Clinical presentation and treatment of bladder pain syndrome/interstitial cystitis (BPS/IC) in India
Bladder pain syndrome/interstitial cystitis (BPS/IC) is a neglected debilitating chronic, inflammatory disorder of the urinary bladder characterized by variable degree of bladder pain, frequency and urinary urgency (1). Twenty-five years ago it was believed that BPS/IC did not exist in India and it was a disease predominantly present in Western world. Symptoms of BPS/IC and tuberculosis are more or less same and as tuberculosis is common in India most of the patients of BPS/IC were diagnosed and treated as tuberculosis. Now in 2015 it is well established that PBS/IC is not uncommon in India and it is estimated that there are more than 1.25 million patients with BPS/IC. Awareness of the disease amongst urologists is much higher compared to family physicians, surgeons, physicians and gynecologists who see the patients initially and treat them. All over the world lot of effort has been put in to find a cure for BPS/IC but no concrete solutions have emerged. It is a unique disease with no clue to etiology (2) its pathology is unknown (3) and no specific treatment exists to affect a cure (4).
Definition
In June 2009, the Indian BPS/IC society framed the Indian interstitial cystitis (IC) guidelines which defined BPS/IC as recurrent pelvic pain or discomfort (pressure, burning, throbbing, etc.), of at least 4-6 weeks duration, which increases with bladder filling and/or decreases with micturition in the absence of definable pathology associated with urinary frequency and/or urgency.
The American Urological Association (AUA) published its guideline in 2011, naming the disease BPS/IC. The condition was defined as “an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract (LUT) symptoms of more than 6 weeks duration, in the absence of infection or other identifiable causes” (5).
According to “The European Society for the Study of Interstitial Cystitis (ESSIC)”—bladder pain syndrome (BPS) is diagnosed on the basis of chronic pelvic pain, pressure, or discomfort perceived to be related to urinary bladder accompanied by at least one other urinary symptom like persistent urge to void or urinary frequency. Confusable diseases as the cause of the symptoms must be excluded (6).
Japan, Taiwan and Korea have their own guidelines and label it as “hypersensitive bladder” (HSB) (7) defined as “bladder hypersensitivity, usually associated with urinary frequency, with or without bladder pain”.
It is of note that the terms IC, BPS/IC, BPS, HSB are used interchangeably and for the same syndrome!
BPS/IC etiopathology
There is no agreement on pathophysiology of BPS/IC, there are however many theories. The following theories have been proposed (Figure 1):
- Leaky epithelium glycosaminoglycan [glycosaminoglycan (GAGs) theory] (9-11);
- Occult infection;
- Neurogenic inflammation (12);
- Mast cell activation (12);
- Autoimmunity (13);
- Vascular.
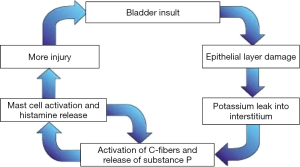
Multifactorial etiology of BPS/IC
Though there are many theories for the etiology of BPS/IC, the most popular is a multi-factorial causation. Following infection, inflammation, pelvic surgery, childbirth or urological instrumentation bladder epithelial damage occurs. Normally epithelial surfaces heal following injury but in some patients the process of epithelial healing does not take place completely leading to abnormalities in the GAG layer. Defective GAG layer allows potassium and other urinary metabolites to pass the submucosal layer and cause inflammation leading to activation of mast cells which elicits local tissue damage and vascular constriction. A cascade of events is set up and leads to more bladder damage, injury to detrusor smooth muscles and fibrotic changes. Final result is small capacity bladder. There is also neural up regulation and development of neural changes in the spinal cord (13).
Author’s hypothesis of BPS/IC
The author believes that abnormality of detrusor muscle stretch in response to bladder filling is responsible for the symptoms of BPS/IC. Physiologically the bladder fills to adequate volume and the detrusor stretches to accommodate that amount without an intense desire to pass urine. In normal persons when the bladder is overfull, signal is transmitted to brain and he has to pass urine. The same phenomenon occurs in a patient of BPS/IC when the bladder contains lesser volumes of urine e.g., 20-50 mL and the bladder senses overfilling and sends signals to the brain to initiate micturition. This sensation of an overfull bladder is related to stretch of the detrusor muscle and an abnormality of detrusor stretch reflex sends signals to the brain that bladder is overfull and there is an urgent need to void although the bladder contains minimal urine. This hypothesis is the basis of success of ileocystoplasty surgery in patients of BPS/IC in the author’s personal series.
