Fertility treatment in spinal cord injury and other neurologic disease
Introduction
Patients with neurologic disorders who desire fertility face a number of challenges, including hormonal imbalances, ejaculatory dysfunction, and poor semen quality. The aim of this review is to describe the etiology of problems related to fertility in the population of neurologically impaired patients and to facilitate decision making in their management. Available treatment modalities for ejaculatory dysfunction, non-surgical and surgical sperm retrieval, and fertilization methods are discussed. We also describe the physiology and approach to treatment of erectile dysfunction (ED). As traumatic spinal cord injury (SCI) impacts predominantly younger men of whom more than half are less than 35 years old at the time of their injury (1), fertility in this population has been studied more extensively than in other neurologic conditions, which usually affect an older population. As such, the focus of this chapter is predominantly geared toward the management of infertility of male patients with SCI, although other neurologic conditions including multiple sclerosis (MS) and diabetes are covered as well. This review uses the term SCI to include individuals with spinal cord lesions from traumatic, non-traumatic, congenital, and degenerative lesions of the spinal cord. Several topics related to female fertility in SCI and other neurologic diseases are addressed, however the discussion is limited by a paucity of literature on this subject.
General sexual function
The overwhelming majority (87%) of patients with SCI engage in some form of sexual relationship post-injury. The likelihood of involvement in a sexual relationship positively correlates with number of years post-injury (2). Depending on the clinical setting, from 1–37% of SCI patients are interested in sexual function with the ultimate aim of attaining pregnancy (2,3). An initial infertility work-up of a couple in which a partner is affected by a neurological condition should include an assessment of sexual function (Table 1). The two standardized tools for sexual function assessment utilized in the SCI population are the International Index of Erectile Function (IIEF) and Female Sexual Function Index (FSFI). The IIEF consists of 15 questions that cover five domains of erectile function: erectile and orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. The FSFI assesses six domains including desire, subjective arousal, lubrication, orgasm, sexual satisfaction, and pain (4). While both the IIEF and the FSFI have yet to be validated in the SCI population, their potential applicability to the clinical realm is informed by their increased utilization in research.

Full table
Neural control of erectile function
There are two neurologic pathways which lead to an erection: (I) reflex via tactile stimulus perception by the parasympathetic fibers of the spinal cord segments S2-4, which results in vasodilation and opening of the arteriovenous shunts in the corpora cavernosa, and (II) psychogenic via sympathetic fibers at T11-L2 and the hypogastric plexus (5). In general, a complete upper motor neuron (UMN) injury above T11 can result in reflex erections in the absence of psychogenic erections, while men with a lower motor neuron (LMN) injury affecting the sacral pathway are likely to have psychogenic erections and no reflex erections (6). There is, however, a considerable degree of variability of function, depending on preservation of neurologic function in incomplete injuries. For example, men with incomplete UMN injuries and some preserved neurologic function in the T11-L2 region of the spinal cord may exhibit capacity for both reflex and psychogenic erections (5,7).
In the immediate aftermath of an injury, spinal shock lasts from a few hours to several weeks, and is characterized by complete suppression of all reflex activity below the level of the cord lesion (5). After the resolution of spinal shock, three types of erections after SCI are observed: (I) reflex erection resulting from stimulation below the level of the lesion, with engorgement of both the corpora cavernosa and the corpus spongiosum in patients with a complete UMN injury above T11 (lesions below T11 involve engorgement of the corpora cavernosa only, resulting in a partial erection); (II) psychogenic erection triggered by thoughts and non-tactile stimuli, with a partial erection attained through sympathetic outflow from the thoracolumbar regions in SCI patients with lesions below L2; and (III) mixed (reflex and psychogenic) erection, which may occur when the lesion is between L2 and S2 (5,8).
Treatment of erectile dysfunction (ED)
The use of phosphodiesterase-5 inhibitors (PDE-5I) is the first line of management for ED in men with SCI. Efficacy, as defined by ability to achieve and maintain an erection sufficient for penetration, as well as satisfaction were found to be equivalent with sildenafil, tadalafil, and vardenafil compared to placebo. Sildenafil is associated with improved function at lower doses than tadalafil or vardenafil, making it the preferred agent for patients who experience adverse side effects at higher doses (9). Lesions above the level of the sacral spinal have been found to be associated with better therapeutic response to PDE-5I, compared to lesions of the sacral spinal segments (9). Adverse events occur in about 13% of men taking an PDE-5I and most commonly include headache, flushing, hypotension, nasal congestion and dyspepsia (10).
In the case of failure of PDE-5I, intracavernosal injections of vasoactive medications such as papaverine and phentolamine may be used. It should be noted that patients with SCI are much more sensitive to intracavernous injections compared to patients with vasculopathic impotence, and thus should be started at an initial lower dosage in order to avoid priapism. Other adverse reactions include difficulties with insertion of needle, dysesthesias, autonomic dysreflexia (AD), seizures, and intracorporeal fibrosis (7,11).
Prosthetic devices have been used for management of ED; however the rate of complications is considerable. In men with SCI who underwent placement of a semirigid prosthesis, the erosion rates range from 16–33% (12-14). Inflatable prosthesis are associated with a lower (8%) rate of erosion, however this is still considerably higher than the 1% erosion rate in the general population (14).
Neural control of ejaculatory function
Ejaculation is the result of coordination of the sympathetic, parasympathetic, and somatic nervous systems. It is important to note that this process is independent of whether or not an erection is achieved. Afferent input is received via stimulation of the dorsal penile nerve, triggering the activation of efferent sympathetic fibers from thoracolumbar segments T11-L2. These fibers transmit a signal via the hypogastric nerve plexus that elicits a coordinated contraction of the epididymis, vas deferens, ejaculatory ducts, and seminal vesicles, as well as closure of the bladder neck. Together these events comprise emission, which is the first step of ejaculation. This process ends with deposition of ejaculatory fluids and sperm in the posterior urethra. Expulsion is the second step, involving efferent signaling via the parasympathetic center (S2-S4), and includes: (I) parasympathetic outflow via the pelvic nerve stimulating secretions of the prostate and seminal vesicles; and (II) somatic outflow via the perineal branch of the pudendal nerve resulting in contraction of the bulbocavernosal and ischiocavernosal muscles as well as the relaxation of the external urethral sphincter. This sequence of events results in forceful pulsatile antegrade ejaculation (5,15,16). During the expulsive phase, a closed bladder neck prevents the reflux of semen into the bladder as the urethral pressure increases. Failure of closure of the bladder neck results in reflux of semen into the bladder, termed retrograde ejaculation. Clinically, this manifests in a low-volume ejaculate, with sperm identified in the post-ejaculatory urine (17).
Anejaculation, defined as the absence of either antegrade or retrograde ejaculation, is common in patients with SCI. Overall, only 9–15% of men with SCI are able to ejaculate in response to normal stimuli (18-20), however there is some variability depending on the level of injury. When subdivided according to level of lesion, 4–11% of men with complete UMN lesions and 32% of men with incomplete UMN lesions retain the ability to ejaculate. In men with complete LMN spinal cord injuries, 18% are reported to ejaculate, while incomplete LMN injuries are associated with a 70% likelihood of ejaculation. A greater likelihood of ejaculation is associated with the capacity for psychogenic erection (7,15,21).
