Increased bladder permeability in interstitial cystitis/painful bladder syndrome
Introduction
The urinary bladder functions to collect urine and then to expel it under the voluntary control. It generally performs this function well until later life when problems often arise with voluntary control (incontinence), urgency/frequency [overactive bladder (OAB)] or urgency-frequency-pain [interstitial cystitis/painful bladder syndrome (IC/PBS)], although the latter can occur at any age (1). Urine contains a number of noxious substances that need to be kept out of the bladder tissue. Therefore, the bladder urothelium evolved to be the most impermeable membrane in the mammalian body (2). In this article we review the structure of the urothelium, particularly discussing how the structure of the urothelium contributes to its unique function how it can fail in disease, and how loss of barrier function may be a major factor in bladder disorders. Our hypothesis is that the loss of impermeability of the bladder urothelium is not only responsible for the symptoms of pain and urgency but also is the trigger for degenerative changes often seen in the urothelium that may be irreversible. We also will show that the urothelium can become permeable both as the result of endogenous factors (i.e., neural modulation) as well as from failure of the bladder defenses against urine substances such as organic cations. Thus, instead of viewing the organs of the lower abdomen in isolation, they must be viewed as an interconnected system and that disorders of one organ may perturb the homeostasis of others.
Structure of urothelium and its barrier function
Anatomical structure
The urothelium consist of three layers of cells (2-4). Adjacent to the lamina propria is a layer of basal cells that likely contain stem cells with each stem cell being responsible for a monoclonal patch (5,6). Increasing knowledge of urothelial stem cells is identifying markers and the lineage of urothelium (7,8). Virtually all cell division is restricted to the basal layer. Above the basal cells is a layer of partially differentiated intermediate cells. Their function is to rapidly terminally differentiate when one of the highly specialized apical or umbrella cells is lost. This outer layer comprises the main protective barrier against urine. These cells are relatively long-lived (turnover more than 6 months) (9), so the normal level of mitosis in the healthy bladder is very low (10). In case of injury or infection, in which the apical cells typically slough off and carry infecting bacteria with them, the intermediate layer cells rapidly differentiate into apical layer cells and cell division is ramped up in the basal layer and replaces the intermediate layer cells that differentiated into apical layer cells (11). Even before the intermediate cell is fully differentiated into an umbrella cell, it forms tight junctions (12), which illustrates the importance of the urothelial barrier. When repairing damage, the normally quiescent urothelium expresses among the highest levels of cell division of any epithelium in the body (13).
The apical cells are highly evolved for their function of providing an impermeable barrier to urine in the bladder lumen. The barrier function is comprised of multiple defensive molecules—tight junctions, uroplakin plaques, and a dense layer of glycosaminoglycan (GAG) on the apical surface (the “GAG layer”) The apical cells highly express tight junction proteins on their basolateral surfaces (12,14,15) that provide a barrier to urine passing between cells. The apical surface of the apical cells also is composed of plaques of uroplakins, which are tetraspannin proteins that form hydrophobic plaques on the cell surface (16,17). Loss of uroplakin in knockout mice increases the permeability about 2-fold (18). The urothelium also expresses a unique means for stretching and contracting. When contracted, small segments of membrane are removed from the cell surface and then stored in specialized vesicles, and when the bladder again is stretched, this stored membrane is reintegrated with the luminal surface (19).
Figure 1 schematically illustrates the structure of the bladder and illustrates the relationships among the urothelium and nerves and blood vessels. The urothelium itself is not enervated nor do capillaries penetrate within it. Therefore nutrients must diffuse across the lamina propria, a relatively large distance as compared to the vascularization observed in other tissues. Urothelial cells also have some very unique properties. They are both immune cells and also have characteristics of neurosensory cells in the form of receptors that are typically found in neural cells (20-22).