Prevalence of BPS/IC
BPS/IC is a rare disease and prevalence is different in different countries. More and more patients are being diagnosed as awareness of the disease is increasing. BPS/IC is under-diagnosed. There is big range as China has minimum prevalence of 100 per lakh [100,000] (14) to USA where it is estimated to be 2,600 per lakh [100,000] (Berry) (15). When strict criteria is used for inclusion in the definition the prevalence is less and if a more inclusive definition is used the prevalence is more. In India there are no epidemiological studies, but even going by minimum population prevalence of 100 per 100,000, India has minimum 1.25 million patients with BPS/IC (16).
Symptoms of BPS/IC
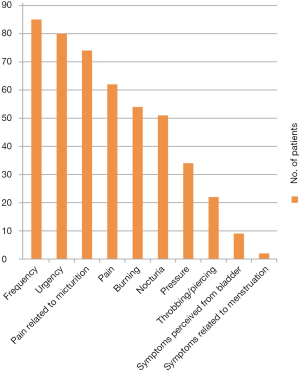
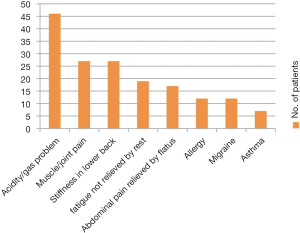
Pelvic discomfort with urinary frequency and urgency is most common presentation of BPS/IC (Table 1). Pain is important cause of pelvic discomfort but patients also complain of unusual pressure sensation, burning, throbbing or, piercing or childbirth like pain. Pelvic discomfort increases with bladder filling and decreases on voiding. In severe cases pelvic discomfort is continuous (18% of patients in our series, 17 of 92 patients). It is felt in the suprapubic, retropubic, infrapubic, urethral, genital, rectal regions and/or deep pelvic area. In most of the patients the frequency is more than 8 times in a day. Once the patient gets desire to pass urine they cannot postpone it and the discomfort increases. An important point is that patients do not leak urine. To start with patient may have only one symptom initially but develop fully fledged syndrome over next 4-5 years. Patients with severe disease void every 5-10 minutes and live a very miserable life and prefer to travel by train over bus because of the availability of toilets. Patients are sometimes forced to urinate in public or even wear diapers so that they can pass urine in them. Very few patients perceive their symptoms to be from the bladder (Figure 2). This is an important distinction from the AUA definition which gives importance to the perception of pain being from bladder. It is also to be noted that symptoms are not related to menstruation.
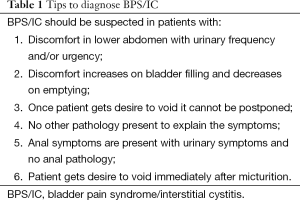
Full table
Unusual obstructive urinary symptoms of BPS/IC
BPS/IC is basically a disease of bladder sensation. Patients feel that the bladder is full with urine even when bladder is having minimal urine. Patients complain of obstruction in the urinary passage and inability to completely evacuate bladder. In fact there is no obstruction in urinary passage in these patients. In severe cases patients sit in the toilet for hours as they feel that bladder is full and there is continuous urge to pass urine. The obstructive symptoms are incomplete evacuation, thin stream, dribbling and straining and are present in about 50% (range, 38-54%) patients of PBS/IC. Presence of obstructive symptoms leads to wrong diagnosis of urethral stricture in these patients (Figure 3). This point is very important as the general perception is that BPS/IC presents with irritative symptoms only and a different diagnosis is considered when patients complain of obstructive symptoms.
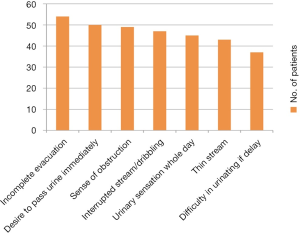
Unusual non-urinary symptoms of BPS/IC
Non-urinary symptoms—anal discomfort, vulvar and glandular pruritis and burning, dyspareunia, painful ejaculation and difficulty in walking and sitting—occur in up to 25% of patients. Post intercourse symptom flares do occur in both men and women. Increased tone of pelvic muscles or inability to relax pelvic muscles may be responsible for some of these symptoms (Figure 4). BPS/IC must be thought in a patient with urinary symptoms presenting with anal pain (28% of our 92 patients).
