Similarities between interstitial cystitis/bladder pain syndrome and vulvodynia: implications for patient management
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic multifaceted illness whose clinical hallmarks include perceived bladder pain and irritative voiding symptoms, in the absence of easily identifiable pathology. Similarly, vulvodynia is a broad term imparted to varied forms of persistent vulvar pain of unknown origin. Once thought to be rare clinical entities, recent epidemiology suggests that these conditions are not only rather common, but also frequently co-exist. Furthermore, IC/BPS and vulvodynia often share co-morbidities. The apparent close clinical association between these syndromes may relate to similar embryological origins and/or multiple forms of shared pathologies. This article will examine the similarities between these conditions and therapeutic implications.
Common embryological derivations
Urogenital tissues share common embryological origins, which may account for the frequent coexistence of IC/BPS and vulvodynia (Figure 1). McCormack first reported on 36 patients with focal vulvitis (vulvodynia) of those, 11 also had comorbid IC/BPS (1). Fitzpatrick et al. then published case reports of three women with coexisting IC/BPS and vulvodynia and discussed the significance of a disorder of the urogenital sinus (UGS)-derived epithelium for both diagnosis and treatment (2). Similar embryological derivations between tissues may also account for common complaints of urethral pain (a region that bridges the bladder and genital tissue) in both patient groups.
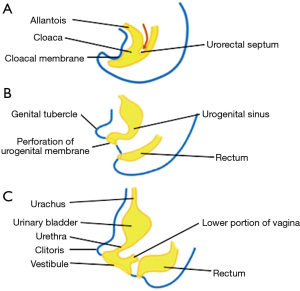
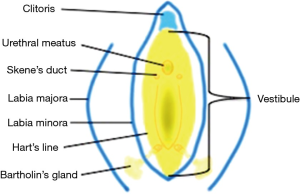
Indeed, urogenital tissues are of endodermal origin and derived from the cloacal membrane, a structure that is formed at the third fetal week. By the seventh week, a urorectal septum divides the cloacal membrane, thus separating it into an anterior urogenital and a posterior anal membrane (3). The urogenital membrane perforates to create the primary UGS, a structure that communicates with the amniotic cavity. A definitive UGS is formed with further partitioning that includes caudal phallic and pelvic regions. These regions will ultimately develop into the vaginal introitus and the female urethra/lower portion of vagina, respectively. The uterus, cervix, and upper portion of the vagina are derived from the Müllerian ducts (mesodermal tissue) after the ducts fuse and canalize with the UGS. Other endodermal derivatives of clinical importance are the Skene’s and Bartholin’s glands. “Hart’s line” found on the inner aspect of the labia minor marks the final boundary between endodermal (UGS) and ectodermal derivatives (Figure 2). The upper portion of the UGS develops into the urinary bladder. Past embryological dogma suggested that the trigone of the bladder was derived from mesoderm; however, more recent investigations suggest that the entire bladder is of endodermal origin (4).
Pathology
Over the past two decades, much basic research has been invested to defining the cause of both pain syndromes. Although lines of investigation for these conditions differ in many aspects, there are some commonalities of pathology that have been identified.
Mast cell involvement
Typically, mast cells are associated with allergic disorders and acute inflammatory responses, secreting numerous proinflammatory mediators; contributing to a variety of chronic inflammatory conditions such as intestinal ulceration, rheumatoid arthritis and Crohn’s disease. Additionally, they are found in stress-induced neuroinflammatory diseases including multiple sclerosis, migraines, atopic dermatitis, chronic urticaria, irritable bowel syndrome and IC/BPS (5). Vasoactive, nociceptive and proinflammatory molecules released from activated mast cells produce neuronal sensitization and secretion of neurotransmitters that further stimulate mast cells. It is this cycle that may contribute to the chronic voiding and pain symptoms associated with IC/BPS (6).
Similarly, enhanced mast cell activity has been identified in vulvodynia patients where histamine release is thought to stimulate peripheral pain transmitting neurons (C and A-δ fibers) (7-9). Borenstein et al. compared vestibular tissue from women with severe vulvodynia (n=40) for mast cell count and degranulation and nerve cell total area compared to controls (n=7). They found a significant increase in the total mast cell count (P=0.006; P=0.15) and degranulated mast cells (P=0.0001) in the vulvodynia patients compared to controls. The total nerve fiber area was found to be 10 times higher in the vulvodynia group (4,190 μm2) compared to the control group (425 μm2) (P=0.01) (10).
Neurogenic inflammation, neuropathic pain, central sensitization
The role of the peripheral and central nervous systems in the pathogenesis of IC/BPS and vulvodynia is complex and profoundly incomplete. Clinical observations suggest that both of these pain syndromes have subtypes where pain is derived from the “end organ” and/or those where pain is generated at higher levels within the nervous system. Traditionally, treatment for these patients has been targeted to peripheral pathology; however, these therapies may be insufficient due to altered central nervous system (CNS) processing.
