 51 men with approximately 20 years follow-up. Perfect continence, defined as at least a 4-hour period between catheterizations without urinary incontinence, was reported in 60% of patients. The tradeoff was again a high revision rate with 48% of patients requiring revision, removal and replacement of the AUS. Replacement of the device averaged 6 years in this group, again illustrating the average limited life span of the AUS regardless of placement location (9).
Modifications for artificial urinary sphincter (AUS) placement in patients with neurogenic bladder
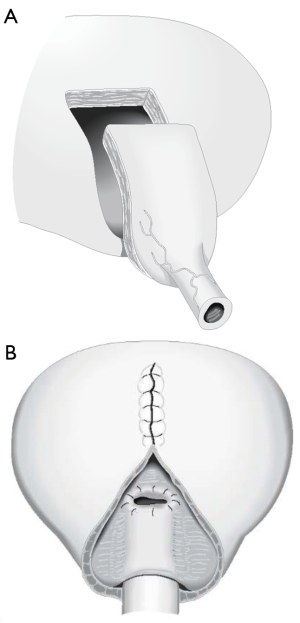
There are two publications that offer intriguing modifications of AUS placement that may decrease erosion rates when they are used in neurogenic patients. The first study looked at patients with neurogenic bladder from mostly spinal cord injury and placement of the AUS at the bladder neck (10). Rather than connecting the cuff of the AUS to a pump that is placed in the scrotum, a tissue expander port was instead connected to the AUS cuff and inserted under the abdominal wall (Figure 2). This tissue expander acted to connect the reservoir to the cuff with a static pressure. Patients then had their reservoir and cuff filled via the tissue expander port until a minimum pressure prevented leakage from the urethra as detected with fluoroscopy. The theoretical advantage of this system is that much less pressure is needed to maintain continence, especially in neurogenic patients that are often augmented, rather than standard pressure in normal AUS reservoir of 61–70 or 71–80 cmH2O. In theory this lower pressure may lead to less atrophy, erosion, and also the lack of the pump mechanism may minimize mechanical failure. In support of these theoretical advantages, the authors found the total revision rate was 35%, and 10 (20%) total cuffs had to be revised, removed, or replaced after a mean follow-up of 96 months. A 20% rate of cuff related revision at a mean follow-up of 96 months is a better outcome than those reported in the previous study of close to 48–85% revision rate—with most of the patients requiring removal, revision, or replacement of the cuff (8,9).
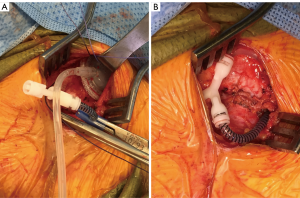
In another study, surgeons from the Mayo clinic placed the AUS cuff at the bladder neck and did not place the reservoir and pump at all. They relied upon the coaptation of the AUS around the bladder neck alone to place enough pressure to obstruct the outlet from leak. This was done at the time of simultaneous augmentation cystoplasty. They placed the modified AUS in 13 patients. Unfortunately only four patients ended up with continence that lasted greater than 2 years; however, these patients had virtually no need for AUS revisions. The authors also reported on the nine patients that had incontinence who were subsequently connected to a reservoir and pump, in addition to five patients that had initially had a complete AUS device placed at the time of augmentation cystoplasty. These 14 patients had a 36% rate of revision due to common issues (mechanical failure, erosion, and pump problems) (10). Although the 31% continence rate in patients with the AUS cuff placement alone is not a strong argument for this approach, the study does illustrate that patients may do well and have longer term success with lower revision rates if the lowest possible AUS pressure system is used while balancing clinical continence.
The role of AUS placement in adults with neurogenic bladder is very poorly defined. The procedure is certainly possible and AUS is thought to be more durable when it is placed at the bladder neck compared to bulbar urethral placement. Patients who may really benefit from this approach are those that have good bladder capacity, do not need augmentation cystoplasty, and void spontaneously without the assistance of a catheter before surgery. In these patients spontaneous voiding may be preserved with AUS placement, although there may be a need for augmentation cystoplasty in the future if there is a change in bladder function.
Artificial urinary sphincter (AUS) placement: summary
- Placement of AUS at the bladder neck is often a preferred approach in neurogenic bladder patients;
- Some patients can preserve spontaneous voiding;
- The AUS has a very high revision rate even when it is placed at the bladder neck;
- Patients undergoing AUS placement have a high rate of needing subsequent augmentation;
- Cystoplasty;
- Bladder neck AUS placement can be done safely at the time of augmentation cystoplasty.