Management of retrograde ejaculation
Retrograde ejaculation can occur as a result of SCI, neuropathy due to diabetes or MS, or surgical interventions such as a retroperitoneal lymph node dissection (RPLND). Pharmacologic therapy with an alpha agonist can be used to achieve antegrade ejaculation in patients with peripheral lesions and normal sensation, such as in early stages of diabetic or MS neuropathy and in iatrogenic peripheral nerve lesions such as that observed in nerve sparing RPLND (22). In these populations, sympathomimetics act by augmenting the insufficient degree of neural stimulation via endogenous pathways. Medications which have been successfully used include imipramine, ephedrine, pseudoephedrine, midodrine, and phenylpropanolamine (17,23). We typically employ pseudoephedrine 60 mg 4 hours prior to collection and again 1 hour prior to collection. The dose can be titrated as necessary, although tachycardia and associated discomfort are usually the limiting factors.
Retrograde ejaculation refractory to pharmacologic treatment can be managed with sperm retrieval from the bladder. The central aim of this technique is minimization of contact of sperm with urine and the acidic environment of the bladder, both of which can severely damage sperm and decrease their ability to fertilize an oocyte. The original technique proposed by Hotchkiss in 1955 consists of emptying the bladder, washing the bladder with Ringer’s Lactate via catheterization, and then instilling a small amount of the same solution into the bladder before removing the catheter. Following ejaculation, the bladder contents were obtained either via voiding or catheterization (24). This required same-day intrauterine insemination (IUI) or an assisted reproductive technology (ART) lab in close proximity. The modified Hotchkiss technique, which replaced the instilled solution with 40 mL of sterile culture buffered medium for gametes, permits the recovered spermatozoa to be cryopreserved (25). Additional techniques recommended for preservation of sperm integrity are the use of plastic, rather than silicone catheters, and a non-spermicidal lubricant (26). The collected specimen can then be used either for vaginal insemination or IUI if the sperm concentration is adequate, or in-vitro fertilization with or without intracytoplasmic sperm injection (ICSI). In a series of patients treated with the modified Hotchkiss technique and ART, 60% of couples achieved at least one pregnancy (27).
Neurostimulation for anejaculation
For a man with anejaculation who desires fertility, the initial means of sperm retrieval consist of assisted ejaculation. The rates of successfully attaining ejaculation are 97–100%, when utilizing a step-wise approach of vibratory stimulation, followed by electroejaculation (EEJ) (18,28). Subcutaneous physostigmine was previously the first line treatment of anejaculation, with attainment of antegrade ejaculate in more than 2/3 of SCI subjects (29). However, due to side effects, including AD, nausea, vomiting, hallucination and dizziness, this technique is no longer employed.
Penile vibratory stimulation (PVS)
PVS is the current recommended first choice in treatment of anejaculation in spinal cord injured men, as it is non-invasive, simple to use, does not require anesthesia, and can be employed in clinic as well as at home by the patient or his partner. The technique requires neurologically intact dorsal penile nerve as well as an intact T11-S4 spinal cord to attain a coordinated propulsive ejaculation (30) (Figure 1). Afferent penile dorsal nerve (S2-4) stimulation is provided by application of a medical-grade vibrator against the ventral or dorsal surface of the glans penis for periods of 2½–3 minutes or until ejaculation occurs. The approved device which is commercially available for this technique is the FertiCare vibrator (Multicept Frederiksberg, Denmark) (16). The optimal vibrator parameters associated with the highest rates of antegrade or retrograde ejaculation are an amplitude of excursion of 2.5 mm and a frequency of 100 Hz (31,32). If no ejaculation occurs, a rest period of 1–2 minutes is recommended before stimulation begins again. In majority (89%) of men in whom PVS is effective, ejaculation occurs in the first 2 minutes of stimulation (33).
Overall rates of successful ejaculation using vibroejaculation range from 67% to 88% (3,31,34). Among men who have a favorable response to PVS, approximately 2/3 achieve antegrade and 1/3 achieve retrograde ejaculation (31). Lesions above T10 are associated with high rates of success with PVS (81–86%) (18,32). The higher the level of injury, the more likely the patient is to respond (33). Another predictor of success is presence of the hip flexion reflex (L2-S1) and the bulbocavernosus reflex (32,35). Conversely, lesions at and below T10 and the absence of the hip flexion and bulbocavernous reflexes are associated with poor or absent ejaculatory response to PVS in most men with SCI (19,36,37). PVS has also been noted to fail in most men with injuries of less than 6–18 months duration (15,36). As cortical inhibition in the clinic setting may inhibit successful ejaculation with PVS, a better success rate may be achieved when the technique is employed in the privacy of a home environment (16). Another approach that has been associated with an increased treatment response is the simultaneous application of two vibrators to the penis, one on the ventrum and the other on the dorsum (38).
If semen parameters are adequate, PVS can be used in conjunction with vaginal self-insemination at home, scheduled at the time of ovulation. Intravaginal insemination (IVI) conducted after PVS has been observed to result in a pregnancy rate of 22% per cycle (3) and an overall pregnancy rate of 22–62% per couple (30,34).
The initial PVS session must be performed in a monitored setting before utilizing the technique at home because of the risk of inducing AD in patients with injury at T6 and above. The symptoms of AD include hypertension, bradycardia, sweating, and headache and are typically triggered by an irritating stimulus below the level of injury. In extreme instances, the hypertension can be sufficiently severe to result in stroke and event in death. Pharmacologic options for management of AD include nifedpine, nitroglycerin, or prazosin (33). Nifedipine 20 mg sublingually 15 minutes prior to stimulation onset is the recommended starting dose which may be adjusted based on the blood pressure response observed during PVS (15). Prazosin appears to reduce the severity of AD, lowering peak systolic blood pressures by about 40 mmHg during PVS in patients with SCI, without affecting resting blood pressure (39).
As a secondary benefit, scheduled use of PVS over the course of 4 weeks has been associated with decreased bladder spasticity on EMG as well as with an increased bladder capacity at leakpoint pressures (34). Relative contraindications to PVS are presence of penile prosthesis which could result in hardware erosion and severe irritation of the glans, such as that incurred by patients utilizing a condom catheter (15).
Electroejaculation (EEJ)
For men whose injury is at or below the T10 thoracolumbar sympathetic center and who therefore do not have an intact ejaculatory arc, EEJ can be employed to obtain ejaculate (37). While EEJ is relatively invasive compared to PVS, its widespread acceptance has been driven by the high rate of success (80–100%) of attaining semen after failure of PVS (3,28,30,36). Unlike PVS, it is as successful within 6 months of injury as in chronic injury, and may succeed even if the hip flexion reflex is absent (36). Additionally, the level or completeness of injury do not predict success of EEJ; similar rates of ejaculation are elicited in cervical patients (60%), compared with lumbar patients (50%), and those patients with complete (71%) versus incomplete (61%) lesions (30).
The recommended protocol was developed by Halstead in 1987 (40). EEJ is carried out with the patient in a lateral decubitus position, with positioning of the electrical probe in contact with the anterior rectal wall in the area of the prostate and seminal vesicles. The electrical stimulation is administered in a wave-like pattern with voltage progressively increasing in 1–2 V increments until ejaculation occurs, at which time voltage is discontinued. Voltage and current that have been reported to successfully produce ejaculation at a range from 5 to 25 V and 100–600 mA, respectively (20). Between 15 and 35 stimulations can be required per session. Interrupted, rather than continuous current delivery, with discontinuation of current at the peak of contraction of the external sphincter has been shown to yield a higher antegrade semen volume, total sperm count, and total motile sperm count (TMSC) (41).