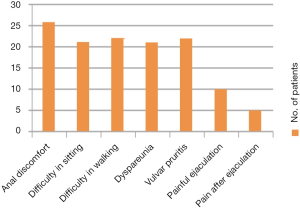
Associated diseases
Gastrointestinal symptoms are commonly associated with BPS/IC in India (50% of 92 patients). Typical irritable bowel syndrome (IBS) is present in few patients. Allergy, migraine and asthma are not very common (Figure 5).
BPS/IC and chronic abacterial prostatitis (CAP): same disease
BPS/IC is more common in females. Male patients with symptoms as BPS/IC are diagnosed as CAP. At our center patients with pelvic discomfort, urgency and frequency are analyzed on the basis of relation of symptoms with micturition. If the symptoms increase with micturition and decrease after passing urine they are considered as suffering from BPS/IC and treated accordingly. If the patient has only pelvic discomfort not related to micturition, he is considered as suffering from chronic prostatitis and these patients are not treated as BPS/IC. A group of urologist believe BPS/IC and CAP as same disease. NIDDK used umbrella term “urologic chronic pelvic pain syndrome (UCPPS)”, to refer to pain syndromes in both men and women believing in the philosophy of similar disease in different sexes.
In 70% of men with symptoms of nonbacterial prostatitis and prostatodynia cystoscopy revealed ecchymosis and petechial hemorrhage on cystoscopy under anaesthesia (17). In our series 80% of patients have petechiae and ecchymosis on cystoscopy and hydrodistension. Discomfort with bladder filling is found in 45% patients with BPS/IC (18). Mayo et al. described hypersensitivity in 30% of patients with chronic prostatitis (19). Clinically it is very difficult to diagnose BPS/IC in male patients as the symptoms of both CAP and BPS/IC overlap. The best strategy is to think BPS/IC in all patients with CAP where the therapy for prostatitis fails.
Investigations
Urine culture
BPS/IC patients usually have negative urine cultures. But BPS/IC should be suspected even in a patient with culture positive urine if the symptoms do not resolve with appropriate antibiotic treatment.
Frequency volume chart
This is very useful assessment of the patient’s voiding pattern. In BPS/IC patients void small amount of urine many times. A 3-day voiding log is considered representative. We use frequency volume chart when we have doubt about the diagnosis.
Ultrasound of kidney ureter and bladder
Ultrasonography is a common initial investigation in India for patients with urinary symptoms. In BPS/IC ultrasound of the kidneys, ureter and bladder is usually normal with no or minimal post-void residual volume. Because patients cannot hold their urine, the pre-void volumes are low and are characterized as having small capacity bladders. Tuberculous bladders also have small bladder capacity (thimble bladders) but differ from BPS/IC as thimble bladders have thick bladder wall with upper tract changes on ultrasonography.
Cystoscopy
All patients suspected of BPS/IC undergo cystoscopy under spinal anaesthesia. Initially all cystoscopies were done under general anaesthesia but it was observed that as soon as patients awaken from general anaesthesia they would have severe pain and urinary sensation. Now all cystoscopies are done under spinal anaesthesia and post anaesthesia patient is very comfortable and patients can tolerate a Foley catheter for 60-90 minutes. The catheter is removed as soon as patients start moving their legs (16).
Advantages of cystoscopy (16)
The following are advantages of cystoscopy:
- To rule out other disease as cause of symptoms e.g., carcinoma in situ, tuberculosis (20);
- To biopsy any suspicious lesion present before the bladder is distended. Red petechial hemorrhage and ecchymosis develop as the bladder is distended or after bladder is evacuated. Biopsy of such new lesions is not of any significance;
- To measure capacity of bladder. If bladder capacity is less than 150 mL under anaesthesia, it is better to advise surgical intervention;
- Therapeutic hydrodistension can be performed at the same sitting;
- If Hunner’s lesion is found, it can be fulgurated or resected or treated with a laser.
Technique
The saline reservoir is kept at a height of 80 cm from pubic symphysis and the bladder is filled under gravity. The bladder is then evacuated and refilled. The colour of the evacuated fluid is noted. It is necessary to distend the bladder again as the petechiae and ecchymosis usually develop on evacuation of the bladder and can be observed only when the bladder is redistended. In some cases petechiae and ecchymosis develop even on first distension. Few patients bleed from all over the mucosa while distension is being performed. In therapeutic hydrodistension, the bladder is kept distended for 3 minutes and then drained again. The reservoir height is not increased as it will hydro-dilate the bladder. Our aim is to hydro-distend the bladder not hydro-dilate (16). Hydrodilatation causes breaches in the bladder mucosa. Following hydrodistension, bladder biopsies can be taken. Bladder capacity under anaesthesia varies from less than 100 mL to around 1,000 mL. We do not distend the bladder more than 800 mL for fear of rupture. A Foley catheter is inserted in all the cases (16).