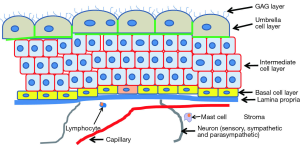
The GAG layer
The apical surface is also densely coated with a layer of GAG that comprises a major component of the permeability barrier. This layer can be seen with Alcian blue staining (23) or by immunohistochemical staining for chondroitin sulfate (23,24). It has long been suggested that this GAG layer was substantially responsible for bladder impermeability (25,26). The GAG layer visualized by Alcian blue and immunochemical staining of bladder tissue for GAG layer components are illustrated in papers from our group (see figures in all three papers, all of which can be accessed from PubMed) (23,27,28). The presence of the GAG layer was demonstrated previously by Hurst and co-workers (28-32), who characterized its composition and demonstrated that its removal with dilute HCl, which causes loss of the apical cells within 24 hrs, leads to enhanced permeability to 86Rb+, a K+ mimetic. Restoring the GAG with exogenous GAG (e.g., intravesical administration of chondroitin sulfate) restores impermeability to 86Rb+ to baseline levels (33) and also substantially inhibits the recruitment of inflammatory cells to permeabilized areas (23). The reason that excluding K+ is important is that one theory for the origin of pain in the bladder in interstitial cystitis (IC) is that penetration of bladder tissue by K+ depolarizes sensory nerves (34-36). This observation led to the development of the potassium sensitivity test (PST) in which 0.1 m KCl (but not 0.1 m NaCl) instilled into the bladder elicits a pain response in most IC patients (35) as well as about 40% of patients diagnosed with OAB (37). These earlier findings were completely confirmed recently by Janssen and co-workers, who also showed that without introducing any damage to the urothelium other than to digest the GAG layer with chondroitinase ABCase the permeability could be increased in a cell culture model (24). Recently our group has demonstrated that in vivo digestion of the GAG layer with chondroitinase ABC led to a decrease of the transepithelial electrical resistance (TEER) as measured in the Ussing chamber. In control and sham treated rat bladders, the TEER measurements were means of 2,524±1,117 vs. 2,623±1,124 vs. 1,175±518 Ωcm2 and 1,080±687 Ωcm2 in the protamine sulfate-treated and chondroitinase-treated rat bladders (P=0.0016 and P=0.0039 respectively). Similar differences were seen in dextran permeability. Thus, treatment with organic cations and specific removal of the GAG layer both produce permeability.
Loss of barrier function in human bladder disorders
IC and loss of barrier function
IC is the disorder most closely associated with loss of bladder permeability. Although IC can appear at any age, it becomes more common in women, during middle age and does not seem to vary with race or ethnicity (38). IC was initially thought to be quite rare following its description by Hunner (39). At that time there was an objective diagnostic criterion, namely the presence of Hunner’s lesion or ulcer upon cystoscopy. As time went by and the disorder was investigated in more detail came the realization that a great many more patients were very similar to the classic description, but had more diffuse symptoms. In 1978 Messing and Stamey (40) introduced a new diagnostic criterion, namely the observation of petechial bleeding on hydrodistention. This was incorporated into the research criteria promulgated by the NIDDK in 1987 (41), but these rapidly became the clinical definition of the disorder in spite of the specific intent of the NIDDK that this not be the case. This conflict between the strict research definition that was intended to improve the power of clinical trials and a looser definition that seemed to be emerging in practice led to a re-examination of the diagnostic criteria (42) and a further broadening of the definition such that the disorder is no longer rare (1). It is diagnosed by the triad of pain, urgency and frequency and is a diagnosis of exclusion (43-45), and no objective diagnostic criteria have stood the test. Although some investigators claim that objective criteria for diagnosis can be derived from histology (46-48), these have not proven specific or sensitive enough for diagnostic use. As a consequence, the syndrome is undoubtedly heterogeneous, which greatly complicates clinical trials because non-responders are automatically included in any clinical trial (49).