As antegrade ejaculate is not produced in a projectile fashion, but rather as an intermittent dribble of semen, the urethra may need to be milked. Given high prevalence of retrograde ejaculation, the bladder is catheterized to empty urine prior to procedure and a buffering medium is instilled. After the procedure, the bladder is catheterized again to retrieve the retrograde fraction. Rectoscopy is performed prior to and following the procedure to exclude rectal injury. In sensate men, the procedure may cause significant discomfort requiring spinal or general anesthesia. As with PVS, EEJ may produce AD, therefore blood pressure monitoring and premedication (if indicated) should be performed (42).
Cryopreservation
In men with SCI, cryopreservation has been associated with decreased viability, as well as decreased total and progressive motility (1). In couples of whom one partner is a male with SCI, the pregnancy rate following ICSI utilizing fresh sperm (65%) is significantly higher compared to ICSI utilizing frozen-thawed sperm (25%). Interestingly, this difference in pregnancy rates was not observed between fresh and frozen sperm in couples with a non-SCI male (43).
Based on these outcomes, it has been recommended that cryopreservation be offered only in specific situations such as when anxiety about future fertility is high or when there is difficulty scheduling semen retrieval with oocyte retrieval (1). Another indication for cryopreservation would be early semen harvest in the first 1–2 weeks following SCI, given the rapid initial deterioration in semen parameters that occurs several weeks following the traumatic event (33,44). After the initial deterioration, a plateau in semen quality is observed. As such, routine cryopreservation of semen samples acquired more than several weeks following injury is not indicated (45).
Semen quality
The principal abnormality observed on semen analyses of men with SCI has been low sperm motility. Multiple studies of men with SCI utilizing unassisted and assisted ejaculation techniques have demonstrated low motility in the setting of normal to high sperm counts (1,28,30,32,35,46-48). Other semen parameter abnormalities have also been observed. For example, compared to non-SCI men undergoing routine semen analysis, the SCI group demonstrated lower ejaculate volume, decreased percentage of normal sperm morphology, and an increase in the round cell and neutrophils counts (49). Other studies found ejaculates from men with SCI often have normal sperm counts but more immotile sperm (50) and lower sperm viability compared to men without SCI (1). In SCI patients with asthenospermia, the majority of sperm showed degenerative changes and significant axonemal defects (51). Necrospermia and DNA fragmentation has also been found in semen of men with SCI at significantly higher rates than in that of men without SCI (52,53).
There is ample evidence to suggest that semen quality of men with SCI differs based on the technique employed to achieve ejaculation; semen attained by masturbation is superior to that which is attained by PVS, which is in turn superior to that attained by EEJ. In a study of 444 SCI subjects, sperm motility was higher in the masturbation group (37%) than the PVS group (26%) or EEJ group (15%), but lower compared with a control masturbation group of 61 non-SCI healthy men (58%). The SCI-masturbation group had similar antegrade sperm concentration (83.3×106/mL) as the PVS group (77.4×106/mL) and control group (82.0×106/mL), but higher than the EEJ group (49.8×106/mL) (54). In men with SCI utilizing EEJ, higher percentages of small sperm heads, vacuolated sperm heads, and sperm with tail defects were found than in manual ejaculates of able-bodied men (55).
Multiple studies comparing semen quality attained by PVS versus EEJ demonstrate comparatively superior motility, viability, and rapid linear acceleration of sperm retrieved utilizing PVS (37,56,57). In a crossover study, using both PVS and EEJ to obtain ejaculate from 11 males with SCI, antegrade PVS-induced ejaculate sperm exhibited higher motility compared to antegrade EEJ specimens. However, a higher total semen volume was obtained using EEJ, yielding similar numbers of motile sperm between the two techniques (37). The causes that have been proposed for the relatively more impaired motility rates with EEJ include electroporation injury and chronic denervation (30).
Comparison of semen derived by antegrade versus retrograde ejaculation utilizing PVS suggest no significant difference in sperm count, mean sperm motility, sperm progressive motility, and percent normal sperm morphology (57,58). Conversely, in men utilizing EEJ, retrograde ejaculates were found to have significantly impaired sperm motility and viability (59).
Factors associated with compromised semen parameters
There are multiple postulated etiologies for compromised semen parameters in men with SCI, which can be broadly classified into issues related to storage, acquisition, infection, and innate biochemical characteristics (Table 2).
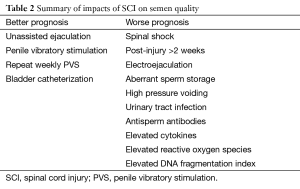
Full table
Storage
Aberrant storage of sperm within the inhospitable environment of the seminal vesicles has been implicated in poor semen quality. Elevated concentrations of senescent sperm with poor motility and viability that are found in the seminal vesicles of SCI men were also found to comprise a substantial portion of ejaculates obtained by PVS and EEJ (60). Sperm stagnation in the seminal ducts because of anejaculation has also been suggested as a cause of compromised semen quality, leading to several studies investigating the role of repeat ejaculation on semen quality. A comparison of baseline semen characteristics with those after repeat weekly PVS for 3 months, were found to have higher sperm concentration, progressive motility, and a decrease in abnormal sperm morphology (61). Another study for SCI patients with antegrade ejaculation assigned to 4–6 months of once weekly PVS, demonstrated improved penetration capacity, as well as increased semen volume and fructose content in the seminal plasma, the latter suggesting improved function of the seminal vesicles and prostate (62). Conversely, repeated EEJ failed to demonstrate improvement in the volume, sperm concentration, motility or the total motile count in the successive antegrade and retrograde samples (63). Testicular hyperthermia is another factor which may contribute to poor semen quality, however data on this topic are conflicting (64,65).
Timing of semen acquisition
During spinal shock, few to no spermatozoa are observed using EEJ. Following resolution of spinal shock, sperm motility and viability improve temporarily and then rapidly deteriorate starting about 2 weeks following injury, reaching the levels observed in males with chronic SCI at day 16 post-injury (44). In the chronically spinal cord-injured individual with an injury older than 1 year, there is no evidence of progressive decline in sperm quality (44,66). The age at which injury is sustained also appears to impact semen quality. For example, it has been observed that SCI sustained before the age of 9 years appears to interfere with spermatogenesis, while those who were injured closer to the onset of puberty exhibited semen quality in adulthood similar to that of subjects who were injured as adults (67).
Infection
In SCI, the incidence of signs of infection in the urine and semen (41% and 56%, respectively) is elevated compared to patients with normal bladder function (0% and 11%). Urinary tract infection has been found to be associated with slightly lower sperm quality and lower pregnancy rates, while leukocytospermia itself has not been demonstrated to have adverse effects (68,69). However, elevated levels of cytokines in the seminal plasma of men with SCI have been associated with impaired sperm motility, suggesting that while leukocytospermia itself may not be harmful, the high numbers of activated T lymphocytes producing cytokines can indicate a poor prognosis (70). There is also some evidence that cytokine neutralization leads to improved motility (71).