Important points about cystoscopy
The following are the important points about cystoscopy:
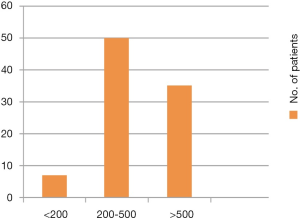
- Patients with normal capacity under anaesthesia can also have BPS/IC. Figure 6 shows distribution of BPS/IC patients as per cystoscopy capacity under anaesthesia. Only about 8% of patients have a small capacity bladder (<200 mL) (Figure 6);
- Bladder mucosa is normal in around 20% cases of BPS/IC (19 out of 92 patients in our series had normal bladder mucosa on distension and redistension). The prevailing concept that normal bladder on cystoscopy rules out BPS/IC is wrong (Figure 7);
- There are no specific pathognomic changes on routine pathologic examination of bladder biopsy in BPS/IC. Bladder biopsy can be absolutely normal in BPS/IC. The belief that if bladder biopsy is normal it is not BPS/IC is incorrect. Cold cup biopsy will mostly show changes of inflammation.
Glomerulations and Hunner’s lesion
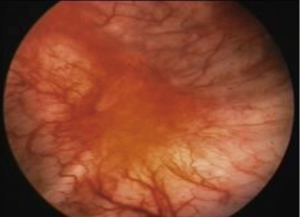
On cystoscopy there are distinct lesions or normal mucosa. The distinct lesions are pinpoint red spots, ecchymosis or Hunner’s lesion. Glomerulations (first mentioned in Campbell Urology textbook in 1978 edition) are “pinpoint” submucosal hemorrhages. Hunner’s ulcer identified by Guy Hunner is not an ulcer but a lesion with specific characteristics.
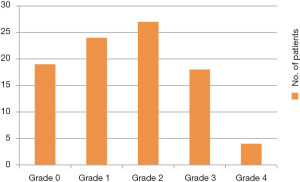
Glomerulations were long considered the hallmark of BPS/IC (2). In my own series of 60 patients, only 30 had glomerulations involving more than 75% area of the bladder surface. The remainder did not develop glomerulations at all or had only few glomerulations. Importantly, in 40 patients with bladder diseases other than BPS/IC none developed glomerulations even on second distension or hydrodistension thus proving that glomerulations are hallmark of BPS/IC (21). I believe that glomerulations may not be present in all cases of BPS/IC but when they are present in patient with pain urgency and frequency with no other pathology they point to the diagnosis of BPS/IC. Glomerulations have also been found in normal women undergoing tubal ligation (22). Although glomerulations are associated with BPS/IC, there is no correlation between glomerulations and degree of histological inflammation (23) or symptoms (24). Glomerulations and Hunner’s lesion are confusing terminology. Moreover, these findings are very subjective and differ from observer to observer. In an attempt to standardize cystoscopic findings, ESSIC has described various grades of bladder mucosa appearance on cystoscopy. In author’s series of 92 patients the commonest grade was grade 1 and around 20% patients had grade 0 (Figure 7, Table 2). It is to be appreciated that Hunner’s lesion are always present on first distension but petechiae and ecchymosis mostly develop either during first distension or after first evacuation so it is very important to redistend bladder to visualize them.

Full table
Cystoscopic appearance of Hunner’s lesion
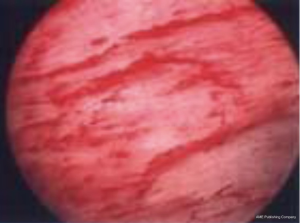
Hunner’s lesion is a distinctive inflammatory lesion with characteristics central fragility, which ruptures on hydrodistension. It is usually a solitary lesion but 2-3 lesions may be present. This lesion is typically visible before the bladder is distended. On cystoscopy, it appears as a circumscript reddened mucosal area with small vessels radiating towards a central scar. This site ruptures with increasing bladder distension; with petechial oozing blood from the lesion in a waterfall fashion (15). In our series of more than 250 cystoscopies in BPS/IC patients, a typical Hunner’s lesion has rarely been observed. In our series we have seen red patches but accompanied by patechiae and ecchymosis all over the bladder and not sure if it is Hunner’s lesion (16) (Figure 9).