From a neuroanatomical perspective, the female urinary and reproductive systems are innervated by the same sacral nerve pathways (11). This nerve innervation is responsible for both the motor/efferent and sensory/afferent pathways of the somatic and visceral systems (12). Additionally, the dorsal root ganglion cells at the thoracolumbar and sacral levels play a role in the transmission of painful sensations from the pelvis to the brain (13).
Much of the pain experienced by IC/BPS and vulvodynia patients appears to be neuropathic. Neuropathic pain is a primary excitatory disorder of the nervous system occurring through a variety of mechanisms such as infection, trauma, or with no apparent etiology. In patients with neuropathic pain, the injured nerve fibers fire spontaneously or at a lower threshold, the latter phenomenon accounting for the local allodynia (painful response to non-noxious stimuli) identified in many patients with IC/BPS and vulvodynia. Neurogenic inflammatory events may be part of this process whereby primary afferent nociceptors (primarily C-fibers but to a lesser extent also A-δ fibers) are stimulated with the subsequent release of inflammation provoking neuropeptides [e.g., substance P, calcitonin gene-related peptide (CGRP), neurokinin A and B] and lead to a state of neurologic “wind-up” (6,8,13,14). Downstream clinical events typically include local vasodilation, edema, hyperalgesia and allodynia; all notable characteristics in certain subgroups of IC/BPS and vulvodynia. Neurogenic inflammation plays a role in local pain and inflammation, but also at referred sites (6,13). This may explain why patients with IC/BPS may experience referred pain in the vulva and patients with vulvodynia may experience referred pain in the bladder and urethra.
Abnormal pain processing, suggesting CNS sensitization, has been identified in IC/BPS and vulvodynia patients. Ness et al. demonstrated generalized lower thermal pain tolerances in IC/BPS patients than healthy controls (15). Previous studies in vulvodynia showed lower peripheral pain thresholds in both painful and non-painful areas when compared to healthy controls. (16-18).
Central processing abnormalities in vulvodynia were further investigated by functional MRI (fMRI). This revealed that when women afflicted with vulvodynia described pressure as painful, they had significantly higher activation levels in the insular and frontal cortical regions than did the healthy control group (19). Hampson et al. recently compared local (vulvar) and remote (thumb) pressure-evoked pain processing via fMRI scans between women with vulvodynia (n=24), age-matched healthy controls (n=13), and positive controls with fibromyalgia (n=24) (for thumb pressure). Vulvodynia and fibromyalgia patients displayed similar insular brain activations that were greater than the healthy control patients in response to thumb pressure (P<0.005). Also, compared to the healthy controls, the vulvodynia group displayed greater levels of activation within the insula, dorsal mid cingulate, posterior cingulate and thalamus during thumb pressure (P<0.005) (20). Grey matter densities in areas that modulate pain and stress (e.g., parahippocampal gyrus, hippocampus and basal ganglia) were significantly higher in women with vulvodynia vs. controls (21).
Recent fMRI studies in IC/BPS demonstrated alterations in resting state oscillations and enhanced connectivity in sensory and motor networks in IC/BPS patients compared to controls. Increased connectivity was greatest in patients reporting pain during bladder filling (22). White matter abnormalities, many of which correlated to pelvic pain severity and urinary symptoms, were detected in female IC/BPS patients using diffusion tensor imaging or fractional anisotropy (23).
Hormonal influences
Endocrine dysregulation has been postulated to account for the development and/or maintenance of IC/BPS and vulvodynia. Symptom flares in these patients may in fact be modulated by the estrogen surge before menses that induces the release of histamine from mast cells resulting in increased amounts of substance P (24,25). A neuromodulatory role of estrogen on pain transmission at various sites has been documented (26). Gonadal hormones are also theorized to influence the CNS activity of neuromodulators involved in nociceptive processing (27). Clinically, this may impact patients with both IC/BPS and vulvodynia. A recent prospective study investigated the effect of local (topical) estrogen on urinary and sexual symptoms in premenopausal women with IC/BPS (28). A total of 34 women with IC/BPS were treated with topical estriol 3 times per week for 12 weeks. A total of 94.1% (32/34 of the women) were diagnosed with concomitant vulvodynia. Results indicate a significant improvement of both urinary symptoms and sexual function (P<0.001, for both). Investigators theorize that women with these conditions are vulnerable to changes in sex steroid milieu thereby predisposing urogenital tissues to abnormal responses (28).
Many IC/BPS patients report symptom exacerbation, or flares, perimenstually or around ovulation. Powell-Boone et al. conducted a small study on IC/BPS patients and healthy controls that showed a correlation between pain and urinary frequency scores and the phases of the menstrual cycle (29).
Several studies have shown a link between combined hormonal oral contraception and vulvodynia (30-32); however, other studies failed to demonstrate this correlation (33,34). Further investigations are warranted to determine the association between endogenous and exogenous gonadal hormones and the development and/or exacerbation of IC/BPS and vulvodynia.