Biochemical factors
Inhibitory factors within seminal plasma of men with SCI may play a role in impaired sperm motility. This theory has been supported by an elegant experiment in which the seminal plasma from ejaculates of SCI men was shown to inhibit motility of sperm from normal men, while seminal plasma from normal men improved motility of sperm from SCI men (72). Additionally, incubation of normal sperm with SCI seminal plasma induced a concentration-dependent decrease in sperm motility (43%) with a concurrent drop in intracellular ATP content (33%) (51). Further evidence that seminal fluid in SCI men contributes to their poor semen profile is provided by the observation of lower sperm viability and motility rates of ejaculated spermatozoa compared to those derived by aspiration of the vas deferens. Notably, controls exhibited no significant difference in sperm motility and viability between ejaculated and aspirated sperm (73). The presence of reactive oxygen species (ROS) in the semen of men with SCI have been inversely correlated with sperm motility, but not morphology (74,75). One of the outcomes of accessory gland dysfunction is a lower semen fructose concentration, and can be attributed to EEJ or neurological injury of accessory glands (76). Antisperm antibodies, which have a disproportionately high prevalence in SCI men, may also contribute to asthenospermia (77).
Modifiable factors for improvement in semen quality
The method of emptying a neurogenic bladder in SCI may affect sperm quality, as evidenced by the lower sperm quality in men with high pressure reflex voiding compared to men who utilize intermittent self-catheterization (ISC) (20,68,78). ISC, indwelling urethral or suprapubic catheters have all been found to have a significantly enhanced semen quality compared to those voiding by reflex or straining. Within the catheter group, ISC patients achieved a higher percentage of motile sperm (78). Similar findings were observed in another study, in which ISC was associated with better sperm quality and a higher pregnancy rate (44%) compared to patients using alternative bladder management (7%) (68).
Antibiotics have been observed to improve sperm counts slightly, without impact on incidence of urine or semen infection. Continuous prophylaxis was associated with microbial resistance and had no advantage compared to a short course of antibiotics (68).
The utilization of bladder chemodenervation with botulinum toxin demonstrated mixed effects. A beneficial feature was a reduction in UTI’s, which was postulated to in turn result in lower semen contamination, possibly improving spermatogenesis and semen quality. One detrimental effect was the high resultant rate of retrograde ejaculation and therefore reduced semen volume, attributed to the reduced tone of the bladder neck and smooth muscles of sexual accessory organs (79).
The observation of increased ROS and sperm damage in semen samples obtained from men with SCI via EEJ raises the question of whether antioxidants could have a role improving sperm quality. While data on this topic is limited, two studies have demonstrated that the addition of pentoxifylline to fresh or thawed sperm retrieved by EEJ enhances sperm motility (80,81).
Pregnancy rates following ejaculatory assistance
What are the overall chances of attaining pregnancy in SCI, regardless of ejaculatory assistance or fertilization technique? DeForge et al., pooled fertility data among couples attempting to bear a child from 1993–2003, demonstrating a 51% pregnancy rate and an estimated live birth rate of 40% (37). Another meta-analysis of nine studies utilizing PVS or EEJ combined with ART such as IUI or in-vitro fertilization with or without ICSI demonstrated a 39–64% pregnancy rate per cycle (34) (Table 3). Another study demonstrated a pregnancy rate per cycle of 18% and a cumulative pregnancy rate per couple of 55% (3). Among couples utilizing only EEJ, about 1/3 of couples are successful in achieving pregnancy that resulted in a live birth (48,82).

Full table
IVI at home is a useful technique for those men with SCI who have been previously evaluated in clinic to be responsive to PVS, demonstrated understanding of safe usage, have adequate TMSCs, and whose partner has predictable normal ovulatory cycles without uterine or tubal abnormalities. In these circumstances, PVS can be used to collect ejaculate into a container, whose contents are then aspirated into a syringe that is used to deposit semen at the cervix. Overall, the rate of success of achieving pregnancy with this method is 25–70% per couple (3,28,83-86).
For couples who fail IVI and yet have adequate semen parameters, IUI may be utilized. In a study of couples with sperm retrieved by EEJ, IUI demonstrated 30% pregnancy rate per cycle, compared to a 5% rate with IVI (3). In other studies, timed IUI yielded a pregnancy rates per treatment cycle of 12% (87) and a pregnancy rate of 28% per couple (88). As with non-SCI couples who are being treated for infertility, pregnancy rates can be improved with administration of clomiphene citrate and hCG injection 38–40 hours prior to insemination (89).
There is no established threshold for TMSC below which IUI cannot be performed, but the literature suggests that it is 5 to 10×106 per mL (90). For example, in a study by Dickey et al., the pregnancy rate was 2.3% per cycle if the TMSC was less than 5×106 and 8.3% if the TMSC was 5 to 10×106 per mL (91). Based on these findings, it has been suggested that if the initial EEJ specimen has a TMSC of less than 5×106 per mL, it is recommended to proceed directly to in vitro fertilization (IVF). In those who fail IUI, IVF has demonstrated a pregnancy rate of about 70% per couple (88) and 37% per cycle (92). Cost analysis data suggests that unless a man requires EEJ with anesthesia, it is preferred to start with EEJ and IUI. Conversely, if anesthesia is required for EEJ, it is cost-effective to use testicular sperm aspiration (TESA) under local anesthesia and IVF as first line (92). In studies of IVF combined with ICSI followed by uterine embryo transfer, pregnancy rates per treatment cycle ranges are 20–82% per cycle and 39–100% per couple (15,78,84,85,87,88).
Surgical approaches for assisted fertility
Surgical methods of sperm retrieval are available for SCI patients in whom ejaculation cannot be attained by PVS or EEJ. Among men with SCI seeking fertility, about 1 out of 10 require surgical extraction (85). A variety of surgical sperm retrieval techniques are available, including TESA, testicular sperm extraction (TESE), microsurgical epididymal sperm aspiration (MESA), and percutaneous epididymal sperm aspiration (PESA) (15).
In a group of men with SCI with severe oligospermia or poor sperm quality after EEJ and obstructive azoospermia, TESA or open TESE resulted in sperm being found on almost 90% of procedures (93). High rates of success with sperm retrieval via TESE from men with SCI are observed regardless of semen analysis, hormone concentrations, level of lesion, patient age, or time since injury (94). In men with SCI and azoospermia on semen analysis after EEJ, TESE or sperm retrieval from the epididymis or vas deferens with ICSI have a relatively high likelihood of success, with a clinical pregnancy rate of 30% per cycle and 59% per couple, and a live birth rate of 63% (93).
Testicular biopsy specimens from men with SCI have shown a unique profile of alterations of the germinal epithelium, a finding that has been named the “neurogenic testicle.” Rather than maturation arrest, the primary pathogenesis in otherwise healthy infertile men, the biopsy tissue demonstrates decreased spermatogenesis with an elevated Sertoli cell-to-spermatid ratio per seminiferous tubule (94-96).
Hormonal abnormalities
In women who have experienced SCI, stress induced prolactin secretion may lead to a temporary amenorrhea. This has been observed in about a quarter of women with SCI of childbearing age, with higher levels of prolactin correlating to a higher likelihood of developing temporary amenorrhea, which usually lasts 5–6 months (97,98). Following resolution of amenorrhea, the physiologic capacity for conception is believed to return to baseline. However, not surprisingly, women who have experienced SCI have a much lower pregnancy rate of 0.34 per person compared to the pregnancy rate of 1.3 per person prior to injury. Those women who had higher and more complete neurological injuries were the least likely to become pregnant compared with those with lower degrees of neurologic impairment (98).