Urethral and bladder neck slings
Sling operations act to increase bladder outlet resistance by compressing the urethra and in some cases, elevate the urethra upward to create resistance. The advantage of slings is the potential long-term durability of the procedure whereas AUS placement is not a permanent solution and associated with a very high revision rate, especially in neurogenic bladder patients (8). A disadvantage of urethral slings compared to AUS placement is that patients cannot be expected to spontaneously void and clean intermittent catheterization (CIC) is almost universally needed.
Although the technique was initially described in 1907 by Giordano, the pubovaginal autologous fascial sling (PVS) repair for the surgical treatment of urinary incontinence was modified and popularized by Drs. Mcguire and Lytton in the late 1970’s (11). PVS is a very effective procedure for the treatment of stress urinary incontinence and ISD in neurologically intact women. The outcomes in these women are excellent (67–93% cure rates) (12-15) and placement is associated with very high patient satisfaction (16,17).
Fascial slings in children with neurogenic bladder
Autologous fascial slings have been utilized for the treatment of pediatric cases of myelomeningocele with neurogenic bladder and ISD. Multiple studies report its effectiveness in children with intractable urinary incontinence (18-22). In one such study, 36 children (14 males and 23 females) underwent myofascial sling repair. At 4 years follow-up, 34 (92%) remained dry between catheterizations (23). In a more contemporary study Snodgrass et al., compared two cohorts of children with ISD who underwent fascial sling repair with or without augmentation cystoplasty. The authors used very strict definitions of incontinence and reported no difference between the two groups (83% overall continence) (24). The point of this comparison, according to the authors, was to illustrate that deterioration in bladder compliance may not be as high risk as it is in children undergoing AUS placement, where up to a third of patients needed subsequent augmentation cystoplasty (6). The relative stability of the bladder, in patients undergoing sling placement, is perhaps due to continued utilization of CIC compared to children undergoing AUS placement where many of the children spontaneously void. Other studies have corroborated that enterocystoplasty is not necessarily needed in these children if they have reasonable bladder dynamics prior to sling placement (25). Higher success rates with PVS in children have also been reported when the sling is concomitantly placed at the time of a BNR like the Leadbetter Mitchell procedure (26).
Fascial slings in adults with neurogenic bladder
Although the evidence for PVS repair is limited in the adult neurogenic population compared to children, there are several studies reporting positive outcomes. Women with neurogenic bladders and stress urinary incontinence have generally been found to have comparable outcomes when compared to non-neurogenic women. In one study, 33 female patients with myelomeningocele or spinal cord injury and ISD (mean age of 37 years) had a 91% satisfaction rate after placement of PVS (27). Twenty-five patients (76%) were totally dry, while five patients (15%) were markedly improved. A prospective evaluation of 21 women (mean age, 27) confirmed these findings with 95.2% of subjects completely dry with utilization of CIC (25). There are also successful reports of the use of bladder neck slings in men with neurogenic bladder. These slings are placed from an abdominal approach where the sling is passed around the bladder neck in a plane between the seminal vesicles and the bladder neck. In one study involving bladder neck placement of slings, in 13 men with neurogenic bladder, 9 (69.2%) were completely dry on CIC, 2 (15.4%) required injection of a bulking agent for improved continence, and 2 failed completely, requiring subsequent procedures (28). The two failures had synthetic Marlex slings and both experienced urethral erosion necessitating eventual transurethral excision. There were no such complications in the autologous fascial sling patients. In another study, utilizing rectus fascial slings in 12 adult men (mean age of 37 years), authors reported an overall success rate of 83% with eight patients completely dry between catheterizations and two significantly improved with only minimal leakage (29).
Synthetic slings in patients with neurogenic bladder
The majority of studies reporting outcomes of adult men undergoing placement of synthetic slings are in men with mild to moderate post-prostatectomy incontinence. In this population perineal sub-urethral sling outcomes are well defined (30,31). In patients with neurogenic bladder synthetic slings have also been used mostly for patients in the setting of either myelomeningocele or spinal cord injury-induced ISD, although the literature is very limited in this population of patients. As an alternative to the AUS, the male sling offers the possibility of continence, a lower risk device and urethral injury with self-catheterization and a lower-likelihood of revision due to device failure (21). In addition, synthetic slings are placed via a perineal approach without harvesting autologous fascia, further simplifying the surgery. One male sling design, the bone anchored perineal synthetic sling (InVance, American Medical Systems, Minnesota, USA), was studied in six adolescent boys with ISD from myelomeningocele. At a median of 33 months of follow-up, five of the patients with sling still in place were fully continent on CIC (32). In a larger study of 20 adults with either spinal cord injury or myelomeningocele, placement of a transobturator perineal synthetic sling (AdVance, American Medical Systems, Minnesota, USA), cured 8 (40%), or improved 5 (25%) patients at 12 months of follow-up (33).