Epidemiology
Epidemiology with regard to IC/BPS and vulvodynia has been primarily hampered by the varied definitions for these syndromes. Nevertheless, most investigations indicate that these conditions not only have a high prevalence and have common co-morbidities; but that they are frequently identified in the same patient.
Among women age 18 and over in the US, recent data suggest between 3.3 and 7.9 million women have symptoms of IC/BPS (35). The prevalence of vulvodynia has been estimated to range between 4-16%, effecting more than 2.4 million women in the US (36-38). The association between IC/BPS and vulvodynia is often reported, with 25% of women with IC/BPS also reporting to have vulvodynia (28,37,39,40). Gordon et al. conducted an Internet-based survey of 428 women with self-reported vulvar pain and found that 11% had an IC/BPS diagnosis (41). Nguyen et al. conducted a survey of 1,457 women with diagnosed vulvodynia [generalized (involves entire vulva) n=467, localized (involves a specific portion or component of the vulva) n=639, or both n=351] and the presence of co-morbid pain conditions. They found that 18.8% of the generalized group, 14.7% of the localized group, and 27.9% of the group with both types of vulvodynia also reported co-morbid IC/BPS (P<0.001) (42).
Patients often have overlapping symptoms making diagnosis of one or both conditions all the more difficult. Frequency of both conditions is often underestimated due to the fact that many in the medical community believe these conditions to be psychological in origin or because women reluctant to discuss their symptoms (43).
Comorbid conditions
IC/BPS and vulvodynia are frequently associated with other pain syndromes. Interestingly, recent non-population based studies suggest that irritable bowel syndrome and fibromyalgia are the two most prevalent comorbid conditions associated with both conditions. (42,44). Other co-morbid conditions commonly seen in both IC/BPS and vulvodynia are temporal mandibular joint and muscle disease (TMD) and chronic fatigue syndrome (CFS). As previously mentioned, IC/BPS and vulvodynia are frequently seen as co-morbid to each other. Both Nickel et al. and Nguyen et al. indicate that the prevalence of multiple comorbidities increases with symptom severity (42,44).
Hypertonic pelvic floor dysfunction (HTPFD) is commonly seen in both conditions. Many patients with IC/BPS and vulvodynia present with muscle tenderness, myofascial pain, spasticity, voiding dysfunction and dyspareunia which are also manifestations of HTPFD. Approximately 50-87% of patients with IC/BPS are diagnosed with HTPFD (45). Yong et al. recently reported the prevalence of PFD (defined as pelvic floor muscle tenderness) in 189 women with chronic pelvic pain compared to 32 healthy controls. A total of 40% of the women with chronic pelvic pain (75/189) had concomitant PFD vs. 13% of controls (4/32) (46). While it is unclear if HTFPD results from chronic pain of IC/BPS and vulvodynia or if it is a precipitating factor, HTPFD can exacerbate symptoms and can itself worsen in response to IC/BPS or vulvodynia flares.
Quality of life (QOL)
Chronic urogenital pain syndromes may have a profound effect upon QOL resulting in feelings of hopelessness and helplessness, depression as well as negatively impacting personal relationships. A case-controlled survey demonstrated a correlation between stress, anxiety, depression and catastrophizing to IC/BPS symptoms and a decreased QOL (44). Similar reports demonstrate when compared to controls, women with vulvar pain reported significantly worse mental health-related QOL, lower levels of happiness in couple relationships, higher trait anxiety, increased somatization and catastrophizing related to dyspareunia (47-49).
Behavioral interventions emphasizing a self-management approach, such as cognitive behavioral therapy (CBT), are effective treatment option for both conditions. Numerous studies have shown that CBT increases perceived control in patients with chronic pain by reducing pain, helplessness and psychological distress (50-54). CBT has been shown to reduce catastrophizing, decrease pain and improve sexual functioning in women with vulvodynia and has been recommended for patients with IC/BPS (55-57).
Sexual function
Sexual dysfunction strongly impacts the QOL in patients with IC/BPS and vulvodynia. It has been postulated that pain mediates sexual dysfunction and its associated effects on QOL. A higher prevalence of moderate to severe sexual dysfunction has been identified in IC/BPS patients compared to controls (58,59).
Bogart et al. conducted a telephone survey of women with IC/BPS (n=1,469) to determine the prevalence of sexual dysfunction compared to the general population. A total of 88% of the women with a sexual partner reported having sexual dysfunction vs. 43% in the general population (57). Gardella et al. evaluated women diagnosed with IC/BPS (n=47) and a control group (n=47) for the characteristics of vulvodynia. The prevalence of vulvodynia was 85.1% in the IC/BPS group compared to 6.4% in controls (P<0.0001). Dyspareunia was described as “unbearable” in 31.9% of the IC/BPS group compared to 4.3% of controls (P=0.0001) (39).
Limiting or avoidance of sexual activity due to associated pain is common in patients with IC/BPS and vulvodynia. What used to be arousing and satisfying for the couple may now be a source of pain. Communication, self-care, changes in sexual scripts and discussion of non-penetrative alternatives are all important aspects of managing sexual function with chronic pelvic pain syndromes. A multidisciplinary approach with urology, physical therapy and sex therapy is fundamental to targeting the multiple facets of chronic urogenital pain and altered sexual function.