In men, hormonal abnormalities observed include hyperprolactinemia, elevated follicle-stimulating hormone (FSH) and elevated serum luteinizing hormone (LH). Serum testosterone levels have been observed to be both elevated and decreased. Interestingly, in some instances, low serum testosterone was found in the absence of elevated gonadotropins, while elevated FSH and/or LH were associated with normal or high serum testosterone. Their findings suggest that hypogonadism in SCI likely does not result from classic primary gonadal failure and that disruptions in feedback in the hypothalamus-pituitary-testicular axis is involved (99). While there has generally been no clear correlation observed between hormone profiles and testis biopsy results in SCI, one study did, however, find abnormal FSH levels to correlate with azoospermia (100).
Myelodysplasia and fertility
While the level of the neurologic lesion in patients with myelodysplasia does not strictly predict erectile and ejaculatory function, the best performance is observed in those with low sacral lesions and intact reflexes. Severe sexual dysfunction affects 90% of women and 59% of men with myelodysplasia (101). Men with lesions higher than T10 were noted to be at an elevated risk of azoospermia (102). There is also an increased risk of neural tube defects in offspring of men with spina bifida (102).
Multiple sclerosis (MS)
Although research is sparse, several sexual and endocrine disturbances have been described in MS patients of both genders, leading to lower fertility rates. Sexual dysfunction affects the majority of men with MS and includes ED in 73%, orgasmic or ejaculatory dysfunction in 50%, and reduced libido in 40% (103). ED can be managed with PDE-5I or intracavernous injections (104) and ejaculatory dysfunction can be treated with PVS.
Disturbances in the hypothalamic-pituitary gonadal axis affect both female and male MS patients. In women, this may induce menstrual disturbances and subsequent infertility secondary to elevated prolactin, LH, FSH, total and free testosterone (105). In men, serum levels for LH, FSH and testosterone are significantly lower compared to controls, and are refractory to GnRH analogue injections (106).
Compromised semen parameters including a lower total sperm count, motility and percent normal morphology have been observed in MS patients compared to controls (106). While endocrine abnormalities may be culprit, medical treatments especially immunosuppressive therapies (e.g., mitoxantrone, cyclophosphamide) may adversely affect spermatozoa and oocytes (104). As a means of managing this problem, sperm/oocyte cryostorage or ovarian fragment cryopreservation and autografting have been proposed (107).
Fertility does not seem to be impaired to a large extent in women with MS, however women who choose to undergo ART have been found to have an elevated risk of MS relapse. The proposed mechanisms for this finding include an interruption of disease modifying medications, stress of undergoing fertility therapy, and a hormonally-induced upregulation in pro-inflammatory markers (108).
Epilepsy
Fertility rates have been found to be reduced in epilepsy patients compared to the general population (109). Part of the mechanism of disturbances in reproductive function is attributed to the disrupted hypothalamic and pituitary function due to electrical discharges, resulting in disrupted release of GnRH, with abnormal LH and testosterone production (110). Antiepileptic drugs (AEDs) add to the adverse neuroendocrine effects on fertility through multiple mechanisms, including: (I) lowering bioavailable testosterone due elevations in sex hormone binding globulin (SHBG) (111); (II) direct spermatotoxicity resulting in a higher frequency of morphologically abnormal sperm, reduced sperm counts and decreased sperm motility (112); and (III) adverse effects on gonadal development, with a lower testicular volumes in men (113) and an increased incidence of polycystic ovary syndrome (PCOS) in women (114). There is evidence, however, that these effects are reversible with the discontinuation of the medications, even after long-term use (115).
Diabetes
Prevalence of subfertility in diabetic men has been cited to be as high as 52%, and is attributed to a combination of vascular disease, endocrine dysfunction, and autonomic neuropathy (116). The prevalence of ED in the diabetic population is 35–73%, and depends on severity and duration of disease (117-119). Ejaculatory dysfunction affects 40% of diabetic men, with an observed step-wise deterioration that starts with delayed ejaculation, progressing to retrograde ejaculation, and ultimately anejaculation (120).
Semen characteristics of diabetic men have included decreased semen volume (121-123), decreased sperm motility and morphology (122,123), as well as increased DNA fragmentation (121). Proposed etiologies have included hormonal disturbances through disruption of the hypothalamus-pituitary-testis axis leading to reduced testosterone production and chronic oxidative stress disrupting sperm maturation. Decreased ejaculate volume may be attributed to partial retrograde ejaculation and decreased seminal fluid excretion due to impaired neurological stimulation of the accessory male sexual glands (124).
Conclusions
Infertility in patients with neurologic disorders is a complex entity, resulting from a combination of hormonal, immunologic, sexual, and ejaculatory disturbances. As such, attaining fertility in neurologic disorders can require a multimodal and stepwise approach. Its involved logistics and potential psychological toll on the patients and their partners require an excellent physician-to-patient relationship. The associated high failure rates require a strong commitment from both the patient and the practitioner.
Acknowledgements
None.
Footnote
Conflicts of Interest: JM Hotaling: StreamDx, Andro360, Nanonc (equity in early stage startup companies). The other author has no conflicts of interest to declare.
References
- Bechoua S, Berki-Morin Y, Michel F, et al. Outcomes with intracytoplasmic sperm injection of cryopreserved sperm from men with spinal cord injury. Basic Clin Androl 2013;23:14. [PubMed]
- Anderson KD, Borisoff JF, Johnson RD, et al. The impact of spinal cord injury on sexual function: concerns of the general population. Spinal Cord 2007;45:328-37. [PubMed]
- Rutkowski SB, Geraghty TJ, Hagen DL, et al. A comprehensive approach to the management of male infertility following spinal cord injury. Spinal Cord 1999;37:508-14. [PubMed]
- Alexander MS, Brackett NL, Bodner D, et al. Measurement of sexual functioning after spinal cord injury: preferred instruments. J Spinal Cord Med 2009;32:226-36. [PubMed]
- Biering-Sørensen F, Sønksen J. Sexual function in spinal cord lesioned men. Spinal Cord 2001;39:455-70. [PubMed]
- Courtois FJ, Goulet MC, Charvier KF, et al. Posttraumatic erectile potential of spinal cord injured men: how physiologic recordings supplement subjective reports. Arch Phys Med Rehabil 1999;80:1268-72. [PubMed]
- Benevento BT, Sipski ML. Neurogenic bladder, neurogenic bowel, and sexual dysfunction in people with spinal cord injury. Phys Ther 2002;82:601-12. [PubMed]
- Chapelle PA, Durand J, Lacert P. Penile erection following complete spinal cord injury in man. Br J Urol 1980;52:216-9. [PubMed]
- Soler JM, Previnaire JG, Denys P, et al. Phosphodiesterase inhibitors in the treatment of erectile dysfunction in spinal cord-injured men. Spinal Cord 2007;45:169-73. [PubMed]
- Rizio N, Tran C, Sorenson M. Efficacy and satisfaction rates of oral PDE5is in the treatment of erectile dysfunction secondary to spinal cord injury: A review of literature. J Spinal Cord Med 2012;35:219-28. [PubMed]
- Lloyd LK, Richards JS. Intracavernous pharmacotherapy for management of erectile dysfunction in spinal cord injury. Paraplegia 1989;27:457-64. [PubMed]
- Rossier AB, Fam BA. Indication and results of semirigid penile prostheses in spinal cord injury patients: long-term followup. J Urol 1984;131:59-62. [PubMed]
- Van Arsdalen KN, Klein FA, Hackler RH, et al. Penile implants in spinal cord injury patients for maintaining external appliances. J Urol 1981;126:331-2. [PubMed]
- Collins KP, Hackler RH. Complications of penile prostheses in the spinal cord injury population. J Urol 1988;140:984-5. [PubMed]
- Brackett NL, Ohl DA, Sønksen J, et al. Abnormalities of ejaculation. In: Lipshultz LI, Howards SS, Niederberger CS, editors. Infertility in the Male. 4th Edition. New York: Cambridge University Press, 2009:454-73.