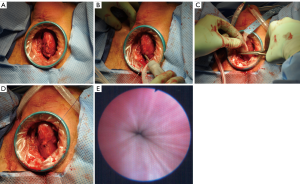
At the University of Utah, our single institution experience with transobturator perineal synthetics slings is very similar. We identified eight patients with neurogenic bladder and ISD who underwent perineal synthetic urethral sling placement (AdVance, American Medical Systems, Minnesota, USA, or modified Virtue, Coloplast, Minnesota, USA) (Figure 3). Two patients (25%) had sling failure that was treated successfully with either an AUS or sling revision surgery, and in 5/7 (71%) patients that have follow-up, the continence outcome was satisfactory. One concern regarding synthetic slings in this setting is erosion due to the need for intermittent catheterization; however, in our patients and the other two reported studies, there were no problems during follow-up with urethral erosion (32,33). The placement of urethral slings for neurogenic bladder patients with ISD is a feasible and safe option with not perfect, but an acceptable level of continence, especially if continence proves to be durable over longer follow-up.
Sling placement: summary
- Autologous fascial slings are a mainstay in treatment of ISD in neurogenic bladder and are successful in multiple clinical scenarios;
- CIC is still required after urethral sling placement for ISD;
- Augmentation cystoplasty is not required, as long as there are reasonable bladder dynamics at the time of sling placement, although careful follow-up is warranted to catch adverse changes in bladder compliance earlier than later;
- Perineal placement of male urethral synthetic mesh slings may be a viable alternative to fascial slings for selected situations.
Bladder neck reconstruction (BNR)
Alternative surgical options to AUS implantation and fascial sling repair for ISD include direct BNR procedures. There are various BNR techniques that have been used to increase bladder outlet resistance. The most commonly reported are the Young-Dees-Leadbetter (YDL), the modified Leadbetter-Mitchell (LM) repair, the Kropp repair, and the Pippi Salle (34). All have shown a reasonable success rate in the hands of specialty trained surgeons (35).
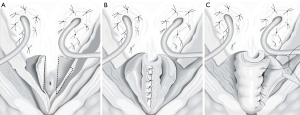
The YDL was initially reported by Young in 1922, the procedure was subsequently modified by Dees and Leadbetter resulting in improved continence rates (Figure 4) (36,37). The YDL procedure entails a vertical incision in the bladder neck, which is then reduced and elongated up to the trigone or beyond by resecting wedges of bladder mucosa from either side of the neo-urethra as it approaches the trigone. The mucosa of the neourethra can be dissected away from the detrusor muscle, which can be wrapped around the urethra reinforcing the continence mechanism. Most studies of the YDL technique report outcomes in patients with epispadias-exstrophy, with continence rates reportedly 70% to 86% (38,39). While this technique appears to achieve good continence rates for this population, it may not be transferable to patients with other causes of ISD. For instance, in one study of 38 subjects, all with neurogenic bladder dysfunction, authors showed that 30 (79%) patients were dry, 7 (18%) were partially dry, and 1 remained incontinent after YDL with or without bladder augmentation. However, to achieve these outcomes, only 26 cases (68%) were patients actually continent after their first operation and 8 required multiple procedures to achieve continence (40). Dr. Leadbetter has emphasized that tubularization must be carried up to the trigone with concomitant bilateral ureteral reimplantation to achieve continence with this procedure (41). A modification of the traditional YDL, the LM technique, was proposed in 1993. A V-flap of bladder neck is incised anteriorly leaving a 15-mm wide strip of bladder neck and urethra posteriorly. As the anterior bladder is closed vertically, the urethra, bladder neck, and trigone are tubularized (42). This method allows for preservation of bladder volume since the V-shaped incision comes to rest at the top of the tubularization and is incorporated into the bladder repair instead of wedges of the bladder being excised (43). Other modifications to the YDL technique have been described. Churchill et al. reported YDL reconstruction of the bladder neck combined with fascial wrap in a lengthening, narrowing, and tightening procedure (LeNT) (44). In this operation, the YDL procedure was performed and then a cadaveric fascia strip was wrapped around the bladder neck like a cuff to reinforce the repair. This was performed in a series of 19 patients and complete continence was found in 78.9% of patients at long-term follow-up. There was no difficulty with CIC in any of the patients postoperatively although five patients underwent concomitant creation of a catheterizable channel and three ultimately had bladder neck closure with creation of catheterizable channel for continued urethral incontinence.
