Patient reported outcome measures in neurogenic bladder
Introduction
Measurement is the science of assigning a standard numerical scale to a concept. It can be something as readily understood as distance, or a more abstract concept such as quality of life (QOL) or functional status (1). Disease specific measurement tools are tailored to a particular condition or population, and can help to quantify disease symptoms, or QOL. The measurement tool can be based on one of several points of view, such as the patients, the caregivers or the healthcare professionals. While each of these sources has its strengths and weaknesses, self-reported data has several advantages: it is easily available, specific to the particular patient, and reflects their subjective experience of a disease state. Arguably, the patient’s own perception of symptoms is the most relevant, especially when considering non-life threatening symptoms or QOL. Measurement tools are ubiquitous in Psychological research, and the importance of a well-developed, valid and reliable measurement tool is well recognised in medical research.
Important concepts in measurement
The first step to choosing a measurement tool is to understand the explicit construct that is being measured. This construct will determine whether the measurement tool is appropriate for a specific research purpose. Selecting a QOL measure when the objective is to measure symptom burden would be inappropriate given that some patients may have significant symptoms, but despite this perceive their QOL to be high. This distinction is particularly important amongst individuals with neurogenic bladder dysfunction. For example, improvements in perceived QOL have been consistently demonstrated among individuals despite continued paralysis during the early years after a spinal cord injury (SCI) (2,3).
Patient reported outcome measures (PROMs) can be applied to three different scenarios, (I) cross-sectional: evaluation of patients at 1 point in time; (II) predictive: predicting improvement or decline in a patient characteristic; and (III) change over time: evaluation of a patient characteristic over time (4). PROMs are usually designed and evaluated for one of these purposes, but in some cases they may be able to be used for multiple purposes. It is important to identify the purpose and to determine whether the PROM of interest can be applied to that purpose. For example, a PROM with questions asking about symptoms over the last 3 months shouldn’t be used to look at a change over a 2-week period. Further, a cross-sectional QOL tool for SCI patients that has items that would rarely change over time would be a poor choice for a clinical trial evaluating patients after an intervention which would only be expected to make a small difference in QOL. Once the scenario of interest has been identified, initial PROMs can be selected, and the details of specific PROMs should be reviewed.
One of these details is item generation, which is the formulation of a group of questions which address a particular construct. There are many methods to generate these items, including the use of items from previous tools, qualitative interviews with patients and caregivers, and from expert consensus. Each of these methods has advantages and disadvantages, and their relative use depends on the measurement objective. At the early stage of development, the items must at least appear to represent the construct in question; this is termed face validity, and is essential to evaluate when considering a measurement tool for use.
Once the initial items in the measurement tool have been assembled, psychometric testing is performed to assess whether the items are all testing the same construct (internal validity). The most common statistical method to examine this is either Cronbach’s alpha or an interclass correlation coefficient (ICC). These are interpreted as the degree of internal consistency of the PROM as measured by the correlation between items. Generally higher values (closer to 1) are desirable, however if the items are too closely correlated, then they may be redundant. While these methods are ideal for use in PROMs which tap into a single hypothetical construct (for example anxiety or depression), they are less useful for QOL PROMs, which may seek to evaluate a constellation of domains. Each of these domains may independently contribute to the overall life satisfaction in an individual, but a patient may not necessarily score themselves at the same level across these domains (5). In these situations, the correlation of specific sub-domains within the entire construct is more appropriate. The next step is to determine if the items actually measure the construct they are designed to measure (external validity). In order to assess this, the items and the overall measurement tool can be evaluated against a “gold-standard”, or specific hypotheses around clinical characteristics can be constructed and tested. Unfortunately, for concepts such as QOL, there is often no single gold standard, and therefore alternative methods (such as comparisons with other PROMs or patients clinical features) may be used.
