How does interstitial cystitis begin?
It has only been in recent years that interstitial cystitis (IC) sometimes referred to as bladder pain syndrome (but many patients do not have pain) has become more widely known and understood. Twenty five years ago IC was thought to be a rare disease that affected only people with severe symptoms and who had a destroyed and shrunken bladder. The median age at diagnosis was over 50 plus years of age and fewer than 50,000 people in Unites States were diagnosed with the disease. It was the lack of knowledge that relegated IC to an orphan disease state (1). But IC like any disease does have a beginning, a time when the symptoms are mild and intermittent and clinicians assign various traditional diagnoses based on their specialty, primary care versus gynecology versus urology. By interviewing IC patients who fulfill the classic severely symptomatic patient it can be recognized that the disease begins with mild intermittent symptoms that progress slowly until the disease is so intense that most doctors would recognize it. But during the “early years” of the disease much effort is wasted on incorrect diagnoses leading to treatments and medical care that does not help the patient. The recurrent symptom flares drive the patient to repeated MD visits, tests and medications that add up to substantial health care costs that are of no real benefit to anyone.
To help IC patients one must correctly diagnose the disease in order to utilize therapy that will help the person and there are good treatments for IC today. This study was done to help develop an understanding of how the disease begins, what the key symptoms are and how it progresses all of which will aid in the correct diagnosis of IC earlier in the disease process. To accomplish this goal 100 patients filled out a questionnaire and were interviewed on when their symptoms began, what they were and are now as well as the various diagnoses that they received before they were determined to have IC.
Materials and methods
Subjects
Subjects were from one urologist’s practice at the University of California San Diego. They were serially selected from patients presenting for either a new patient visit of a return visit. One hundred female subjects were recruited and signed an informed consent. All of them met the clinical NIDDK criteria (no cystoscopy required) for IC (2). They filled out a questionnaire asking about the age their disease presented, their initial and current symptoms, what their original diagnoses were, effect of the menstrual cycle and sexual activity on their symptoms and about any relatives with bladder symptoms or a current diagnosis of IC. Their responses were reviewed with the principal investigator.
Initial symptoms
They patients were asked at what age they first noted bladder symptoms and what they were, frequency, urgency, and/or bladder pain. They were asked if at the onset of their symptoms if they were either intermittent or continuous.
Current symptoms
The patients were asked what their current symptoms are including frequency, urgency, bladder or pelvic pain, and urinary incontinence.
Diagnoses prior to IC diagnosis
The patients were asked what their initial diagnoses were and if they received any associated gynecologic diagnoses. If they had a diagnosis of recurrent urinary tract infections (UTI), they were asked if they had been told that they had negative cultures during a flare of their bladder symptoms.
Gynecologic symptoms
They reported the effect of sexual activity on their pain and whether or not they had post intercourse flares of their bladder symptoms. For those that had a menstrual cycle the effect of it on their symptoms was recorded as to when during their cycle the symptoms flared.
Patients’ that had childhood symptoms of IC
Each subject was queried about childhood bladder symptoms, at what age they presented, whether or not they had seen a doctor for these problems and if they knew the diagnosis they were given if any.
First degree relatives with bladder symptoms
Patients were asked if any male or female relatives had bladder symptoms similar to their own symptoms. They reported on mothers, sisters, daughters, father, brothers or sons who had problems.
Results
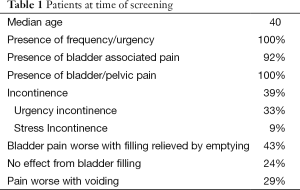
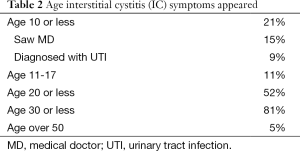
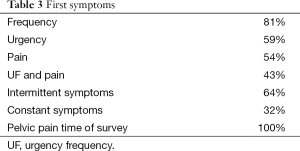
A total of 100 females with IC were evaluated. At the time of this survey the median age of the females was 40 (Table 1). All reported frequency and urgency, 92% reported bladder associated pain and another 8% reported pelvic pain since they were not able to distinguish the bladder as the source. Urinary incontinence was present in 39% and 85% of these patients stated it was urgency incontinence. Of those with bladder associated pain, 43% reported the pain was worse with filling and relieved by emptying, 24% were not worse with filling and 29% had worse pain with voiding (Table 1). The age that IC symptoms began are shown it Table 2 and Table 3 lists the first symptoms that were experienced.