- Fode M, Sønksen JL. Management of male neurologic patients with infertility. In: Vodušek DB, Boller F, editors. Handbook of Clinical Neurology. Amsterdam, Netherlands: Elsevier B.V., 2015:435-49.
- Jefferys A, Siassakos D, Wardle P. The management of retrograde ejaculation: a systematic review and update. Fertil Steril 2012;97:306-12. [PubMed]
- Brackett NL, Ibrahim E, Iremashvili V, et al. Treatment for ejaculatory dysfunction in men with spinal cord injury: an 18-year single center experience. J Urol 2010;183:2304-8. [PubMed]
- Chéhensse C, Bahrami S, Denys P, et al. The spinal control of ejaculation revisited: a systematic review and meta-analysis of anejaculation in spinal cord injured patients. Hum Reprod Update 2013;19:507-26. [PubMed]
- Ohl DA, Bennett CJ, McCabe M, et al. Predictors of success in electroejaculation of spinal cord injured men. J Urol 1989;142:1483-6. [PubMed]
- Bors EH, Comarr AE. Neurological disturbances of sexual function with special reference to 529 patients with spinal cord injury. Urol Surv 1960;110:191-221.
- Gilja I, Parazajder J, Radej M, et al. Retrograde ejaculation and loss of emission: possibilities of conservative treatment. Eur Urol 1994;25:226-8. [PubMed]
- Kamischke A, Nieschlag E. Update on medical treatment of ejaculatory disorders. Int J Androl 2002;25:333-44. [PubMed]
- Hotchkiss RS, Pinto AB, Kleegman S. Artificial insemination with semen recovered from the bladder. Fertil Steril 1954;6:37-42. [PubMed]
- Ranieri DM, Simonetti S, Vicino M, et al. Successful establishment of pregnancy by superovulation and intrauterine insemination with sperm recovered by a modified Hotchkiss procedure from a patient with retrograde ejaculation. Fertil Steril 1995;64:1039-42. [PubMed]
- Shangold GA, Cantor B, Schreiber JR. Treatment of infertility due to retrograde ejaculation: a simple, cost-effective method. Fertil Steril 1990;54:175-7. [PubMed]
- Philippon M, Karsenty G, Bernuz B, et al. Successful pregnancies and healthy live births using frozen-thawed sperm retrieved by a new modified Hotchkiss procedure in males with retrograde ejaculation: first case series. Basic Clin Androl 2015;25:5. [PubMed]
- Sønksen J, Sommer P, Biering-Sørensen F, et al. Pregnancy after assisted ejaculation procedures in men with spinal cord injury. Arch Phys Med Rehabil 1997;78:1059-61. [PubMed]
- Leduc BE, Roy D, Poulin O. The use of physostigmine in men with spinal cord injury with ejaculatory dysfunction. Can J Rehab 1992;5:231-5.
- Sønksen J, Ohl DA. Penile vibratory stimulation and electroejaculation in the treatment of ejaculatory dysfunction. Int J Androl 2002;25:324-32. [PubMed]
- Sønksen J, Biering-Sørensen F, Kristensen JK. Ejaculation induced by penile vibratory stimulation in men with spinal cord injuries. The importance of the vibratory amplitude. Paraplegia 1994;32:651-60. [PubMed]
- Ohl DA, Menge AA, Sønksen J. Penile vibratory stimulation in spinal cord injured men: Optimized vibration parameters and prognostic factors. Arch Phys Med Rehabil 1996;77:903-5. [PubMed]
- Brackett NL, Ferrell SM, Aballa TC, et al. An analysis of 653 trials of penile vibratory stimulation in men with spinal cord injury. J Urol 1998;159:1931-4. [PubMed]
- Biering-Sørensen F, Laeessøe L, Sønksen J, et al. The effect of penile vibratory stimulation on male fertility potential, spasticity and neurogenic detrusor overactivity in spinal cord lesioned individuals. Acta Neurochir Suppl 2005;93:159-63. [PubMed]
- Beckerman H, Becher J, Lankhorst GJ. The effectiveness of vibratory stimulation in anejaculatory men with spinal cord injury. Review article. Paraplegia 1993;31:689-99. [PubMed]
- Brindley GS. The fertility of men with spinal injuries. Paraplegia 1984;22:337-48. [PubMed]
- DeForge D, Blackmer J, Garritty C, et al. Fertility following spinal cord injury: a systematic review. Spinal Cord 2005;43:693-703. [PubMed]
- Brackett NL, Kafetsoulis A, Ibrahim E, et al. Application of 2 vibrators salvages ejaculatory failures to 1 vibrator during penile vibratory stimulation in men with spinal cord injuries. J Urol 2007;177:660-3. [PubMed]
- Phillips AA, Elliott SL, Zheng MM, et al. Selective alpha drenergic antagonist reduces severity of transient hypertension during sexual stimulation after spinal cord injury. J Neurotrauma 2015;32:392-6. [PubMed]
- Halstead LS, VerVoort S, Seager SW. Rectal probe electrostimulation in the treatment of anejaculatory spinal cord injured men. Paraplegia 1987;25:120-9. [PubMed]
- Brackett NL, Ead DN, Aballa TC, et al. Semen retrieval in men with spinal cord injury is improved by interrupting current delivery during electroejaculation. J Urol 2002;167:201-3. [PubMed]
- Steinberger RE, Ohl DA, Bennett CJ, et al. Nifedipine pretreatment for autonomic dysreflexia during electroejaculation. Urology 1990;36:228-31. [PubMed]
- Kanto S, Uto H, Toya M, et al. Fresh testicular sperm retrieved from men with spinal cord injury retains equal fecundity to that from men with obstructive azoospermia via intracytoplasmic sperm injection. Fertil Steril 2009;92:1333-6. [PubMed]
- Mallidis C, Lim T, Hill S, et al. Collection of semen from men in acute phase of spinal cord injury. Lancet 1994;343:1072-3. [PubMed]
- Iremashvili V, Brackett NL, Ibrahim E, et al. Semen quality remains stable during the chronic phase of spinal cord injury: a longitudinal study. J Urol 2010;184:2073-7. [PubMed]
- Chung PH, Yeko TR, Mayer JC, et al. Assisted fertility using electroejaculation in men with spinal cord injury--a review of literature. Fertil Steril 1995;64:1-9. [PubMed]
- Kolettis PN, Lambert MC, Hammond KR, et al. Fertility outcomes after electroejaculation in men with spinal cord injury. Fertil Steril 2002;78:429-31. [PubMed]
- Buch JP, Zorn BH. Evaluation and treatment of infertility in spinal cord injured men through rectal probe electroejaculation. J Urol 1993;149:1350-4. [PubMed]
- da Silva BF, Borrelli M, Fariello RM, et al. Is sperm cryopreservation an option for fertility preservation in patients with spinal cord injury-induced anejaculation? Fertil Steril 2010;94:564-73. [PubMed]
- Brackett NL, Nash MS, Lynne CM. Male fertility following spinal cord injury: facts and fiction. Phys Ther 1996;76:1221-31. [PubMed]