Clinical implications
Similarities in treatment options
IC/BPS and vulvodynia are complex pain syndromes accompanied by multiple pain generators, i.e., HTPFD, where symptom improvement (or lack thereof) rests upon a gauntlet of trial and error therapies. Unfortunately, there is a paucity of literature detailing studies for the treatment of IC/BPS and vulvodynia. Most clinical trials thus far have been limited to small label open or rare placebo-controlled studies, usually too small to yield useful data (43). Nonetheless, some useful information has been garnered from these investigations; and when combined with expert opinion, some interesting similarities between the treatments of IC/BPS and vulvodynia can be identified. These similarities may have potential impact upon streamlining patient care, particularly for those patients who suffer from both ailments. Furthermore, similarities with respect to treatment response may have some bearing on the underlying pathophysiologies of these conditions.
Behavioral modification
First-line treatment for both IC/BPS and vulvodynia employs behavioral modification and self-care measures. Patient empowerment from the initial visit is imperative. This can be accomplished by giving them information pertaining to online resources (e.g., medically accurate websites, advocacy groups), support groups, books, etc.
Stress reduction and relaxation techniques are strongly recommended. Data suggest that women with urogenital pain syndromes have higher levels of stress than healthy controls (60-63). Rothrock et al. conducted a prospective comparison of 45 IC/BPS patients to 31 age-matched controls in order to determine how stress levels in daily life impacts their symptoms. Participants with IC/BPS were asked to track their daily urinary frequency, urgency, bladder pain, and stress for 1 month. Results showed that increased levels of stress were related to greater pain and urgency in the IC/BPS group, but not in the control group (63). Clinical observations suggest that stress exacerbates symptoms and that symptoms tend to worsen stress. As such, multimodal treatments are suggested for both conditions to break frequently identified vicious cycles of pain.
Physical therapy
Physical therapy, once rarely used for the treatment of IC/BPS and vulvodynia, has become a mainstay in the treatment algorithm. Pelvic floor physical therapy is now more widely available and effective in the treatment of both pain syndromes. As previously mentioned, patients with both IC/BPS and vulvodynia often exhibit pain, taut bands and/or trigger points in the muscles of the pelvic floor. Often, patients are found to have involvement of the external muscles of the pelvis, low back, abdomen and lower extremities. Physical therapists with a specialized expertise in chronic urogenital pain employ treatment techniques including internal and external myofascial release and soft tissue mobilization in addition to stabilization, stretching exercises and biofeedback. It is important to note that there is no evidence physical therapy strengthening exercises (Kegel’s) improve symptoms. It has been noted that this type of pelvic floor therapy may in fact worsen pelvic pain conditions (64).
Hartmann et al. performed a retrospective review on women with vulvodynia who were treated with physical therapy (n=24). They reported that 71% showed greater than 50% improvement in symptom reduction (65). Upon interviewing 35 women after completion of a multimodal physical therapy program, Bergeron et al., found that 71% reported complete, great or moderate improvement (66). A small, prospective study of 13 women with vulvodynia completed eight sessions of pelvic floor physical therapy. They demonstrated that physical therapy led to normalization of pelvic floor muscle tone, significant reduction in pain during vaginal palpation, and decreased pain during gynecological exams and sexual intercourse (67).
A large randomized controlled trial (RCT) compared myofascial physical therapy to global therapeutic massage in IC/BPS patients (n=81). Participants were randomized to either group for ten 60-minute sessions over 12 weeks. A total of 59% of the physical therapy group reported moderate or marked improvement, compared to 26% in the massage group on the global response assessment (P=0.0012) (68).
Oral therapies
Many oral therapies prescribed can be used interchangeably for the treatment of both IC/BPS and vulvodynia.
Tricyclic antidepressants (TCA) are commonly used in the treatment of many chronic pain conditions with a neuropathic component. The most common TCAs used in the treatment of IC/BPS and vulvodynia are amitriptyline or nortriptyline and are thought to work by: (I) central and peripheral anticholinergic actions at certain receptor sites; (II) block reuptake of serotonin and norepinephrine; (III) block H1-histaminergic receptors thereby inhibiting mast cell release; (IV) have a sedative effect related to their antihistaminic properties; (V) desensitizes alpha two receptors on central noradrenergic neurons; and (VI) stimulate beta-adrenergic receptors in the bladder (69,70).
Munday et al. reported a 47% complete response to amitriptyline for the treatment of vulvodynia (n=33), however, the investigators were also employing behavioral modification and psychological support (71). Reed et al. evaluated 162 women prescribed a TCA (amitriptyline, desipramine or another tricyclic antidepressant) for vulvodynia. Data show at the first follow-up visit at approximately 3 months, 49 of the 83 women taking the TCAs improved by 50%, compared to 30 of the 79 not taking TCAs (improvement rate =38%, P=0.007). They demonstrated that women taking TCAs for vulvodynia have greater pain improvement than women not taking these medications (72).