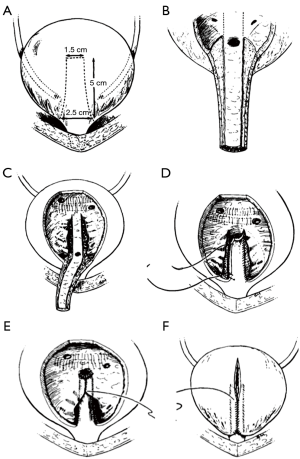
In 1986, Kropp et al. reported an alternative method of reconstruction: tubularization of an anterior bladder wall flap and reimplantation into the posterior bladder wall (45). In this technique, a submucosal tunnel is created in the posterior bladder, where the urethra and anterior bladder tube has been disconnected from the bladder neck. Then the anterior bladder tube is pulled through this tunnel creating a flap valve (Figure 5). The rational for this procedure makes good sense in comparison to the YDL since an actual flap valve is created, very similar to successful approaches in creation of catheterizable channels via the Ghoneim and Skinner-T approach to continent catheterizable pouches (46,47), or ureteral reimplantation for treatment of vesico-ureteral reflux. In some reports, the procedure has been associated with reduced bladder capacity and difficulties with catheterization (in other than Dr. Kropp’s hands) and for this reason it might be best suited to patients also undergoing augmentation cystoplasty and creation of a catheterizable channel (42).
In 1994, Pippi Salle et al. described a BNR with similar principals to the Kropp procedure. Pippi Salle modified creation of the “Kropp” anterior bladder tube, by creating the lengthened urethra from an anterior bladder flap anastomosed to a similar length posterior bladder flap. The posterior bladder flap was created by parallel mucosal incisions from the bladder neck through the trigone. The composite tube would obviously have a serosal layer from the anterior bladder lying within the bladder lumen. In order to create a flap valve and avoid exposed serosa, the posterior-lateral edges of bladder mucosa created by the parallel incisions from the bladder neck to the trigone were brought over the composite lengthened urethra and anastomosed (Figure 6). Ultimately this creates a lengthened urethra that is below a mucosal layer and a similar final configuration to the Kropp procedure. The Pippi Salle, although difficult to conceptualize at first, may be a little more feasible than the technical problems encountered with the Kropp procedure, which include complete disconnection of the urethra, creating a lengthened urethra by sewing it posteriorly from the bladder neck, and the angulation problems in creation of a long posterior based submucosal tunnel (48). Pippi Salle reported continence rates of 70% in 17 patients, with only three experiencing difficulties with catheterization (49). A multi-institutional study in the UK concluded that this technique could be used as first-line treatment for neurogenic bladder related ISD in females, but was less successful for male patients (50). In this study, 13 of 16 female patients reported complete daytime continence, and only 1 of the 13 leaked at night. However, only 5 of 12 males were dry. In another recent study, authors publishing the long-term outcomes of the Pippi Salle technique and showed that of nine patients with neurogenic ISD, 78% achieved complete continence (at median 75 months of follow-up) (51). However, there were a number of significant complications in these patients, all of which were fixed with additional endoscopic interventions.
Bladder neck reconstruction (BNR): summary
- Several techniques have been described for BNR that all report reasonable outcomes;
- YDL repair is the simplest repair that involves lengthening and tightening of the bladder neck. Several modifications of this repair have been described to improve continence outcomes (LM, and LeNT);
- Alternative techniques for bladder neck repair differ from the YDL repair, in that they create a flap valve mechanism for continence as well as lengthening the urethra. These include the Kropp and Pippi Salle procedures.