Full table
Full table
Full table
There were 21% of the patients reported seeing an MD for their symptoms as a child less that age 10 (Table 1). The most common diagnosis was UTI (60%). Other diagnoses included urethral problem, anxiety, small bladder or no diagnosis was given.
Sexual and menstrual cycle effects on symptoms are reported in Table 4. Diagnoses the patients received before an IC diagnosis are in Table 5. UTI was the most common early IC diagnosis, 74% and of those patients with this diagnosis, 93% reported one or more negative urine cultures at the time of symptom flares. First degree relatives with similar bladder symptoms are listed in Table 6.

Full table

Full table

Full table
Discussion
The main reason that these data were obtained was to explore the issue of how does IC begin. I have evaluated more than 9,000 people with IC over the past 35 plus years and during that time have done extensive basic laboratory and clinical research on IC. From this research I have come to believe that there is one primary disease process that causes bladder symptoms in females at all ages and in males less than 55 years of age (3). That process is a dysfunction of the mucus barrier (GAG layer) at the bladder epithelial surface. This layer is the reason that the normal epithelium is relatively impermeable to small molecules but if this mucus is diseased there is an impaired ability to prevent urine solutes from “leaking” into the bladder wall. When an epithelial dysfunction occurs, potassium is the principal solute that leaks into the bladder wall and causes depolarization of bladder nerves and muscle resulting in the symptoms of urgency/frequency, pain or urinary incontinence in any combination (3). The severe phase of this disease process is the traditional IC patient. But IC has to have a beginning and in the early phase of IC symptoms are milder, intermittent, develop slowly and numerous incorrect diagnoses are given as shown by these data.
Patient histories I have taken have many common threads and one does develop an impression for how the disease begins. It is my observation that the first symptom is usually a slow insidiously progressive frequency. The patient does not present for help from a physician until it bothers them such as voiding every hour, multiple episodes of nocturia or has post void urgency. But after time many will note the onset of pain cycles that appear suddenly are intense and drive the patient to seek medical care. These cycles typically occur after sexual activity and last for 3-14 days and then resolve. Most are diagnosed with a urinary infection and will receive antibiotics regardless of the results of a urine culture (often negative). Others will receive no actual diagnosis or treatment. When the flares are multiple in a short time period or last for weeks and months then they are referred to urology. Initially the symptom flares are intermittent but even at base line the patients are likely to have increased frequency of urination that they don’t necessarily perceive as abnormal, they have always had this so it is normal to them. Many of the patients may not develop pain cycles but when their urgency/frequency progresses and becomes bothersome they will seek medical care and receive the “overactive bladder diagnosis” (OAB). This diagnosis is separated from IC only by an arbitrary definition and not on scientific evidence. And in fact, 73% of OAB patients have been reported to have a positive potassium sensitivity test (PST) consistent with an IC diagnosis (4). The current study was conducted to obtain data to determine whether or not these impressions were valid. And the data reported here are consistent with this overall concept.
The onset of bladder symptoms reported by the patients (Table 1) began before age 10 in 21%, by age 20 in 52% and before age 30, 81%. The first symptoms were frequency/urgency in 81% while only 54% reported the presence of pain and 43% had both frequency and pain. Only 5% reported the onset of bladder symptoms after age 50. At the time of this survey 39% reported incontinence of urine, 85% of whom had urgency incontinence. This problem did not appear in most of the patients until after age 35. Symptoms were initially intermittent in the majority of patients, 64%.
Sexually activity cause pain and symptom flares in 74%. There were 65 who currently had menstrual cycles and 74% reported that their cycle affected symptoms. Most experience flares the week before their period, 75% (Table 4).
The classic pain associated with the diagnosis of IC is “pain made worse with bladder filling and relieved by emptying.” But this is usually not the case only 43% reported this symptom. In fact voiding provoked the bladder pain is 29% contrary to the classic definition. Then there is the issue of all the chronic pelvic pain (CPP) patients that see gynecologists most of whom (80%) appear to have the bladder as the source of the pain (5-9). These patients see the gynecologist because they do not perceive the bladder as the cause of the pain or else they would be seeing an urologist. So it would appear that many more IC patients do not have “classic pain.” Unfortunately most urologists including many experts in IC confine their observations to only those patients seeing them for pain and are unaware that they majority of patients with bladder generated pain are not seeing Urologists. These traditional concepts have to be changed incorporating all of the new data so that patients can be correctly diagnosed and treated.