A number of investigators have reported changes in the urothelium that suggest loss of the barrier function may be a factor, at least in many patients. In the classic ulcer patients typically show a very thin urothelium that even erodes away in places, leaving ulcers that can be plainly seen by cystoscopy (43). In the more common non-ulcer form histopathologic changes, including loss of umbrella cells (28,46-48,50), which leads to loss of the GAG layer, have been reported. However, some patients show nearly normal-appearing urothelium (28,47,48). Whether this heterogeneity of loss of the GAG layer is a sampling difference, that is the loss of impermeability is focal and just was not sampled, or whether it is widespread in some bladders and those that do not show this loss represent a different class of patients is not known. However, a recent study in a feline model of IC by our group in which large sections of bladder were available for analysis showed considerable heterogeneity in the molecular pathology of the GAG layer, uroplakin and cell adhesion molecule distribution (51), suggesting that the changes reported in human IC biopsies may be focal. Wider sampling therefore might prove more useful diagnostically, but imposes a greater burden on patients. Several investigators have suggested an altered differentiation program in the IC urothelium could be responsible for these histopathologic changes (29,52-55). Although a number of papers have been published on urothelial differentiation (56-62), the flaw in IC is unknown. In recent years it has been recognized that IC may represent a manifestation of a wider problem called painful bladder syndrome (PBS) that likely is even more heterogeneous than IC itself (1,44,63,64). Evidence for a wider etiology is the observation that patients with IC have a roughly 70% comorbidity with irritable bowel syndrome (IBS). Recent work has shown a link between bowel and bladder such that dysfunction with one organ can lead to dysfunction in the other (65,66). This suggests that the origins of the changes in the urothelium could be quite complex and could result from both neurally modulated mechanisms as well as from intravesical toxins.
Parsons and coworkers in 1983 suggested that a defect in the bladder barrier function was the root cause of IC symptoms (67). It certainly is an attractive theory because it both suggests a diagnostic criterion and therapy. Interestingly, although Parsons demonstrated in 1991 that IC patients absorbed a significantly higher amount of urea instilled into the bladder than did controls (68) the theory has remained controversial. Other, indirect evidence has supported the increased permeability of the bladders of IC patients. A high percentage of IC patients exhibit a positive potassium sensitivity test as compared to controls (43,69,70), and IC patients show a slower elimination of fluorescein administered intravenously, presumably due to resorption through the bladder (71). As discussed above considerable evidence from several laboratories suggests that the urothelium has adopted an aberrant differentiation program that could lead to loss of terminal differentiation of the apical cells or altered protein expression that could lead to loss of the barrier function with increased permeability. Clearly, this is an area for further research, given the heterogeneity within the IC/PBS population. The question of whether phenotypically these patients divide into “leakers” and “non-leakers” that may respond completely differently to various treatments is unresolved (72). In our laboratory we have recently shown (unpublished) that bladder permeability in rats can be measured directly by instilling fluorescein into the bladder and sampling a small volume every 2 minutes. This should be able to be performed on humans as well.
GAG replenishment therapy
Given that we demonstrated a deficiency in the GAG layer in at least some IC patients and that exogenous GAG preferentially adheres to damaged urothelium (33,73), it is surprising that most clinical trials of GAG replacement show that although some patients clearly benefit and often have nearly complete remissions, other patients derive very little benefit (74-80). Clinical trials are often ambiguous because most are underpowered to detect an effect that occurs in only about 50% of the treated population. Similar observations apply to other therapies (81,82). GAG has been delivered orally as well as intravesically. The only GAG to be delivered orally is pentosan polysulfate (Elmiron), which is about 7,000 Da molecular weight and a few percent of the oral dose ends up in circulation, where it is apparently cleared into the urine. There are several possible explanations for the failure of GAG replenishment therapy to be more successful. One could be that the disorder is heterogeneous and comprised of “leakers” and “non-leakers”. A second is the disorder could be progressive, and the non-responders could represent an end stage where progressive damage has altered the bladder to the degree that it can no longer respond. Third, the optimal dosing regimen is incorrect and either the agent needs to be delivered more frequently or the dosing is too frequent and is producing side effects.