There are four additional concepts that can be considered when evaluating PROMs: feasibility, test-retest reliability, responsiveness, and interpretability. Once the PROM has established its validity and reliability, it is important to consider how the PROM will perform in the desired clinical setting. The feasibility of the PROM includes such things as how easy it is for the patients to actually answer the questions (for example the size of the boxes to mark), the literacy required to understand the questions and responses, the estimated time that will be required for completion, and any language barriers or cultural considerations. Test-retest reliability measures the consistency of the PROM when it is applied to the same patient at different time points (assuming there has been no change in the patient’s condition); a scale that is reliable should have a high correlation or ICC between these two sets of scores. This is analogous to measuring the length of an object at different times: if there is no change in the length of the object, then your measurement of it should be consistent. Responsiveness is essential for measuring change over time and is usually demonstrated by showing that a PROM actually changes in a situation when it is hypothesised to change. Some PROMs aren’t sensitive to change, and are therefore are not responsive and inappropriate for “change over time” studies. A standardized response mean (SRM) is the mean change score divided by the standard deviation of the change scores (based on measurements before and after an intervention), and can be used as a unitless comparison of responsiveness between PROMs. A higher SRM indicates a greater sensitivity to clinical change. Interpretability refers to how a certain magnitude of change of the PROM score should be perceived. For example, would a 5-point change on the scale result in a significantly worse category for QOL, or result in an increased risk of surgery in the future? Related to this concept is minimally detectable change (MDC) or minimally clinically important detectable change (MCID). These statistics determine how small a change the PROM score can actually detect, and they take into account the precision of the scale (6).
Which patient reported outcome measure is right for you?
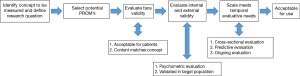
As PROMs continue to proliferate, it is important to understand how to evaluate them for clinical use. To identify an appropriate PROM, start with defining a clear research or clinical question, and then evaluate specific PROMs (7).
Defining the research/clinical question includes three relevant pieces of information: (I) what concept you want to measure; (II) in what population; and (III) for what purpose. Concepts can range from extremely broad (pain scales) to very specific (number of episodes of incontinence per day). Populations can be defined by a demographic (such as income level), a treatment (such as incontinence medication) or a disease (such as individuals with SCI). As previously outlined, the purpose of the research question can be characterized as cross-sectional, predictive, or change over time (8). Explicit formulation of these components is critical to select the appropriate PROM.
A useful tool for the assessment of specific measurement tools has been formulated by Beaton et al. (7) (Figure 1). This step-wise procedure allows clinicians and researchers to quickly eliminate inappropriate PROMs and focus on the most relevant ones.
Patient reported outcome measures (PROM) for neurogenic bladder
The complications of neurogenic bladder have been associated with a lower QOL across several patient populations (9-11), especially in the domains of physical functioning, mental health, and socialization (12). A systematic review of existing PROMs in neurogenic bladder was published by Patel et al. (12). Their systematic review qualitatively synthesized information from articles on PROMs in neurogenic bowel and bladder. They found 39 articles on urinary dysfunction published between Jan 1st 2000– Jan 1st 2014, which used a variety of PROMs. They identified only one outcome measure, the Qualiveen questionnaire (13), which was specifically designed to address bladder dysfunction among individuals with SCI [and subsequently multiple sclerosis (MS)]. The remainder of PROMs identified within the review were not specifically designed to address neurogenic bladder outcomes.
A review of the literature was also performed as part of the development of a patient reported Neurogenic Bladder Symptom Scale by Welk et al. (5). The authors did not identify any existing instruments specifically designed to measure neurogenic bladder symptoms, although nine neurogenic disease specific QOL scales and 29 urinary symptom-specific measurement tools were identified.
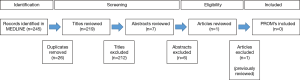
We replicated the search strategy from the Patel et al. article to identify any new articles related to neurogenic bladder published since 2013. We searched Medline using the following strategy (((“urinary incontinence” [MeSH] OR “urinary bladder, neurogenic”[MeSH] AND (“quality of life”[MeSH] OR “patient reported outcomes”) AND (“Multiple Sclerosis”[Mesh] OR “Spinal Cord Injuries”[Mesh] OR “Parkinson Disease”[Mesh] OR “Stroke”[Mesh] OR “Spinal Dysraphism”[Mesh])). This search yielded 245 results (Figure 2). Conference abstracts were not considered and we excluded 26 duplicate records. Titles of all results were reviewed and 212 were excluded for non-relevance. Abstracts of seven results were reviewed and six were excluded. One new paper was identified (neurogenic bladder symptom score) (NBSS) (Figure 2). This additional paper, as well as relevant neurogenic bladder PROMs identified in the previous literature review by Patel et al. (12) and Welk et al. (5) are summarised in Table 1. The highlighted PROMs are representative of all identified PROMs specific to neurogenic bladder, which are discussed in more detail below. General neurogenic bladder QOL measures with a bladder function component, and a selection of commonly used PROM which have not been validated in neurogenic bladder patients are included in Table 1 and are briefly summarised below.