Bulking agents
Endoscopic treatment of stress urinary incontinence in patients with neurogenic bladder dysfunction and ISD involves the injection of implantable bulking materials at the bladder neck or posterior urethra in order to increase bladder outlet resistance. These bulking agents have been used as either primary or adjuvant treatments after other procedures have failed to resolve incontinence from ISD (52). Injectable prosthetic materials are not a new concept and were first proposed in 1974 for adult female stress incontinence using polytetrafluoroethylene (Teflon/Polytef) paste (53).
Since the first procedures were performed, a variety of different injectable bulking agents have been used. After the initial success of Polytef, glutaraldehyde cross-linked bovine collagen (GAX), dextanomer/hyaluronic acid (Deflux), and polydimethylsiloxane (Macroplastique) were also developed as alternative bulking agents. There are numerous reports of these injections for neurogenic bladder related ISD within the pediatric population (54-60). Results have been mixed, often showing short-term improvement, but long-term recurrence of incontinence (56,57). In a prospective study of 45 patients aged 5 to 20 years old with more than 2 years of follow-up, treatment was effective in approximately half of the participants. Of the 45 patients, 19 (39.6%) were completely dry, 6 (12.5%) experienced improvement, and 23 (47.9%) had no significant improvement (54). On the other hand, another study, which injected either dextranomer/hyaluronic acid (Deflux) or polytetrafluoroethylene (Polytef), reported only 2 out 20 patients were dry for longer than 6 months (61). Neither the number of injections, nor the bulking material used seemed to alter the results. Another report in children showed that injection of polydimethylsiloxane (Macroplastique) at the bladder neck did not result incontinence resolution at all and patients only had a 42% “improvement” rate (62).
In contrast to children, studies of injectable agents in adults with neurogenic bladder related ISD are even more limited. In one study, Polytef was injected in six females with neurogenic bladder related ISD (62). At limited follow-up, all women achieved complete urinary control with use of CIC. Promising results were also reported in one study of 11 patients treated with GAX; seven patients were cured or significantly improved, four were only slightly improved or no better after injection (63).
Although primary injection of bulking agents has proven to be of limited value in treating neurogenic sphincter deficiency, surgeons have postulated that it could be useful as a supplemental procedure. However, evaluation of bladder neck injection for persistent low pressure incontinence after fascial sling repair yielded disappointing results. In this study, after long-term follow-up of 8 years, only 2 of the 27 patients were continent, despite repeat injections in many participants (1).
Limitations of these studies are small sample size, differing definitions of continence or cure, and variable reporting of concomitant or prior procedures such as augmentation cystoplasty. The overall utility of injection of bulking agents in patients with neurogenic bladder related ISD seems to be very low, although it is certainly commonly done, especially as a “Hail Mary” after other failed bladder outlet procedures.
Bulking agents: summary
- Bulking agents have a very poor success as either a primary or secondary treatment of neurogenic ISD;
- Even though bulking agents are not very successful, they may still be helpful due to their low complication rate.
Special considerations for operations for intrinsic sphincteric deficiency (ISD)
Another area that is not well defined in the surgical literature on bladder outlet surgery for ISD in patients with neurogenic bladder is ability to catheterize after the procedure. Many reports on bladder outlet procedures in ISD tout that the procedures do not cause difficulty with catheterization, or the incidence of catheterization problems is very low (44,45,49). However, if a bladder outlet procedure addresses leakage very effectively but the outlet becomes too tight or tortuous to catheterize, then the result is a very dissatisfied patient (and surgeon) who often resort to a suprapubic tube after what can be very invasive surgery to reconstruct the bladder outlet. In order to avoid this situation, many surgeons do not perform bladder neck procedures without creating a catheterizable channel (24,64).
Over time, creation of a catheterizable channel during bladder outlet surgery has progressively become our practice at University of Utah. Exceptions to this are when simple perineal surgeries can be done like the male synthetic perineal sling. In this circumstance, abdominal reconstruction can mostly be avoided or approached in a staged fashion if needed. Another example where creation of a catheterizable channel would not be warranted is the use of an AUS when the expectation from patients is spontaneous voiding rather than reliance on CIC. In other repairs, however, like BNR, we feel much more comfortable if patients agree to have a catheterizable channel created preferably with augmentation cystoplasty if there is any evidence of marginal bladder compliance or detrusor overactivity incontinence.