Bladder-bowel intercommunication
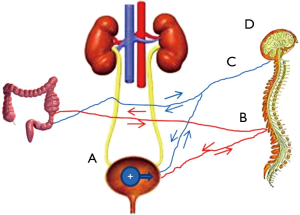
One of the more interesting recent developments in research into the clinical disorder of PBS/IC is the link between the bladder and the bowel. It has long been known that IBS shows a high comorbidity with IC/PBS, and a recent meta-analysis confirmed this finding, albeit with significant criticisms of experimental design (83). Other disorders with significant comorbidity include fibromyalgia and other generalized lower abdomen pain syndromes. Buffington attempted to identify a stress-related subset of patients (66) because stress was shown to be a major factor in feline IC (84). Whether the stress experienced by these patients is a product of the disorder or a causative factor in the disorder remains to be resolved. As is discussed above these findings have led some to suspect that IC actually represents a urologic manifestation of a more generalized pelvic pain syndrome and possibly whether there is a causative relationship between bowel and bladder symptoms. Supporting the latter hypothesis have been a number of recent papers demonstrating visceral organ crosstalk, as summarized in a recent review (85). The main question is whether the intercommunication arises from cellular communication by migratory cells such as mast cells or whether information is transmitted through neural communication and release of neurosecretory proteins that can alter one organ according to the status of another. There is evidence for both theories. Mast cells were implicated in a recent study that showed the cross-communication was not observed in Kit–/– mice lacking mast cells, but whether this was an effect of the loss of kit or of mast cells is unclear. Recent exciting work by Kevin Tracey and colleagues has shown that inflammatory cells such as mast cells and macrophage respond to neural signals in a kind of neuroinflammation, the so-called inflammatory reflex (86-90). Interestingly, an anti-inflammatory network also has been identified (90,91). Up-regulation of innervation has been reported in IC (92), as upregulation of neuropilins and VEGF receptors.(93). This intercommunication is not restricted to IC and IBS. Constipation can worsen symptoms of OAB, and treatment of OAB with antimuscarinics can worsen constipation (85). Our group has recently shown that in rats, induction of bowel inflammation with trinitrobenzenesulfonic acid produces increased bladder permeability within 24 hrs as measured ex vivo in the Ussing chamber, and conversely, induction of bladder permeability with dilute protamine sulfate (which did not produce overt physical damage) resulted in increased bowel permeability (94). Moreover, the increased permeability was also detectable by magnetic resonance imaging (MRI), suggesting the technique could be used clinically to stratify patients according to permeability and to monitor response to therapy (95).
Directions of future research and summary
Figure 2 summarizes the mechanisms that could produce bladder permeability. It should be obvious from the figure and discussion that the bladder, bowel (and possibly other organs as well), cytokine-responding and secreting cells, and the neuroendocrine system form a complex and interacting system that can no longer be considered as individual parts in isolation. Clearly one of the most pressing clinical needs is to better be able to classify patients. Finding effective treatments would be greatly improved if patients could be stratified into more homogeneous groups for clinical trials. An effective method of measuring bladder permeability could also represent an important step forward in this regard because it could offer an improved diagnostic as well as an objective measure of response to therapy that could be used to optimize therapies. Our group has recently shown that MRI can clearly demonstrate increased bladder permeability that correlates with patient condition (96). The mechanisms by which information concerning the status of one pelvic organ is communicated to another, and how this information affects the other organ is critical to know and is being actively investigated by a number of groups. Finally, understanding the role of the brain, stress, and past life experiences also needs to be investigated.