- Monga M, Dunn K, Rajasekaran M. Characterization of ultrastructural and metabolic abnormalities in semen from men with spinal cord injury. J Spinal Cord Med 2001;24:41-6. [PubMed]
- Brackett NL, Bloch WE, Lynne CM. Predictors of necrospermia in men with spinal cord injury. J Urol 1998;159:844-7. [PubMed]
- Brackett NL, Ibrahim E, Grotas JA, et al. Higher sperm DNA damage in semen from men with spinal cord injuries compared with controls. J Androl 2008;29:93-9; discussion 100-1. [PubMed]
- Kathiresan AS, Ibrahim E, Aballa TC, et al. Comparison of in vitro fertilization/intracytoplasmic sperm injection outcomes in male factor infertility patients with and without spinal cord injuries. Fertil Steril 2011;96:562-6. [PubMed]
- Sedor JF, Hirsch IH. Evaluation of sperm morphology of electroejaculates of spinal cord-injured men by strict criteria. Fertil Steril 1995;63:1125-7. [PubMed]
- Brackett NL, Padron OF, Lynne CM. Semen quality of spinal cord injured men is better when obtained by vibratory stimulation versus electroejaculation. J Urol 1997;157:151-7. [PubMed]
- Ohl DA, Sønksen J, Menge AC, et al. Electroejaculation versus vibratory stimulation in spinal cord injured men: sperm quality and patient preference. J Urol 1997;157:2147-9. [PubMed]
- Chen D, Hartwig D, Roth E. Comparison of sperm quantity and quality in antegrade V retrograde ejaculates obtained by vibratory penile stimulation in males with spinal cord injury. Am J Phys Med Rehabil 1999;78:46-51. [PubMed]
- Hirsch IH, Sedor J, Jeyendran RS, et al. The relative distribution of viable sperm in the antegrade and retrograde portions of ejaculates obtained after electrostimulation. Fertil Steril 1992;57:399-401. [PubMed]
- Ohl DA, Menge A, Jarow J. Seminal vesicle aspiration in spinal cord injured men: insight into poor sperm quality. J Urol 1999;162:2048-51. [PubMed]
- Beretta G, Chelo E, Zanollo A. Reproductive aspects in spinal cord injured males. Paraplegia 1989;27:113-8. [PubMed]
- Siösteen A, Forssman L, Steen Y, et al. Quality of semen after repeated ejaculation treatment in spinal cord injury men. Paraplegia 1990;28:96-104. [PubMed]
- Das S, Dodd S, Soni BM, et al. Does repeated electro-ejaculation improve sperm quality in spinal cord injured men? Spinal Cord 2006;44:753-6. [PubMed]
- Wang YH, Huang TS, Lin MC, et al. Scrotal temperature in spinal cord injury. Am J Phys Med Rehabil 1993;72:6-9. [PubMed]
- Brackett NL, Lynne CM, Weizman MS, et al. Scrotal and oral temperatures are not related to semen quality of serum gonadotropin levels in spinal cord-injured men. J Androl 1994;15:614-9. [PubMed]
- Brackett NL, Ferrell SM, Aballa TC, et al. Semen quality in spinal cord injured men: Does it progressively decline postinjury? Arch Phys Med Rehabil 1998;79:625-8. [PubMed]
- Celigoj FA, Ibrahim E, Aballa TC, et al. Semen quality in men who sustained a spinal cord injury during the prepubertal period. J Urol 2012;188:521-5. [PubMed]
- Ohl DA, Denil J, Fitzgerald-Shelton K, et al. Fertility of spinal cord injured males: effect of genitourinary infection and bladder management on results of electroejaculation. J Am Paraplegia Soc 1992;15:53-9. [PubMed]
- Aird IA, Vince GS, Bates MD, et al. Leukocytes in semen from men with spinal cord injuries. Fertil Steril 1999;72:97-103. [PubMed]
- Basu S, Lynne C, Ruiz P, et al. Cytofluorographic identification of activated T-cell subpopulations in the semen of men with spinal cord injuries. J Androl 2002;23:551-6. [PubMed]
- Cohen DR, Basu S, Randall JM, et al. Sperm motility in men with spinal cord injuries is enhanced by inactivating cytokines in the seminal plasma. J Androl 2004;25:922-5. [PubMed]
- Brackett NL, Davi RC, Padron OF, et al. Seminal plasma of spinal cord injured men inhibits sperm motility of normal men. J Urol 1996;155:1632-5. [PubMed]
- Brackett NL, Lynne CM, Aballa TC, et al. Sperm motility from the vas deferens of spinal cord injured men is higher than from the ejaculate. J Urol 2000;164:712-5. [PubMed]
- Padron OF, Lynne CM, Brackett NL, et al. Seminal reactive oxygen species and sperm motility and morphology in men with spinal cord injury. Fertil Steril 1997;67:1115-20. [PubMed]
- de Lamirande E, Leduc B, Iwasaki A, et al. Increased reactive oxygen species formation in semen of patients with spinal cord injury. Fertil Steril 1995;63:637-42. [PubMed]
- Hirsch IH, Jeyendran RS, Sedor J, et al. Biochemical analysis of electroejaculates in spinal cord injured men: comparison to normal ejaculates. J Urol 1991;145:73-6. [PubMed]
- Hirsch IH, Sedor J, Callahan HJ, et al. Antisperm antibodies in seminal plasma of spinal cord-injured men. Urology 1992;39:243-7. [PubMed]
- Rutkowski SB, Middleton JW, Truman G, et al. The influence of bladder management on fertility in spinal cord injured males. Paraplegia 1995;33:263-6. [PubMed]
- Caremel R, Courtois F, Charvier K, et al. Side effects of intradetrusor botulinum toxin injections on ejaculation and fertility in men with spinal cord injury: preliminary findings. BJU Int 2012;109:1698-702. [PubMed]
- Kolon TF, Philips KA, Buch JP. Pentoxifylline enhancement of post-thaw motility in cryopreserved semen of spinal cord-injured men. Int J Fertil Menopausal Stud 1995;40:156-60. [PubMed]
- Sikka SC, Hellstrom WJ. The application of pentoxifylline in the stimulation of sperm motion in men undergoing electroejaculation. J Androl 1991;12:165-70. [PubMed]
- McGuire C, Manecksha RP, Sheils P, et al. Electroejaculatory stimulation for male infertility secondary to spinal cord injury: the Irish experience in National Rehabilitation Hospital. Urology 2011;77:83-7. [PubMed]
- Dahlberg A, Ruutu M, Hovatta O. Pregnancy results from a vibrator application, electroejaculation, and a vas aspiration programme in spinal-cord injured men. Hum Reprod 1995;10:2305-7. [PubMed]
- Nehra A, Werner MA, Bastuba M, et al. Vibratory stimulation and rectal probe electroejaculation as therapy for patients with spinal cord injury: semen parameters and pregnancy rates. J Urol 1996;155:554-9. [PubMed]
- Löchner-Ernst D, Mandalka B, Kramer G, et al. Conservative and surgical semen retrieval in patients with spinal cord injury. Spinal Cord 1997;35:463-8. [PubMed]
- Leduc BE. Treatment of infertility in 31 men with spinal cord injury. Can J Urol 2012;19:6432-6. [PubMed]