Interestingly 21% of the patients reported childhood bladder problems at age 10 or less, most, 75% saw an MD for this problem. The most common diagnosis was urinary infection, 60%. Others were told they had a urethral problem, anxiety, small bladder or the physician did not know what caused the symptoms. An important point is most of these patients after having a number of flares of symptoms go into a remission until they become sexually active. This emphasizes the intermittent nature of the disease and the fact that IC often does not fulfill the NIDDK criteria but then they were never meant to be the final answer for an IC diagnosis but only criteria for research purposes.
Table 5 lists the diagnoses patients received before being told they had IC. Not surprisingly urinary infection was number one at 74%, of interest, 93% were told of having at least one or more negative cultures when having a symptomatic flare. There is a concept that somehow UTIs may play a causal role in IC. But I believe and my experience has taught me that recurrent UTIs in a female are quite rare. This is supported by these data where over 90% of the patients had negative cultures with symptom flares. The key point is that most doctors incorrectly diagnose the acute flares of IC as UTI until cultures are repeatedly negative, occur multiple times or the symptoms fail to resolve with antibiotic therapy. Even one negative culture with a flare should alert the doctor to the fact that these episodes are not infectious and point to the only other diagnosis the patient could have IC (or if one prefers early IC).
The patients in this study were seeing an urologist but many had seen a gynecologist for their symptoms and received diagnoses of yeast vaginitis, endometriosis or vulvodynia. It does appear from the literature that a substantial majority of IC patients is actually seeing gynecologists for their bladder pain and are misdiagnosed by them as vulvodynia, endometriosis, yeast vaginitis or just chronic pelvic pain (origin not known) (5-9).
In the absence of knowledge concerning the pathophysiology and diagnosis of a medical disease (syndrome) there are often attempts to rename the disease frequently by its symptoms in order to begin a new era of research activity. In urology, prostatitis is a good example of this phenomenon. It has been reclassified 3 times in the last 40 years. In recent years a similar attempt has been made to rename IC as “bladder pain syndrome” in the absence of any scientific evidence to support this reclassification. Often patients with IC have no pain and these data show that many patients present only with frequency issues and develop pain at a later time. But many seek a doctor for their frequency problems and what do they have at this point in time in terms of diagnosis? They have “early” IC or simply IC. Similar problem with the term OAB, these patients were so diagnosed because they have no pain and it is often argued that they do not have IC. But these data show patients saw MDs before they had experienced pain. So by definition they would have “OAB” in this earlier time period but ultimately they were diagnosed with IC. Scientific evidence shows that OAB patients have the same epithelial dysfunction as does the IC patient (4,10). There are those that argue that there is an overlap of these two conditions but the scientific evidence supports the fact that both are one disease, bladder epithelial dysfunction (3). Since the urethra is often involved the term lower urinary dysfunctional epithelium (LUDE) is more accurate (3). Perhaps it is time to remove the syndrome label from IC and OAB since they can now be defined by their pathophysiology, LUDE (3).
The genetic issue is quite real. There were 51% who had a first degree female relative with bladder symptoms and 13% a first degree male relative who had similar bladder symptoms. These results are summarized in Table 6.
These data provide the clinician with useful information to aid in the diagnosis of IC. It is often said it is a difficult diagnosis to make but this not true. It is an easy diagnosis when you realize the disease begins with urinary frequency and progresses slowly with intermittent flares of symptoms of urinary frequency/urgency and/or pain. These flares usually occur after sexual activity and sex is painful. Significant symptoms are already present in over 80% of people who develop IC by age 30. The problem is the median age of diagnosis (which is steadily dropping) is still close to age 40 so most patients are incorrectly diagnosed at a time when they would respond better to therapy. So at the end of the day if a urologist is seeing a 25-year-old female for the following reasons, recurrent symptoms of urinary urgency/frequency and/or bladder pain or voiding associated pain, a history of negative urinary cultures when symptomatic (no how many were “positive”). Sexual activity is painful, her symptoms are worse just before or during her menses, has a mother or sister with similar symptoms and today she is symptomatic only one test is needed, urine analysis or catheterized urine for culture that shows no infection. There is only one thing she could have, IC. The diagnosis is easy so proceed to therapy.