Acknowledgements
Funding: This work was supported, in part, by a grant from the National Institute of Diabetes and Digestive Kidney Diseases, National Institutes of Health, P20 DK097799 (REH).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kusek JW, Nyberg LM. The epidemiology of interstitial cystitis: is it time to expand our definition? Urology 2001;57:95-9. [PubMed]
- Hicks RM, Ketterer B, Warren RC. The ultrastructure and chemistry of the luminal plasma membrane of the mammalian urinary bladder: a structure with low permeability to water and ions. Philos Trans R Soc Lond B Biol Sci 1974;268:23-38. [PubMed]
- Congiu T, Radice R, Raspanti M, et al. The 3D structure of the human urinary bladder mucosa: a scanning electron microscopy study. J Submicrosc Cytol Pathol 2004;36:45-53. [PubMed]
- Jost SP, Gosling JA, Dixon JS. The morphology of normal human bladder urothelium. J Anat 1989;167:103-15. [PubMed]
- Tsai YC, Simoneau AR, Spruck CH 3rd, et al. Mosaicism in human epithelium: macroscopic monoclonal patches cover the urothelium. J Urol 1995;153:1697-700. [PubMed]
- Gaisa NT, Graham TA, McDonald SA, et al. The human urothelium consists of multiple clonal units, each maintained by a stem cell. J Pathol 2011;225:163-71. [PubMed]
- Hatina J, Schulz WA. Stem cells in the biology of normal urothelium and urothelial carcinoma. Neoplasma 2012;59:728-36. [PubMed]
- Ho PL, Kurtova A, Chan KS. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat Rev Urol 2012;9:583-94. [PubMed]
- Cooper EH. The biology of bladder cancer. Ann R Coll Surg Engl 1972;51:1-16. [PubMed]
- Limas C, Bigler A, Bair R, et al. Proliferative activity of urothelial neoplasms: comparison of BrdU incorporation, Ki67 expression, and nucleolar organiser regions. J Clin Pathol 1993;46:159-65. [PubMed]
- Schilling JD, Mulvey MA, Hultgren SJ. Dynamic interactions between host and pathogen during acute urinary tract infections. Urology 2001;57:56-61. [PubMed]
- Kreft ME, Sterle M, Veranic P, et al. Urothelial injuries and the early wound healing response: tight junctions and urothelial cytodifferentiation. Histochem Cell Biol 2005;123:529-39. [PubMed]
- Farsund T. Cell kinetics of mouse urinary bladder epithelium. II. Changes in proliferation and nuclear DNA content during necrosis regeneration, and hyperplasia caused by a single dose of cyclophosphamide. Virchows Arch B Cell Pathol 1976;21:279-98. [PubMed]
- Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 2009;297:F1477-501. [PubMed]
- Varley CL, Garthwaite MA, Cross W, et al. PPARgamma-regulated tight junction development during human urothelial cytodifferentiation. J Cell Physiol 2006;208:407-17. [PubMed]
- Sun TT, Zhao H, Provet J, et al. Formation of asymmetric unit membrane during urothelial differentiation. Mol Biol Rep 1996;23:3-11. [PubMed]
- Wu XR, Lin JH, Walz T, et al. Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J Biol Chem 1994;269:13716-24. [PubMed]
- Hu P, Deng FM, Liang FX, et al. Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol 2000;151:961-72. [PubMed]
- Truschel ST, Wang E, Ruiz WG, et al. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 2002;13:830-46. [PubMed]
- Gonzalez EJ, Merrill L, Vizzard MA. Bladder sensory physiology: neuroactive compounds and receptors, sensory transducers, and target-derived growth factors as targets to improve function. Am J Physiol Regul Integr Comp Physiol 2014;306:R869-78. [PubMed]
- Le PT, Pearce MM, Zhang S, et al. IL22 regulates human urothelial cell sensory and innate functions through modulation of the acetylcholine response, immunoregulatory cytokines and antimicrobial peptides: assessment of an in vitro model. PLoS One 2014;9:e111375. [PubMed]
- Merrill L, Girard B, Arms L, et al. Neuropeptide/Receptor expression and plasticity in micturition pathways. Curr Pharm Des 2013;19:4411-22. [PubMed]
- Engles CD, Hauser PJ, Abdullah SN, et al. Intravesical chondroitin sulfate inhibits recruitment of inflammatory cells in an acute acid damage "leaky bladder" model of cystitis. Urology 2012;79:483.e13-7.
- Janssen DA, van Wijk XM, Jansen KC, et al. The distribution and function of chondroitin sulfate and other sulfated glycosaminoglycans in the human bladder and their contribution to the protective bladder barrier. J Urol 2013;189:336-42. [PubMed]
- Lilly JD, Parsons CL. Bladder surface glycosaminoglycans is a human epithelial permeability barrier. Surg Gynecol Obstet 1990;171:493-6. [PubMed]
- Parsons CL, Boychuk D, Jones S, et al. Bladder surface glycosaminoglycans: an epithelial permeability barrier. J Urol 1990;143:139-42. [PubMed]
- Hauser PJ, Dozmorov MG, Bane BL, et al. Abnormal expression of differentiation related proteins and proteoglycan core proteins in the urothelium of patients with interstitial cystitis. J Urol 2008;179:764-9. [PubMed]
- Slobodov G, Feloney M, Gran C, et al. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol 2004;171:1554-8. [PubMed]
- Hurst RE, Moldwin RM, Mulholland SG. Bladder defense molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology 2007;69:17-23. [PubMed]
- Hurst RE, Roy JB, Parsons CL. The role of glycosaminoglycans in normal bladder physiology and the pathophysiology of interstitial cystitis. In: Sant GR, editor. Interstitial Cystitis. Philadelphia: Lippincott-Raven, 1997:93-100.