Conclusions
The literature describing bladder outlet procedures for patients with ISD from neurogenic bladder is limited and mainly based upon experience in children with myelomeningocele. In this review, we have described several commonly used strategies for treatment of ISD, but there are other treatment options that may also prove to be very effective. We hope this review provides an understanding of the evidence to treat ISD in adults with neurogenic bladder and gives surgeons the tools necessary to tailor treatment options for this challenging surgical problem.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Redshaw JD, Elliott SP, Rosenstein DI, et al. Procedures needed to maintain functionality of adult continent catheterizable channels: a comparison of continent cutaneous ileal cecocystoplasty with tunneled catheterizable channels. J Urol 2014;192:821-6. [PubMed]
- Khoury AE, Dave S, Peralta-Del Valle MH, et al. Severe bladder trabeculation obviates the need for bladder outlet procedures during augmentation cystoplasty in incontinent patients with neurogenic bladder. BJU Int 2008;101:223-6. [PubMed]
- Medel R, Ruarte AC, Herrera M, et al. Urinary continence outcome after augmentation ileocystoplasty as a single surgical procedure in patients with myelodysplasia. J Urol 2002;168:1849-52. [PubMed]
- Kim SP, Sarmast Z, Daignault S, et al. Long-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective review from the University of Michigan. J Urol 2008;179:1912-6. [PubMed]
- Kahlon B, Baverstock RJ, Carlson KV. Quality of life and patient satisfaction after artificial urinary sphincter. Can Urol Assoc J 2011;5:268-72. [PubMed]
- Catti M, Lortat-Jacob S, Morineau M, et al. Artificial urinary sphincter in children--voiding or emptying? An evaluation of functional results in 44 patients. J Urol 2008;180:690-3; discussion 693. [PubMed]
- Wang R, McGuire EJ, He C, et al. Long-term outcomes after primary failures of artificial urinary sphincter implantation. Urology 2012;79:922-8. [PubMed]
- Murphy S, Rea D, O'Mahony J, et al. A comparison of the functional durability of the AMS 800 artificial urinary sphincter between cases with and without an underlying neurogenic aetiology. Ir J Med Sci 2003;172:136-8. [PubMed]
- Chartier Kastler E, Genevois S, Gamé X, et al. Treatment of neurogenic male urinary incontinence related to intrinsic sphincter insufficiency with an artificial urinary sphincter: a French retrospective multicentre study. BJU Int 2011;107:426-32. [PubMed]
- Viers BR, Elliott DS, Kramer SA. Simultaneous augmentation cystoplasty and cuff only artificial urinary sphincter in children and young adults with neurogenic urinary incontinence. J Urol 2014;191:1104-8. [PubMed]
- Mcguire EJ, Lytton B. Pubovaginal sling procedure for stress incontinence. J Urol 1978;119:82-4. [PubMed]
- Cross CA, Cespedes RD, McGuire EJ. Our experience with pubovaginal slings in patients with stress urinary incontinence. J Urol 1998;159:1195-8. [PubMed]
- Chaikin DC, Rosenthal J, Blaivas JG. Pubovaginal fascial sling for all types of stress urinary incontinence: long-term analysis. J Urol 1998;160:1312-6. [PubMed]
- Mitsui T, Tanaka H, Moriya K, et al. Clinical and urodynamic outcomes of pubovaginal sling procedure with autologous rectus fascia for stress urinary incontinence. Int J Urol 2007;14:1076-9. [PubMed]
- Dmochowski RR, Blaivas JM, Gormley EA, et al. Update of AUA guideline on the surgical management of female stress urinary incontinence. J Urol 2010;183:1906-14. [PubMed]
- Haab F, Trockman BA, Zimmern PE, et al. Results of pubovaginal sling for the treatment of intrinsic sphincteric deficiency determined by questionnaire analysis. J Urol 1997;158:1738-41. [PubMed]
- Hassouna ME, Ghoniem GM. Long-term outcome and quality of life after modified pubovaginal sling for intrinsic sphincteric deficiency. Urology 1999;53:287-91. [PubMed]
- McGuire EJ, Wang CC, Usitalo H, et al. Modified pubovaginal sling in girls with myelodysplasia. J Urol 1986;135:94-6. [PubMed]