Hanno and Wein were the first to report a therapeutic response in an IC/BPS patient being concurrently treated for depression with amitriptyline (73). Results of a placebo-controlled, double-blind trial of 48 patients with IC/BPS showed that after 4 months on amitriptyline (25-75 mg, self-titrating dose), 42% of patients (n=10) on the medication had a greater than 30% decrease in overall symptom score compared to 12.5% of those on placebo (n=3) (P<0.001). Pain and urgency improved in the amitriptyline group compared to placebo (P<0.001) and 63% (n=15) of the amitriptyline group reported good or excellent satisfaction with treatment outcome vs. 4% (n=1) of the placebo group (P<0.001) (74). Additional investigations have shown similar efficacy of TCAs for IC/BPS (75-79).
The biggest problem with the use of TCAs in treating both conditions is the potential adverse effects that can develop. Some adverse effects that may preclude continuation with the medication include anticholinergic effects (constipation, dry mouth, urinary retention), fatigue, weight gain, sexual dysfunction and cardiac arrhythmias (70).
Anticonvulsants are often employed for the treatment of IC/BPS and vulvodynia. Anticonvulsants such as gabapentin, and pregabalin, have been effective in treating diabetic neuropathy, post herpetic neuralgia and other diverse pain syndromes of neuropathic origin. The mechanism of action by which they are thought to improve pain is through binding to the alpha 2-delta subunit of voltage-gated of calcium channels thereby decreasing the release of glutamate, norepinephrine and substance P (80). Pregabalin has also exhibited anxiolytic effects in RCTs of generalized anxiety disorder, which may prove beneficial for patients with chronic urogenital pain conditions (81,82).
Ben-David and Friedman first published efficacy with using gabapentin for treating women with vulvodynia (n=17). A total of 82% (n=14) had partial or complete symptom relief with gabapentin therapy by 2 to 4 weeks of treatment. Follow-up extended between 26 and 32 weeks without any reported cases of late failure of therapy (83). A retrospective chart review of 152 women with vulvodynia treated with gabapentin by Harris and colleagues showed 98 (64%) had resolution of at least 80% of their symptoms, and 49 (32%) did not have adequate response. They also found that patients with a longer period of untreated illness seemed to have a less favorable response to therapy (P value not less than 0.05) (84). Further reports show that gabapentin improves vulvodynia symptoms either as monotherapy or as adjuvant therapy (85,86). Currently underway is the first double-blind, placebo-controlled, crossover study to evaluate the efficacy of extended release gabapentin, up to 300 mg/day in the treatment of vulvodynia (87).
The use of gabapentin in the treatment of IC/BPS is mainly from anecdotal reports. Sasaki et al. evaluated 21 patients with refractory of genitourinary pain, including eight with interstitial cystitis and two with vulvar pain. Ten of the 21 patients treated with gabapentin reported subjective improvement in their symptoms; five of the eight patients with IC/BPS reported improvement (88).
Antihistamines have been used in the treatment of IC/BPS for decades. The most common medication used is hydroxyzine, which can block neuronal activation of mast cells (89). Sedation is a common side effect with this medication, so some clinicians have begun to use montelukast, a leukotriene receptor antagonist. Montelukast at 10 mg/day has been used in the treatment of both IC/BPS and vulvodynia with promising results (90,91). Authors proposed that leukotriene receptor antagonist has antihistamine action and may modulate the exaggerated proinflammatory activity.
Conclusions
Many similarities exist between IC/BPS and vulvodynia including clinical presentation, co-morbid conditions, and similar responses to medical interventions. Closer examination reveals commonalities in embryologic origins. Recognition of possible neuropathic involvement and central sensitization in conjunction with fMRI changes has far-reaching implications for improving treatment options. Further research is needed to determine whether these conditions are manifestations of a more global chronic urogenital pain syndrome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- McCormack WM. Two urogenital sinus syndromes. Interstitial cystitis and focal vulvitis. J Reprod Med 1990;35:873-6. [PubMed]
- Fitzpatrick CC, DeLancey JO, Elkins TE, et al. Vulvar vestibulitis and interstitial cystitis: a disorder of urogenital sinus-derived epithelium? Obstet Gynecol 1993;81:860-2. [PubMed]
- Healey A. Embryology of the female reproductive tract. In: Mann GS, Blair JC, Garden AS, editors. Imaging of Gynecological Disorders in Infants and Children. Berlin Heidelberg: Springer-Verlag, 2012:21-30.
- Tanaka ST, Ishii K, Demarco RT, et al. Endodermal origin of bladder trigone inferred from mesenchymal-epithelial interaction. J Urol 2010;183:386-91. [PubMed]
- Hanno PM. Painful bladder syndrome/interstitial cystitis and related disorders. In: Wein AJ, editor. Campbell-Walsh Urology. 9th ed. Philadelphia: Saunders, 2007:330-70.