- Hurst RE, Roy JB, Min KW, et al. A deficit of chondroitin sulfate proteoglycans on the bladder uroepithelium in interstitial cystitis. Urology 1996;48:817-21. [PubMed]
- Hurst RE, Zebrowski R. Identification of proteoglycans present at high density on bovine and human bladder luminal surface. J Urol 1994;152:1641-5. [PubMed]
- Hauser PJ, Buethe DA, Califano J, et al. Restoring barrier function to acid damaged bladder by intravesical chondroitin sulfate. J Urol 2009;182:2477-82. [PubMed]
- Parsons CL. Interstitial cystitis and lower urinary tract symptoms in males and females-the combined role of potassium and epithelial dysfunction. Rev Urol 2002;4 Suppl 1:S49-55. [PubMed]
- Bernie JE, Hagey S, Albo ME, et al. The intravesical potassium sensitivity test and urodynamics: implications in a large cohort of patients with lower urinary tract symptoms. J Urol 2001;166:158-61. [PubMed]
- Hohlbrugger G. Leaky urothelium and/or vesical ischemia enable urinary potassium to cause idiopathic urgency/frequency syndrome and urge incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1996;7:242-55. [PubMed]
- Chung MK, Butrick CW, Chung CW. The overlap of interstitial cystitis/painful bladder syndrome and overactive bladder. JSLS 2010;14:83-90. [PubMed]
- Clemens JQ, Link CL, Eggers PW, et al. Prevalence of painful bladder symptoms and effect on quality of life in black, Hispanic and white men and women. J Urol 2007;177:1390-4. [PubMed]
- Hunner GL. A rare type of bladder ulcer in women: Report of cases. J Boston Med Surg 1915;172:660-5.
- Messing EM, Stamey TA. Interstitial cystitis: early diagnosis, pathology, and treatment. Urology 1978;12:381-92. [PubMed]
- Gillenwater JY, Wein AJ. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis, National Institutes of Health, Bethesda, Maryland, August 28-29, 1987. J Urol 1988;140:203-6. [PubMed]
- Hanno PM, Landis JR, Matthews-Cook Y, et al. The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J Urol 1999;161:553-7. [PubMed]
- Evans RJ, Sant GR. Current diagnosis of interstitial cystitis: an evolving paradigm. Urology 2007;69:64-72. [PubMed]
- Stanford EJ, Dell JR, Parsons CL. The emerging presence of interstitial cystitis in gynecologic patients with chronic pelvic pain. Urology 2007;69:53-9. [PubMed]
- Erickson DR, Tomaszewski JE, Kunselman AR, et al. Do the National Institute of Diabetes and Digestive and Kidney Diseases cystoscopic criteria associate with other clinical and objective features of interstitial cystitis? J Urol 2005;173:93-7. [PubMed]
- Elbadawi A. Interstitial cystitis: a critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis. Urology 1997;49:14-40. [PubMed]
- Leiby BE, Landis JR, Propert KJ, et al. Discovery of morphological subgroups that correlate with severity of symptoms in interstitial cystitis: a proposed biopsy classification system. J Urol 2007;177:142-8. [PubMed]
- Tomaszewski JE, Landis JR, Russack V, et al. Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology 2001;57:67-81. [PubMed]
- Propert KJ, Payne C, Kusek JW, et al. Pitfalls in the design of clinical trials for interstitial cystitis. Urology 2002;60:742-8. [PubMed]
- Elbadawi AE, Light JK. Distinctive ultrastructural pathology of nonulcerative interstitial cystitis: new observations and their potential significance in pathogenesis. Urol Int 1996;56:137-62. [PubMed]
- Hauser PJ, VanGordon SB, Seavey J, et al. Abnormalities in Expression of Structural, Barrier and Differentiation Related Proteins, and Chondroitin Sulfate in Feline and Human Interstitial Cystitis. J Urol 2015;194:571-7. [PubMed]