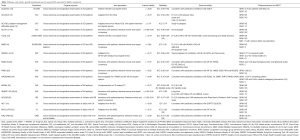
Full table
PROMs specific to neurogenic bladder
The Qualiveen was developed by Costa et al. (13) to measure the urinary QOL of patients with SCI. It contains 30 items rated on a Likert scale developed from interviews with patients, expert review of items and previous studies. The Qualiveen questionnaire was the most commonly used measure designed for the assessment of bladder related QOL in a recent review on patient reported outcomes in neurogenic bladder (12). A short form (SF) of this PROM is available (38) and it has been validated for use in patients with MS. It is available in several languages including English, French, Dutch and Italian.
The NBSS was developed (4) to measure symptoms and consequences associated with neurogenic bladder dysfunction. It contains 24 items (arranged in three domains) scored on a Likert scale and was developed through patient interviews (Including patients with SCI, MS and spina), expert opinion, and a literature review. This measure examines the full spectrum of neurogenic bladder symptoms and associated complications including areas often overlooked with incontinence based PROMs (4).
The Actionable Bladder Symptom Screening Tool (ABSST)-SF, developed by Bates et al. (28) is a PROM which can be used to identify MS patients with urinary symptoms who may benefit from a more precise diagnosis or referral to a Urologist. It contains eight items rated on a Likert scale and one dichotomous item developed as a SF of the validated ABSST. Authors argue that this tool is easy to administer and use in a clinical setting, is specific to MS, and is sensitive and multidimensional (28). The purpose of this measurement is to identify patients who would benefit from a Urological assessment.
The Incontinence Quality of Life Questionnaire (IQOL), developed by Patrick et al., is an incontinence-specific assessment developed for use in individuals with stress incontinence and overactive bladder (39). This index contains 22 items rated on a Likert scale, which covers three subscales: (I) avoidance and limiting factors, (II) psychosocial impact, and (III) social embarrassment. This PROM was then validated in a population of SCI patients with incontinence (19). This PROM was one of the earliest to be validated in the neurogenic bladder population and remains in wide usage.
General neurogenic quality of life (QOL) measures, and general bladder-related patient reported outcome measures (PROMs)
These PROMs are not specifically designed to assess bladder related QOL or neurogenic bladder symptoms. However, they have a bladder component, or have been used as unvalidated instruments in the neurogenic bladder population previously. A general description is provided below:
- Spinal Cord Injury Secondary Conditions Scale (SCI-SCS): a 16-item QOL scale examining secondary conditions (e.g., functional, medical and psychosocial) associated with SCI (15);
- Spinal Cord Injury Quality of Life (SCI-QOL): a multidimensional, computer adaptive PROM with a total of 19 item banks, which measure the physical, emotional, and social aspects of health-related QOL among patients with SCI. There is a 15-question item bank related to bladder management difficulties (16);
- Spinal Cord Injury Functional Index (SCI-FI): a scale containing 90 items on self-care which provides a comprehensive assessment of functional abilities of individuals with SCI (17);
- Multiple Sclerosis Quality of Life 54-item scale (MSQOL-54): a 52-item scale distributed across 12 subscales with 2 additional items. This scale assesses multiple dimensions of QOL associated with MS (9);
- Multiple Sclerosis Impact Scale 29-item (MSIS-29): a 39-item scale examining the physical and psychological impact of MS acceptable for patient-level assessment (22);
- Functional Assessment of Multiple Sclerosis (FAMS): a 59-item scale which broadly examines personal and social QOL domains for individuals with MS (23);
- Hamburg Quality of Life Questionnaire in MS (HAQUAMS): a 38-item questionnaire for QOL assessment across individuals with varying levels of disease severity associated with MS (24);
- Multiple Sclerosis Quality of Life Inventory (MSQLI): an inventory containing 10 scales including a bladder control scale for comprehensive assessment of many domains of QOL affected by MS. This scale is valid across varying levels of cognitive dysfunction (26);
- Quality of Life in Spina Bifida scale (QOLSB): two indexes designed to assess QOL, one for completion by parents and by the child with spina bifida. The scales contain 44 and 47 items (30);
- Incontinence Impact Questionnaire 7-item (IIQ-7): a 7-item non-disease specific scale designed to evaluate the impact of urinary incontinence across the domains of emotional health, physical activity and social health (31). This has not been specifically validated among neurogenic bladder patients;
- Kings Health Questionnaire Lower Urinary Tract (KHQ-LUTS): a 24-item scale designed to assess health related QOL associated with lower urinary tract symptoms and urinary incontinence (32);
- International Consultation on Incontinence Questionnaire Overactive Bladder (ICIQ-OAB): contains an 8-item symptom bother scale and 25-item health related QOL scale designed to evaluate symptoms of overactive bladder (35).