- Gormley EA, Bloom DA, McGuire EJ, et al. Pubovaginal slings for the management of urinary incontinence in female adolescents. J Urol 1994;152:822-5; discussion 826-7. [PubMed]
- Bauer SB, Peters CA, Colodny AH, et al. The use of rectus fascia to manage urinary incontinence. J Urol 1989;142:516-9; discussion 520-1. [PubMed]
- Raz S, McGuire EJ, Ehrlich RM, et al. Fascial sling to correct male neurogenic sphincter incompetence: the McGuire/Raz approach. J Urol 1988;139:528-31. [PubMed]
- Elder JS. Periurethral and puboprostatic sling repair for incontinence in patients with myelodysplasia. J Urol 1990;144:434-7; discussion 443-4. [PubMed]
- Mingin GC, Youngren K, Stock JA, et al. The rectus myofascial wrap in the management of urethral sphincter incompetence. BJU Int 2002;90:550-3. [PubMed]
- Snodgrass W, Keefover-Hicks A, Prieto J, et al. Comparing outcomes of slings with versus without enterocystoplasty for neurogenic urinary incontinence. J Urol 2009;181:2709-14; discussion 2714-6. [PubMed]
- Fontaine E, Bendaya S, Desert JF, et al. Combined modified rectus fascial sling and augmentation ileocystoplasty for neurogenic incontinence in women. J Urol 1997;157:109-12. [PubMed]
- Snodgrass W, Barber T. Comparison of bladder outlet procedures without augmentation in children with neurogenic incontinence. J Urol 2010;184:1775-80. [PubMed]
- Athanasopoulos A, Gyftopoulos K, McGuire EJ. Treating stress urinary incontinence in female patients with neuropathic bladder: the value of the autologous fascia rectus sling. Int Urol Nephrol 2012;44:1363-7. [PubMed]
- Herschorn S, Radomski SB. Fascial slings and bladder neck tapering in the treatment of male neurogenic incontinence. J Urol 1992;147:1073-5. [PubMed]
- Daneshmand S, Ginsberg DA, Bennet JK, et al. Puboprostatic sling repair for treatment of urethral incompetence in adult neurogenic incontinence. J Urol 2003;169:199-202. [PubMed]
- Cornu JN, Sèbe P, Ciofu C, et al. The AdVance transobturator male sling for postprostatectomy incontinence: clinical results of a prospective evaluation after a minimum follow-up of 6 months. Eur Urol 2009;56:923-7. [PubMed]
- Gozzi C, Becker AJ, Bauer R, et al. Early results of transobturator sling suspension for male urinary incontinence following radical prostatectomy. Eur Urol 2008;54:960-1. [PubMed]
- Dean GE, Kunkle DA. Outpatient perineal sling in adolescent boys with neurogenic incontinence. J Urol 2009;182:1792-6. [PubMed]
- Groen LA, Spinoit AF, Hoebeke P, et al. The AdVance male sling as a minimally invasive treatment for intrinsic sphincter deficiency in patients with neurogenic bladder sphincter dysfunction: a pilot study. Neurourol Urodyn 2012;31:1284-7. [PubMed]
- Dave S, Salle JL. Current status of bladder neck reconstruction. Curr Opin Urol 2008;18:419-24. [PubMed]
- Cole EE, Adams MC, Brock JW 3rd, et al. Outcome of continence procedures in the pediatric patient: a single institutional experience. J Urol 2003;170:560-3; discussion 563. [PubMed]
- Leadbetter GW Jr. Surgical correction of total urinary incontinence. J Urol 1964;91:261-6. [PubMed]
- Dees JE. Congenital epispadias with incontinence. J Urol 1949;62:513-22. [PubMed]
- Perlmutter AD, Weinstein MD, Reitelman C. Vesical neck reconstruction in patients with epispadias-exstrophy complex. J Urol 1991;146:613-5. [PubMed]
- McMahon DR, Cain MP, Husmann DA, et al. Vesical neck reconstruction in patients with the exstrophy-epispadias complex. J Urol 1996;155:1411-3. [PubMed]
- Donnahoo KK, Rink RC, Cain MP, et al. The Young-Dees-Leadbetter bladder neck repair for neurogenic incontinence. J Urol 1999;161:1946-9. [PubMed]
- Hinman F, Baskin L. Hinman's Atlas Of Pediatric Urologic Surgery. 2nd Edition. Saunders Elsevier, 2008.
- Esposito C, Guys JM, Gough D, et al, editors. Pediatric Neurogenic Bladder Dysfunction. Diagnosis, Treatment, Long-Term Follow-up. Springer-Verlag Berlin Heidelberg, 2006.