- Schwalenberg T, Stolzenburg JU, Ho TP, et al. Enhanced urothelial expression of human chorionic gonadotropin beta (hCGβ) in bladder pain syndrome/interstitial cystitis (BPS/IC). World J Urol 2012;30:411-7. [PubMed]
- Erickson DR, Schwarze SR, Dixon JK, et al. Differentiation associated changes in gene expression profiles of interstitial cystitis and control urothelial cells. J Urol 2008;180:2681-7. [PubMed]
- Southgate J, Varley CL, Garthwaite MA, et al. Differentiation potential of urothelium from patients with benign bladder dysfunction. BJU Int 2007;99:1506-16. [PubMed]
- Laguna P, Smedts F, Nordling J, et al. Keratin expression profiling of transitional epithelium in the painful bladder syndrome/interstitial cystitis. Am J Clin Pathol 2006;125:105-10. [PubMed]
- Sun TT. Altered phenotype of cultured urothelial and other stratified epithelial cells: implications for wound healing. Am J Physiol Renal Physiol 2006;291:F9-21. [PubMed]
- Mudge CS, Klumpp DJ. Induction of the urothelial differentiation program in the absence of stromal cues. J Urol 2005;174:380-5. [PubMed]
- Romih R, Korosec P, de Mello W Jr, et al. Differentiation of epithelial cells in the urinary tract. Cell Tissue Res 2005;320:259-68. [PubMed]
- Varley CL, Stahlschmidt J, Lee WC, et al. Role of PPARgamma and EGFR signalling in the urothelial terminal differentiation programme. J Cell Sci 2004;117:2029-36. [PubMed]
- Veranic P, Romih R, Jezernik K. What determines differentiation of urothelial umbrella cells? Eur J Cell Biol 2004;83:27-34. [PubMed]
- Daher A, de Boer WI, El-Marjou A, et al. Epidermal growth factor receptor regulates normal urothelial regeneration. Lab Invest 2003;83:1333-41. [PubMed]
- Kreft ME, Romih R, Sterle M. Antigenic and ultrastructural markers associated with urothelial cytodifferentiation in primary explant outgrowths of mouse bladder. Cell Biol Int 2002;26:63-74. [PubMed]
- Ibrahim IA, Diokno AC, Killinger KA, et al. Prevalence of self-reported interstitial cystitis (IC) and interstitial-cystitis-like symptoms among adult women in the community. Int Urol Nephrol 2007;39:489-95. [PubMed]
- Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology 1997;49:2-9. [PubMed]
- Wu EQ, Birnbaum H, Mareva M, et al. Interstitial Cystitis: Cost, treatment and co-morbidities in an employed population. Pharmacoeconomics 2006;24:55-65. [PubMed]
- Buffington CA. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol 2004;172:1242-8. [PubMed]
- Parsons CL, Schmidt JD, Pollen JJ. Successful treatment of interstitial cystitis with sodium pentosanpolysulfate. J Urol 1983;130:51-3. [PubMed]
- Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urol 1991;145:732-5. [PubMed]
- Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 2007;69:9-16. [PubMed]
- Parsons CL, Stein PC, Bidair M, et al. Abnormal sensitivity to intravesical potassium in interstitial cystitis and radiation cystitis. Neurourol Urodyn 1994;13:515-20. [PubMed]
- Buffington CA, Woodworth BE. Excretion of fluorescein in the urine of women with interstitial cystitis. J Urol 1997;158:786-9. [PubMed]
- Gupta SK, Pidcock L, Parr NJ. The potassium sensitivity test: a predictor of treatment response in interstitial cystitis. BJU Int 2005;96:1063-6. [PubMed]
- Kyker KD, Coffman J, Hurst RE. Exogenous glycosaminoglycans coat damaged bladder surfaces in experimentally damaged mouse bladder. BMC Urol 2005;5:4. [PubMed]
- Lai MC, Kuo YC, Kuo HC. Intravesical hyaluronic acid for interstitial cystitis/painful bladder syndrome: a comparative randomized assessment of different regimens. Int J Urol 2013;20:203-7. [PubMed]
- Nickel JC, Hanno P, Kumar K, et al. Second multicenter, randomized, double-blind, parallel-group evaluation of effectiveness and safety of intravesical sodium chondroitin sulfate compared with inactive vehicle control in subjects with interstitial cystitis/bladder pain syndrome. Urology 2012;79:1220-4. [PubMed]