- Sant GR, Kempuraj D, Marchand JE, et al. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology 2007;69:34-40. [PubMed]
- Chaim W, Meriwether C, Gonik B, et al. Vulvar vestibulitis subjects undergoing surgical intervention: a descriptive analysis and histopathological correlates. Eur J Obstet Gynecol Reprod Biol 1996;68:165-8. [PubMed]
- Bohm-Starke N, Hilliges M, Falconer C, et al. Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest 1998;46:256-60. [PubMed]
- Letourneau R, Pang X, Sant GR, et al. Intragranular activation of bladder mast cells and their association with nerve processes in interstitial cystitis. Br J Urol 1996;77:41-54. [PubMed]
- Bornstein J, Goldschmid N, Sabo E. Hyperinnervation and mast cell activation may be used as histopathologic diagnostic criteria for vulvar vestibulitis. Gynecol Obstet Invest 2004;58:171-8. [PubMed]
- Dell JR, Mokrzycki ML, Jayne CJ. Differentiating interstitial cystitis from similar conditions commonly seen in gynecologic practice. Eur J Obstet Gynecol Reprod Biol 2009;144:105-9. [PubMed]
- Myers DL, Aguilar VC. Gynecologic manifestations of interstitial cystitis. Clin Obstet Gynecol 2002;45:233-41. [PubMed]
- Wesselmann U. Neurogenic inflammation and chronic pelvic pain. World J Urol 2001;19:180-5. [PubMed]
- Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Ann Neurol 2013;74:630-6. [PubMed]
- Ness TJ, Lloyd LK, Fillingim RB. An endogenous pain control system is altered in subjects with interstitial cystitis. J Urol 2014;191:364-70. [PubMed]
- Pukall CF, Binik YM, Khalifé S, et al. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. Pain 2002;96:163-75. [PubMed]
- Giesecke J, Reed BD, Haefner HK, et al. Quantitative sensory testing in vulvodynia patients and increased peripheral pressure pain sensitivity. Obstet Gynecol 2004;104:126-33. [PubMed]
- Johannesson U, de Boussard CN, Brodda Jansen G, et al. Evidence of diffuse noxious inhibitory controls (DNIC) elicited by cold noxious stimulation in patients with provoked vestibulodynia. Pain 2007;130:31-9. [PubMed]
- Pukall CF, Strigo IA, Binik YM, et al. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain 2005;115:118-27. [PubMed]
- Hampson JP, Reed BD, Clauw DJ, et al. Augmented central pain processing in vulvodynia. J Pain 2013;14:579-89. [PubMed]
- Schweinhardt P, Kuchinad A, Pukall CF, et al. Increased gray matter density in young women with chronic vulvar pain. Pain 2008;140:411-9. [PubMed]
- Kilpatrick LA, Kutch JJ, Tillisch K, et al. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol 2014;192:947-55. [PubMed]
- Farmer MA, Huang L, Martucci K, et al. Brain White Matter Abnormalities in Female Interstitial Cystitis/Bladder Pain Syndrome: A MAPP Network Neuroimaging Study. J Urol 2015;194:118-26. [PubMed]
- Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology 2001;57:47-55. [PubMed]
- Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology 2007;69:24-33. [PubMed]
- Amandusson Å, Blomqvist A. Estrogenic influences in pain processing. Front Neuroendocrinol 2013;34:329-49. [PubMed]
- Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev 2000;24:485-501. [PubMed]
- Gardella B, Iacobone AD, Porru D, et al. Effect of local estrogen therapy (LET) on urinary and sexual symptoms in premenopausal women with interstitial cystitis/bladder pain syndrome (IC/BPS). Gynecol Endocrinol 2015.1-5. [Epub ahead of print]. [PubMed]
- Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol 2005;174:1832-6. [PubMed]
- Burrows LJ, Goldstein AT. The treatment of vestibulodynia with topical estradiol and testosterone. Sex Med 2013;1:30-3. [PubMed]
- Bouchard C, Brisson J, Fortier M, et al. Use of oral contraceptive pills and vulvar vestibulitis: a case-control study. Am J Epidemiol 2002;156:254-61. [PubMed]
- Johannesson U, Sahlin L, Masironi B, et al. Steroid receptor expression in the vulvar vestibular mucosa--effects of oral contraceptives and menstrual cycle. Contraception 2007;76:319-25. [PubMed]
- Edgardh K, Abdelnoor M. Vulvar vestibulitis and risk factors: a population-based case-control study in Oslo. Acta Derm Venereol 2007;87:350-4. [PubMed]
- Goetsch MF, Morgan TK, Korcheva VB, et al. Histologic and receptor analysis of primary and secondary vestibulodynia and controls: a prospective study. Am J Obstet Gynecol 2010;202:614.e1-8.
- Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 2011;186:540-4. [PubMed]
- Eppsteiner E, Boardman L, Stockdale CK. Vulvodynia. Best Pract Res Clin Obstet Gynaecol 2014;28:1000-12. [PubMed]
- Peters K, Girdler B, Carrico D, et al. Painful bladder syndrome/interstitial cystitis and vulvodynia: a clinical correlation. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:665-9. [PubMed]
- Harlow BL, Kunitz CG, Nguyen RH, et al. Prevalence of symptoms consistent with a diagnosis of vulvodynia: population-based estimates from 2 geographic regions. Am J Obstet Gynecol 2014;210:40.e1-8.
- Gardella B, Porru D, Ferdeghini F, et al. Insight into urogynecologic features of women with interstitial cystitis/painful bladder syndrome. Eur Urol 2008;54:1145-51. [PubMed]
- Reed BD, Harlow SD, Sen A, et al. Relationship between vulvodynia and chronic comorbid pain conditions. Obstet Gynecol 2012;120:145-51. [PubMed]
- Gordon AS, Panahian-Jand M, Mccomb F, et al. Characteristics of women with vulvar pain disorders: responses to a Web-based survey. J Sex Marital Ther 2003;29 Suppl 1:45-58. [PubMed]
- Nguyen RH, Veasley C, Smolenski D. Latent class analysis of comorbidity patterns among women with generalized and localized vulvodynia: preliminary findings. J Pain Res 2013;6:303-9. [PubMed]
- Groysman V. Vulvodynia: new concepts and review of the literature. Dermatol Clin 2010;28:681-96. [PubMed]
- Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol 2010;184:1358-63. [PubMed]
- Cervigni M, Natale F. Gynecological disorders in bladder pain syndrome/interstitial cystitis patients. Int J Urol 2014;21 Suppl 1:85-8. [PubMed]
- Yong PJ, Mui J, Allaire C, et al. Pelvic floor tenderness in the etiology of superficial dyspareunia. J Obstet Gynaecol Can 2014;36:1002-9. [PubMed]
- Sargeant HA, O'Callaghan FV. The impact of chronic vulval pain on quality of life and psychosocial well-being. Aust N Z J Obstet Gynaecol 2007;47:235-9. [PubMed]
- Granot M, Lavee Y. Psychological factors associated with perception of experimental pain in vulvar vestibulitis syndrome. J Sex Marital Ther 2005;31:285-302. [PubMed]
- Brotto LA, Sadownik LA, Thomson S, et al. A comparison of demographic and psychosexual characteristics of women with primary versus secondary provoked vestibulodynia. Clin J Pain 2014;30:428-35. [PubMed]
- McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine (Phila Pa 1976) 2002;27:2564-73. [PubMed]
- López-Martínez AE, Esteve-Zarazaga R, Ramírez-Maestre C. Perceived social support and coping responses are independent variables explaining pain adjustment among chronic pain patients. J Pain 2008;9:373-9. [PubMed]
- Sinclair VG, Wallston KA. Predictors of improvement in a cognitive-behavioral intervention for women with rheumatoid arthritis. Ann Behav Med 2001;23:291-7. [PubMed]
- Castro MM, Daltro C, Kraychete DC, et al. The cognitive behavioral therapy causes an improvement in quality of life in patients with chronic musculoskeletal pain. Arq Neuropsiquiatr 2012;70:864-8. [PubMed]
- Seminowicz DA, Shpaner M, Keaser ML, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain 2013;14:1573-84. [PubMed]
- Landry T, Bergeron S, Dupuis MJ, et al. The treatment of provoked vestibulodynia: a critical review. Clin J Pain 2008;24:155-71. [PubMed]
- Masheb RM, Kerns RD, Lozano C, et al. A randomized clinical trial for women with vulvodynia: Cognitive-behavioral therapy vs. supportive psychotherapy. Pain 2009;141:31-40. [PubMed]
- Bogart LM, Suttorp MJ, Elliott MN, et al. Prevalence and correlates of sexual dysfunction among women with bladder pain syndrome/interstitial cystitis. Urology 2011;77:576-80. [PubMed]
- Peters KM, Killinger KA, Carrico DJ, et al. Sexual function and sexual distress in women with interstitial cystitis: a case-control study. Urology 2007;70:543-7. [PubMed]
- Nickel JC, Tripp D, Teal V, et al. Sexual function is a determinant of poor quality of life for women with treatment refractory interstitial cystitis. J Urol 2007;177:1832-6. [PubMed]
- Arnold LD, Bachmann GA, Rosen R, et al. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol 2006;107:617-24. [PubMed]
- Khandker M, Brady SS, Vitonis AF, et al. The influence of depression and anxiety on risk of adult onset vulvodynia. J Womens Health (Larchmt) 2011;20:1445-51. [PubMed]
- Lutgendorf SK, Kreder KJ, Rothrock NE, et al. Stress and symptomatology in patients with interstitial cystitis: a laboratory stress model. J Urol 2000;164:1265-9. [PubMed]
- Rothrock NE, Lutgendorf SK, Kreder KJ, et al. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology 2001;57:422-7. [PubMed]
- Hanno PM, Erickson D, Moldwin R, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol 2015;193:1545-53. [PubMed]
- Hartmann EH, Nelson C. The Perceived Effectiveness of Physical Therapy Treatment on Women Complaining of Vulvar Pain and Diagnosed With Either Vulvar Vestibulitis Syndrome or Dysesthetic Vulvodynia. J Sect Women's Health 2001;25:13-18.