Conclusions and future directions in this area
PROMs for the assessment of QOL in patients with neurogenic bladder continue to be developed, however there are only a few tools specifically developed for this population. It is essential for clinicians and researchers to have a basic understanding of the principals of PROM development and systematic evaluation so they can effectively select the most appropriate tool for their clinical or research purpose.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nunnally JC. Psychometric theory. McGraw-Hill; 1978:730.
- Krause JS, Crewe NM. Prediction of long-term survival of persons with spinal cord injury: An 11 year prospective study. Rehabil Psychol 1987;32:205-13.
- Stensman R. Adjustment to traumatic spinal cord injury. A longitudinal study of self-reported quality of life. Paraplegia 1994;32:416-22. [PubMed]
- Welk B, Morrow S, Madarasz W, et al. The validity and reliability of the neurogenic bladder symptom score. J Urol 2014;192:452-7. [PubMed]
- Welk B, Morrow SA, Madarasz W, et al. The conceptualization and development of a patient-reported neurogenic bladder symptom score. Res Rep Urol 2013;5:129-37. [PubMed]
- Streiner DL. Being inconsistent about consistency: when coefficient alpha does and doesn’t matter. J Pers Assess 2003;80:217-22. [PubMed]
- Beaton DE, Terwee CB, Singh JA, et al. A Call for Evidence-based Decision Making When Selecting Outcome Measurement Instruments for Summary of Findings Tables in Systematic Reviews: Results from an OMERACT Working Group. J Rheumatol 2015. [Epub ahead of print]. [PubMed]
- Wright JG, McLeod RS, Lossing A, et al. Measurement in surgical clinical research. Surgery 1996;119:241-4. [PubMed]
- Vickrey BG, Hays RD, Harooni R, et al. A health-related quality of life measure for multiple sclerosis. Qual Life Res 1995;4:187-206. [PubMed]
- Ku JH. The management of neurogenic bladder and quality of life in spinal cord injury. BJU Int 2006;98:739-45. [PubMed]
- Sawin KJ, Bellin MH. Quality of life in individuals with spina bifida: a research update. Dev Disabil Res Rev 2010;16:47-59. [PubMed]
- Patel DP, Elliott SP, Stoffel JT, et al. Patient reported outcomes measures in neurogenic bladder and bowel: A systematic review of the current literature. Neurourol Urodyn 2016;35:8-14. [PubMed]
- Costa P, Perrouin-Verbe B, Colvez A, et al. Quality of life in spinal cord injury patients with urinary difficulties. Development and validation of qualiveen. Eur Urol 2001;39:107-13. [PubMed]
- Bonniaud V, Bryant D, Parratte B, et al. Qualiveen: a urinary disorder-specific instrument for use in clinical trials in multiple sclerosis. Arch Phys Med Rehabil 2006;87:1661-3. [PubMed]
- Kalpakjian CZ, Scelza WM, Forchheimer MB, et al. Preliminary reliability and validity of a Spinal Cord Injury Secondary Conditions Scale. J Spinal Cord Med 2007;30:131-9. [PubMed]
- Tulsky DS, Kisala PA, Tate DG, et al. Development and psychometric characteristics of the SCI-QOL Bladder Management Difficulties and Bowel Management Difficulties item banks and short forms and the SCI-QOL Bladder Complications scale. J Spinal Cord Med 2015;38:288-302. [PubMed]
- Jette AM, Tulsky DS, Ni P, et al. Development and initial evaluation of the spinal cord injury-functional index. Arch Phys Med Rehabil 2012;93:1733-50. [PubMed]
- Heinemann AW, Dijkers MP, Ni P, et al. Measurement properties of the Spinal Cord Injury-Functional Index (SCI-FI) short forms. Arch Phys Med Rehabil 2014;95:1289-1297.e5.