- Jones JA, Mitchell ME, Rink RC. Improved results using a modification of the Young-Dees-Leadbetter bladder neck repair. Br J Urol 1993;71:555-61. [PubMed]
- Churchill BM, Bergman J, Kristo B, et al. Improved continence in patients with neurogenic sphincteric incompetence with combination tubularized posterior urethroplasty and fascial wrap: the lengthening, narrowing and tightening procedure. J Urol 2010;184:1763-7. [PubMed]
- Kropp KA, Angwafo FF. Urethral lengthening and reimplantation for neurogenic incontinence in children. J Urol 1986;135:533-6. [PubMed]
- Elshal AM, Abol-Enein H, Mosbah A, et al. Serous-lined unidirectional valve for construction of continent cutaneous urinary reservoir: the test of time. Urology 2012;80:452-8. [PubMed]
- Marino G, Laudi M. Ileal T-pouch as a urinary continent cutaneous diversion: clinical and urodynamic evaluation. BJU Int 2002;90:47-50. [PubMed]
- Mouriquand PD, Sheard R, Phillips N, et al. The Kropp-onlay procedure (Pippi Salle procedure): a simplification of the technique of urethral lengthening. Preliminary results in eight patients. Br J Urol 1995;75:656-62. [PubMed]
- Salle JL, McLorie GA, Bägli DJ, et al. Urethral lengthening with anterior bladder wall flap (Pippi Salle procedure): modifications and extended indications of the technique. J Urol 1997;158:585-90. [PubMed]
- Hayes MC, Bulusu A, Terry T, et al. The Pippi Salle urethral lengthening procedure; experience and outcome from three United Kingdom centres. BJU Int 1999;84:701-5. [PubMed]
- Nakamura S, Hyuga T, Kawai S, et al. Long-Term Outcome of the Pippi Salle Procedure for Intractable Urinary Incontinence in Patients with Severe Intrinsic Urethral Sphincter Deficiency. J Urol 2015;194:1402-6. [PubMed]
- De Vocht TF, Chrzan R, Dik P, et al. Long-term results of bulking agent injection for persistent incontinence in cases of neurogenic bladder dysfunction. J Urol 2010;183:719-23. [PubMed]
- Politano VA, Small MP, Harper JM, et al. Periurethral teflon injection for urinary incontinence. J Urol 1974;111:180-3. [PubMed]
- Alova I, Margaryan M, Bernuy M, et al. Long-term effects of endoscopic injection of dextranomer/hyaluronic acid based implants for treatment of urinary incontinence in children with neurogenic bladder. J Urol 2012;188:1905-9. [PubMed]
- Guys JM, Breaud J, Hery G, et al. Endoscopic injection with polydimethylsiloxane for the treatment of pediatric urinary incontinence in the neurogenic bladder: long-term results. J Urol 2006;175:1106-10. [PubMed]
- Godbole P, Bryant R, MacKinnon AE, et al. Endourethral injection of bulking agents for urinary incontinence in children. BJU Int 2003;91:536-9. [PubMed]
- Kassouf W, Capolicchio G, Berardinucci G, et al. Collagen injection for treatment of urinary incontinence in children. J Urol 2001;165:1666-8. [PubMed]
- Silveri M, Capitanucci ML, Mosiello G, et al. Endoscopic treatment for urinary incontinence in children with a congenital neuropathic bladder. Br J Urol 1998;82:694-7. [PubMed]
- Chernoff A, Horowitz M, Combs A, et al. Periurethral collagen injection for the treatment of urinary incontinence in children. J Urol 1997;157:2303-5. [PubMed]
- Leonard MP, Decter A, Mix LW, et al. Treatment of urinary incontinence in children by endoscopically directed bladder neck injection of collagen. J Urol 1996;156:637-40; discussion 640-1. [PubMed]
- Dyer L, Franco I, Firlit CF, et al. Endoscopic injection of bulking agents in children with incontinence: dextranomer/hyaluronic acid copolymer versus polytetrafluoroethylene. J Urol 2007;178:1628-31. [PubMed]
- Lewis RI, Lockhart JL, Politano VA. Periurethral polytetrafluoroethylene injections in incontinent female subjects with neurogenic bladder disease. J Urol 1984;131:459-62. [PubMed]
- Bennett JK, Green BG, Foote JE, et al. Collagen injections for intrinsic sphincter deficiency in the neuropathic urethra. Paraplegia 1995;33:697-700. [PubMed]
- Snodgrass WT, Elmore J, Adams R. Bladder neck sling and appendicovesicostomy without augmentation for neurogenic incontinence in children. J Urol 2007;177:1510-4; discussion 1515. [PubMed]