- Bergeron S, Brown C, Lord MJ, et al. Physical therapy for vulvar vestibulitis syndrome: a retrospective study. J Sex Marital Ther 2002;28:183-92. [PubMed]
- Goldfinger C, Pukall CF, Gentilcore-Saulnier E, et al. A prospective study of pelvic floor physical therapy: pain and psychosexual outcomes in provoked vestibulodynia. J Sex Med 2009;6:1955-68. [PubMed]
- FitzGerald MP, Payne CK, Lukacz ES, et al. Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. J Urol 2012;187:2113-8. [PubMed]
- Baldessarini RJ. Drugs and the treatment of psychiatric disorders. In: Gilman AG, Rall TW, Nies AS, et al, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York: Pergamon Press, 1990:383-435.
- Dworkin RH, O'Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007;132:237-51. [PubMed]
- Munday PE. Response to treatment in dysaesthetic vulvodynia. J Obstet Gynaecol 2001;21:610-3. [PubMed]
- Reed BD, Caron AM, Gorenflo DW, et al. Treatment of vulvodynia with tricyclic antidepressants: efficacy and associated factors. J Low Genit Tract Dis 2006;10:245-51. [PubMed]
- Hanno PM, Wein AJ. Medical treatment of interstitial cystitis (other than Rimso-50/Elmiron). Urology 1987;29:22-6. [PubMed]
- van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol 2004;172:533-6. [PubMed]
- Renshaw DC. Desipramine for interstitial cystitis. JAMA 1988;260:341. [PubMed]
- Pranikoff K, Constantino G. The use of amitriptyline in patients with urinary frequency and pain. Urology 1998;51:179-81. [PubMed]
- Kirkemo A, Peabody M, Diokno AC, et al. Associations among urodynamic findings and symptoms in women enrolled in the Interstitial Cystitis Data Base (ICDB) Study. Urology 1997;49:76-80. [PubMed]
- van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol 2005;174:1837-40. [PubMed]
- Hertle L, van Ophoven A. Long-term results of amitriptyline treatment for interstitial cystitis. Aktuelle Urol 2010;41 Suppl 1:S61-5. [PubMed]
- Taylor CP. The biology and pharmacology of calcium channel alpha2-delta proteins Pfizer Satellite Symposium to the 2003 Society for Neuroscience Meeting. Sheraton New Orleans Hotel, New Orleans, LA November 10, 2003. CNS Drug Rev 2004;10:183-8. [PubMed]
- Montgomery SA, Tobias K, Zornberg GL, et al. Efficacy and safety of pregabalin in the treatment of generalized anxiety disorder: a 6-week, multicenter, randomized, double-blind, placebo-controlled comparison of pregabalin and venlafaxine. J Clin Psychiatry 2006;67:771-82. [PubMed]
- Rickels K, Pollack MH, Feltner DE, et al. Pregabalin for treatment of generalized anxiety disorder: a 4-week, multicenter, double-blind, placebo-controlled trial of pregabalin and alprazolam. Arch Gen Psychiatry 2005;62:1022-30. [PubMed]
- Ben-David B, Friedman M. Gabapentin therapy for vulvodynia. Anesth Analg 1999;89:1459-60. [PubMed]
- Harris G, Horowitz B, Borgida A. Evaluation of gabapentin in the treatment of generalized vulvodynia, unprovoked. J Reprod Med 2007;52:103-6. [PubMed]
- Ventolini G, Barhan S, Duke J. Vulvodynia, a step-wise therapeutic prospective cohort study. J Obstet Gynaecol 2009;29:648-50. [PubMed]
- Jeon Y, Kim Y, Shim B, et al. A retrospective study of the management of vulvodynia. Korean J Urol 2013;54:48-52. [PubMed]
- Brown CS, Foster DC, Wan JY, et al. Rationale and design of a multicenter randomized clinical trial of extended release gabapentin in provoked vestibulodynia and biological correlates of response. Contemp Clin Trials 2013;36:154-65. [PubMed]
- Sasaki K, Smith CP, Chuang YC, et al. Oral gabapentin (neurontin) treatment of refractory genitourinary tract pain. Tech Urol 2001;7:47-9. [PubMed]
- Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology 1997;49:108-10. [PubMed]
- Bouchelouche K, Nordling J, Hald T, et al. Treatment of interstitial cystitis with montelukast, a leukotriene D(4) receptor antagonist. Urology 2001;57:118. [PubMed]
- Kamdar N, Fisher L, MacNeill C. Improvement in vulvar vestibulitis with montelukast. J Reprod Med 2007;52:912-6. [PubMed]