This study has several weaknesses. First the data obtained was from recall but most subjects felt they were reasonably accurate about their responses and this may not be a significant problem due to the nature of the questions that were asked. Recall of childhood problems with the bladder was only reported as positive if the subject was definite about it. Some people were not sure or could not remember so the actual incidence of childhood bladder problems may be higher. Another weakness is that these were urologic patients presenting to an urologist so the bias is that they would only see a GU if they had obvious bladder symptoms of urgency/frequency or definite pelvic pain they associated with the bladder. Many people with IC are not bothered by their frequency (they think their 12 voids a day is normal, it is for them) so when they have pelvic pain affected by their menstrual cycle and have pain with sex they seek a gynecologist for their problem because it appears to them to be gynecologic in nature. The gynecologist rarely solicits information about voiding symptoms and since the patients don’t perceive they have any frequency they end up with a gynecologic diagnosis. As mentioned previously when gynecologic CPP patients were screened for IC in five reported studies with a symptom questionnaire that included urinary frequency (7) and a PST of their bladder which is positive only when the bladder is the likely cause of their pelvic pain, 85% had urinary frequency and 80% had a positive PST (5-9). Gynecologists see over 15 million women with CPP they so they probably see far more IC patients than urologists. This study was done by an urologist so gynecologic CPP patients were not screened.
In summary IC begins with mild symptoms usually frequency/urgency (33% will ultimately develop urgency incontinence) that is slowly and insidiously progressive. Pain is often a later symptom but many patients may never develop it. In the early phase of IC the symptom flares are intermittent in most patients. Over time symptoms increase and pain cycles may appear and last for 3-14 days. When these cycles become more frequent and last longer they are likely to be referred to a specialist. The most common misdiagnosis is urinary infection followed by yeast vaginitis, endometriosis and vulvodynia. Sexual activity is usually painful and causes a symptom flare in most and symptoms are affected by the menstrual cycle, increasing the week before and during the menses. Over 50% of patients will have a first degree relative with similar symptoms. If one is seeing a woman with this history and her urine culture is negative then the diagnosis is easy, she has interstitial cystitis and therapy should be initiated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: Patient data reported in this article was approved by the ethics committee at UCSD and patients signed consent forms for the use of their information.
References
- Teichman JM, Parsons CL. Contemporary clinical presentation of interstitial cystitis. Urology 2007;69:41-7. [PubMed]
- Gillenwater JY, Wein AJ. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis, National Institutes of Health, Bethesda, Maryland, August 28-29, 1987. J Urol 1988;140:203-6. [PubMed]
- Parsons CL. The role of a leaky epithelium and potassium in the generation of bladder symptoms in interstitial cystitis/overactive bladder, urethral syndrome, prostatitis and gynaecological chronic pelvic pain. BJU Int 2011;107:370-5. [PubMed]
- Minaglia S, Ozel B, Bizhang R, et al. Increased prevalence of interstitial cystitis in women with detrusor overactivity refractory to anticholinergic therapy. Urology 2005;66:702-6. [PubMed]
- Parsons CL, Bullen M, Kahn BS, et al. Gynecologic presentation of interstitial cystitis as detected by intravesical potassium sensitivity. Obstet Gynecol 2001;98:127-32. [PubMed]
- Parsons CL, Dell J, Stanford EJ, et al. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol 2002;187:1395-400. [PubMed]
- Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology 2002;60:573-8. [PubMed]
- Stanford EJ, Dell JR, Parsons CL. The emerging presence of interstitial cystitis in gynecologic patients with chronic pelvic pain. Urology 2007;69:53-9. [PubMed]
- Kahn BS, Tatro C, Parsons CL, et al. Prevalence of interstitial cystitis in vulvodynia patients detected by bladder potassium sensitivity. J Sex Med 2010;7:996-1002. [PubMed]
- Philip J, Willmott S, Irwin P. Interstitial cystitis versus detrusor overactivity: a comparative, randomized, controlled study of cystometry using saline and 0.3 M potassium chloride. J Urol 2006;175:566-70; discussion 570-1. [PubMed]