- Taylor Z, Molloy D, Hill V, et al. Contribution of the assisted reproductive technologies to fertility in males suffering spinal cord injury. Aust N Z J Obstet Gynaecol 1999;39:84-7. [PubMed]
- Heruti RJ, Katz H, Menashe Y, et al. Treatment of male infertility due to spinal cord injury using rectal probe electroejaculation: the Israeli experience. Spinal Cord 2001;39:168-75. [PubMed]
- Pryor JL, Kuneck PH, Blatz SM, et al. Delayed timing of intrauterine insemination results in a significantly improved pregnancy rate in female partners of quadriplegic men. Fertil Steril 2001;76:1130-5. [PubMed]
- Van Voorhis BJ, Barnett M, Sparks A, et al. Effect of the total motile sperm count on the efficacy and cost-effectiveness of intrauterine insemination and in vitro fertilization. Fertil Steril 2001;75:661-8. [PubMed]
- Dickey RP, Pyrzak R, Lu PY, et al. Comparison of the sperm quality necessary for successful intrauterine insemination with World Health Organization threshold values for normal sperm. Fertil Steril 1999;71:684-9. [PubMed]
- Ohl DA, Wolf LJ, Menge AC, et al. Electroejaculation and assisted reproductive technologies in the treatment of anejaculatory infertility. Fertil Steril 2001;76:1249-55. [PubMed]
- Raviv G, Madgar I, Elizur S, et al. Testicular sperm retrieval and intra cytoplasmic sperm injection provide favorable outcome in spinal cord injury patients, failing conservative reproductive treatment. Spinal Cord 2013;51:642-4. [PubMed]
- Elliott SP, Orejuela F, Hirsch IH, et al. Testis biopsy findings in the spinal cord injured patient. J Urol 2000;163:792-5. [PubMed]
- Leriche A, Berard E, Vauzelle JL, et al. Histological and hormonal testicular changes in spinal cord patients. Paraplegia 1977;15:274-9. [PubMed]
- Hirsch IH, McCue P, Allen J, et al. Quantitative testicular biopsy in spinal cord injured men: comparison to fertile controls. J Urol 1991;146:337-41. [PubMed]
- Rutberg L, Fridén B, Karlsson A. Amenorrhoea in newly spinal cord injured women: an effect of hyperprolactinaemia? Spinal Cord 2008;46:189-91. [PubMed]
- Charlifue SW, Gerhart KA, Menter RR, et al. Sexual issues of women with spinal cord injuries. Paraplegia 1992;30:192-9. [PubMed]
- Wang YH, Huang TS, Lien IN. Hormone changes in men with spinal cord injuries. Am J Phys Med Rehabil 1992;71:328-32. [PubMed]
- Brackett NL, Lynne CM, Weizman MS, et al. Endocrine profiles and semen quality of spinal cord injured men. J Urol 1994;151:114-9. [PubMed]
- Lee NG, Andrews E, Rosoklija I, et al. The effect of spinal cord level on sexual function in the spina bifida population. J Pediatr Urol 2015;11:142.e1-6.
- Bong GW, Rovner ES. Sexual health in adult men with spina bifida. ScientificWorldJournal 2007;7:1466-9. [PubMed]
- Haensch CA, Jörg J. Autonomic dysfunction in multiple sclerosis. J Neurol 2006;253 Suppl 1:I3-9. [PubMed]
- Prévinaire JG, Lecourt G, Soler JM, et al. Sexual disorders in men with multiple sclerosis: evaluation and management. Ann Phys Rehabil Med 2014;57:329-36. [PubMed]
- Grinsted L, Heltberg A, Hagen C, et al. Serum sex hormone and gonadotropin concentrations in premenopausal women with multiple sclerosis. J Intern Med 1989;226:241-4. [PubMed]
- Safarinejad MR. Evaluation of endocrine profile, hypothalamic-pituitary-testis axis and semen quality in multiple sclerosis. J Neuroendocrinol 2008;20:1368-75. [PubMed]
- Cavalla P, Rovei V, Masera S, et al. Fertility in patients with multiple sclerosis: current knowledge and future perspectives. Neurol Sci 2006;27:231-9. [PubMed]
- Hellwig K, Correale J. Artificial reproductive techniques in multiple sclerosis. Clin Immunol 2013;149:219-24. [PubMed]
- Webber MP, Hauser WA, Ottman R, et al. Fertility in persons with epilepsy: 1935-1974. Epilepsia 1986;27:746-52. [PubMed]
- Bauer J, Dierkes H, Burr W, et al. Disease- and treatment-related effects on the pituitary-gonadal functional axis: a study in men with epilepsy. J Neurol 2011;258:1080-4. [PubMed]
- Isojärvi JI, Repo M, Pakarinen AJ, et al. Carbamazepine, phenytoin, sex hormones, and sexual function in men with epilepsy. Epilepsia 1995;36:366-70. [PubMed]
- Isojärvi JI, Löfgren E, Juntunen KS, et al. Effect of epilepsy and antiepileptic drugs on male reproductive health. Neurology 2004;62:247-53. [PubMed]
- El-Khayat HA, Shatla HM, Ali GK, et al. Physical and hormonal profile of male sexual development in epilepsy. Epilepsia 2003;44:447-52. [PubMed]
- Zhou JQ, Zhou LM, Chen LJ, et al. Polycystic ovary syndrome in patients with epilepsy: a study in 102 Chinese women. Seizure 2012;21:729-33. [PubMed]
- Lossius MI, Taubøll E, Mowinckel P, et al. Reversible effects of antiepileptic drugs on reproductive endocrine function in men and women with epilepsy--a prospective randomized double-blind withdrawal study. Epilepsia 2007;48:1875-82. [PubMed]
- La Vignera S, Calogero A, Condorelli R, et al. Andrological characterization of the patient with diabetes mellitus. Minerva Endocrinol 2009;34:1-9. [PubMed]
- McCulloch DK, Campbell IW, Wu FC, et al. The prevalence of diabetic impotence. Diabetologia 1980;18:279-83. [PubMed]
- Derosa G, Romano D, Tinelli C, et al. Prevalence and associations of erectile dysfunction in a sample of Italian males with type 2 diabetes. Diabetes Res Clin Pract 2015;108:329-35. [PubMed]
- Siu SC, Lo SK, Wong KW, et al. Prevalence of and risk factors for erectile dysfunction in Hong Kong diabetic patients. Diabet Med 2001;18:732-8. [PubMed]
- Dunsmuir WD, Holmes S. The aetiology and management of erectile, ejaculatory, and fertility problems in men with diabetes mellitus. Diabet Med 1996;13:700-8. [PubMed]
- Agbaje IM, Rogers D, McVicar CM, et al. Insulin dependent diabetes mellitus: implications for male reproductive function. Hum Reprod 2007;22:1871-7. [PubMed]
- Ali ST, Shaikh RN, Siddiqi NA, et al. Semen analysis in insulin-dependent/non-insulin-dependent diabetic men with/without neuropathy. Arch Androl 1993;30:47-54. [PubMed]
- Padrón RS, Dambay A, Suárez R, et al. Semen analyses in adolescent diabetic patients. Acta Diabetol Lat 1984;21:115-21. [PubMed]
- La Vignera S, Condorelli RA, Di Mauro M, et al. Seminal vesicles and diabetic neuropathy: ultrasound evaluation. J Androl 2011;32:478-83. [PubMed]