- Nickel JC, Egerdie RB, Steinhoff G, et al. A multicenter, randomized, double-blind, parallel group pilot evaluation of the efficacy and safety of intravesical sodium chondroitin sulfate versus vehicle control in patients with interstitial cystitis/painful bladder syndrome. Urology 2010;76:804-9. [PubMed]
- Nickel JC, Forrest JB, Tomera K, et al. Pentosan polysulfate sodium therapy for men with chronic pelvic pain syndrome: a multicenter, randomized, placebo controlled study. J Urol 2005;173:1252-5. [PubMed]
- van Ophoven A, Heinecke A, Hertle L. Safety and efficacy of concurrent application of oral pentosan polysulfate and subcutaneous low-dose heparin for patients with interstitial cystitis. Urology 2005;66:707-11. [PubMed]
- Davis EL, El Khoudary SR, Talbott EO, et al. Safety and efficacy of the use of intravesical and oral pentosan polysulfate sodium for interstitial cystitis: a randomized double-blind clinical trial. J Urol 2008;179:177-85. [PubMed]
- Hwang P, Auclair B, Beechinor D, et al. Efficacy of pentosan polysulfate in the treatment of interstitial cystitis: a meta-analysis. Urology 1997;50:39-43. [PubMed]
- van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol 2004;172:533-6. [PubMed]
- Mayer R, Propert KJ, Peters KM, et al. A randomized controlled trial of intravesical bacillus calmette-guerin for treatment refractory interstitial cystitis. J Urol 2005;173:1186-91. [PubMed]
- Bullones Rodríguez MÁ, Afari N, Buchwald DS, et al. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol 2013;189:S66-74. [PubMed]
- Westropp JL, Kass PH, Buffington CA. Evaluation of the effects of stress in cats with idiopathic cystitis. Am J Vet Res 2006;67:731-6. [PubMed]
- Kaplan SA, Dmochowski R, Cash BD, et al. Systematic review of the relationship between bladder and bowel function: implications for patient management. Int J Clin Pract 2013;67:205-16. [PubMed]
- Tracey KJ. Understanding immunity requires more than immunology. Nat Immunol 2010;11:561-4. [PubMed]
- Olofsson PS, Rosas-Ballina M, Levine YA, et al. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev 2012;248:188-204. [PubMed]
- Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat Rev Endocrinol 2012;8:743-54. [PubMed]
- Reardon C, Duncan GS, Brüstle A, et al. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc Natl Acad Sci U S A 2013;110:1410-5. [PubMed]
- Matteoli G, Gomez-Pinilla PJ, Nemethova A, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 2014;63:938-48. [PubMed]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun 2005;19:493-9. [PubMed]
- Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology 2007;69:24-33. [PubMed]
- Saban R, Saban MR, Maier J, et al. Urothelial expression of neuropilins and VEGF receptors in control and interstitial cystitis patients. Am J Physiol Renal Physiol 2008;295:F1613-23. [PubMed]
- Greenwood-Van Meerveld B, Mohammadi E, Tyler K, et al. Mechanisms of Visceral Organ Crosstalk: Importance of Alterations in Permeability in Rodent Models. J Urol 2015;194:804-11. [PubMed]
- Towner RA, Smith N, Saunders D, et al. Contrast enhanced magnetic resonance imaging as a diagnostic tool to assess bladder permeability and associated colon cross talk: preclinical studies in a rat model. J Urol 2015;193:1394-400. [PubMed]
- Towner RA, Wisniewski AB, Wu DH, et al. A Feasibility Study to Determine whether Clinical Contrast-Enhanced MRI can Detect Increased Bladder Permeability in Patients with Interstitial Cystitis. J Urol 2015. [Epub ahead of print]. [PubMed]