- Schurch B, Denys P, Kozma CM, et al. Reliability and validity of the Incontinence Quality of Life questionnaire in patients with neurogenic urinary incontinence. Arch Phys Med Rehabil 2007;88:646-52. [PubMed]
- Possavino F, Preti M, Carone R, et al. Psychometric validation of the Italian version of the I-QoL questionnaire: clinical and urodynamic findings. Int Urogynecol J 2013;24:2125-30. [PubMed]
- Giordano A, Pucci E, Naldi P, et al. Responsiveness of patient reported outcome measures in multiple sclerosis relapses: the REMS study. J Neurol Neurosurg Psychiatry 2009;80:1023-8. [PubMed]
- Hobart J, Lamping D, Fitzpatrick R, et al. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain 2001;124:962-73. [PubMed]
- Cella DF, Dineen K, Arnason B, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology 1996;47:129-39. [PubMed]
- Gold SM, Heesen C, Schulz H, et al. Disease specific quality of life instruments in multiple sclerosis: validation of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS). Mult Scler 2001;7:119-30. [PubMed]
- Gold SM, Schulz H, Stein H, et al. Responsiveness of patient-based and external rating scales in multiple sclerosis: head-to-head comparison in three clinical settings. J Neurol Sci 2010;290:102-6. [PubMed]
- Marrie RA, Miller DM, Chelune GJ, et al. Validity and reliability of the MSQLI in cognitively impaired patients with multiple sclerosis. Mult Scler 2003;9:621-6. [PubMed]
- National Multiple Sclerosis Society. Multiple Sclerosis Quality of Life Inventory (MSQLI). Cited Sep 2, 2015. Available online: http://www.nationalmssociety.org/For-Professionals/Researchers/Resources-for-Researchers/Clinical-Study-Measures/Multiple-Sclerosis-Quality-of-Life-Inventory-(MSQL
- Bates D, Burks J, Globe D, et al. Development of a short form and scoring algorithm from the validated actionable bladder symptom screening tool. BMC Neurol 2013;13:78. [PubMed]
- Burks J, Chancellor M, Bates D, et al. Development and validation of the actionable bladder symptom screening tool for multiple sclerosis patients. Int J MS Care 2013;15:182-92. [PubMed]
- Parkin PC, Kirpalani HM, Rosenbaum PL, et al. Development of a health-related quality of life instrument for use in children with spina bifida. Qual Life Res 1997;6:123-32. [PubMed]
- Moore KN, Jensen L. Testing of the Incontinence Impact Questionnaire (IIQ-7) with men after radical prostatectomy. J Wound Ostomy Continence Nurs 2000;27:304-12. [PubMed]
- Okamura K, Nojiri Y, Osuga Y. Reliability and validity of the King’s Health Questionnaire for lower urinary tract symptoms in both genders. BJU Int 2009;103:1673-8. [PubMed]
- Viana R, Viana S, Neto F, et al. Adaptation and validation of the King’s Health Questionnaire in Portuguese women with urinary incontinence. Int Urogynecol J 2015;26:1027-33. [PubMed]
- Kelleher CJ, Cardozo LD, Khullar V, et al. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol 1997;104:1374-9. [PubMed]
- Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res 2002;11:563-74. [PubMed]
- Wolpe RE, Toriy AM, Da Silveira GF, et al. Assessing the impact of urinary incontinence on quality of life: systematic review of instruments in Portuguese. MTP&RehabJournal 2014;12:201.
- Irwin PP, Harris M. Patient-reported outcomes in overactive bladder due to idiopathic detrusor overactivity: A correlation of two multi-domain questionnaires with a focus on quality of life and lifestyle goals. J Clin Urol 2014;7:403-8.
- Bonniaud V, Bryant D, Parratte B, et al. Development and validation of the short form of a urinary quality of life questionnaire: SF-Qualiveen. J Urol 2008;180:2592-8. [PubMed]
- Patrick DL, Martin ML, Bushnell DM, et al. Quality of life of women with urinary incontinence: further development of the incontinence quality of life instrument (I-QOL). Urology 1999;53:71-6. [PubMed]