Treatment options in BPS/IC
As the etiopathology is multifactorial, it is logical to treat the patients with multimodal therapy (8). Treatment options available are hydrodistension, oral therapy, intravesical therapy, intravesical botox injection, interstim, fulguration and resection of Hunner’s lesion, behavioral and physical therapy. In India botox has not been found effective and there has been no experience of interstim.
Staged treatment
The problem with BPS/IC is its uncertainty in responding to treatment. There is no way to know which patient will respond to which treatment. Multiple treatment options are available (Figure 10, Tables 3,4). A staged treatment policy has been our standard approach with all patients treated with the same protocol (16). Staged therapy has been followed over the last 15 years and minor changes are made depending on advances in understanding the disease, the response of the patient and the availability of the therapeutic agent. The AUA guideline 2011 recommended staged therapy as the preferred way to manage BPS/IC (5). We have no experience of physical therapy behavioral therapy and interstim so it is not included in our protocol. Similarly we have not found intravesical botox injection effective so have removed it from our protocol and do not offer it to the patients. Behavior modification and stress management are first line treatments as per AUA guidelines. Physical therapy is mentioned as 2nd line therapy in the same guidelines (5).

Full table
Full table
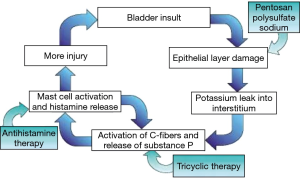
Staged therapy protocol
Stage I: cystoscopy hydrodistension with oral therapy
All patients undergo cystoscopy and therapeutic hydrodistension. Patients with small bladder capacity less than 150 mL or Hunner’s lesions are advised to undergo surgical therapy. Hunner’s lesion can be fulgurated or resected at the same sitting. Experience with fulguration of Hunner’s lesion is minimum. All other patients are put on triple drug therapy including amitriptyline, hydroxyzine and pentosan polysulfate sodium (PPS)/gabapantin for 3 months. After 3 months, management is reviewed and changes made accordingly (16).
Stage II: intravesical therapy
Patients who do not respond or experience a flare-up during oral therapy are treated with an intravesical rescue solution—a mixture of an anesthetic agent, steroid and heparin. The solution is placed in the bladder for 30 minutes and six treatments are given at intervals of 2 weeks. The rescue solution consists of 40 mL sensorcaine-0.5%, 2 cc dexamethasone and 25,000 units of heparin. If the patient does not respond to this rescue solution, other intravesical therapies such as dimethyl sulfoxide (DMSO) and hyaluronic acid are utilized based on local availability.
Stage III: surgery
Surgery is offered as a last resort to those patients who have poor quality of life and have failed all other therapies. Various procedures including augmentation cystoplasty, substitution cystoplasty, and neobladder with or without cystectomy are available but with variable surgical treatment outcomes. Surgery is the option of first choice in patients with Hunner’s lesion or small bladder capacity. Hunner’s lesions are resected or fulgurated with cautery or lased with satisfying results (25,26).
Five augmentation ileocystoplasties have been done at this centre with excellent results.
Foundation of multimodal BPS/IC therapy
As there is no cure for patients of BPS/IC, the aim of management is to decrease the symptoms and make the patient comfortable. The etiology of the condition is unknown but thought to be multifactorial—hence the concept of multimodal therapy. Multimodal therapy is based on the principle of repairing endothelial dysfunction, neural function modulation and mast cell stablization.
PPS
PPS is the first and the only US FDA-approved oral drug for the treatment of BPS/IC. Chemically and structurally PPS is heparin-like macromolecular carbohydrate derivative resembling glycosaminoglycan (GAGs). It repairs endothelium lining of bladder. The recommended dose is 100 mg 3 times a day empty stomach. It may take 3-6 weeks for the effect to be noticed. Important side effects are headache, gastrointestinal upset, hair loss and rectal bleeding. Patient compliance is a major problem with this drug. There have been doubts about the efficacy of PPS in recent study (27).
Amitriptyline hydrochloride
It is most widely and commonly used drug for BPS/IC. It elevates mood, decreases pain and frequency and aids in sleeping. Mostly given at night in dose of 10 to 100 mg. It will be more effective if dose is more than 75 mg but side effects are common at that dose. We mostly prescribe 25 mg at night and in some case advise 25 mg twice in the day. Common side effects include nausea, constipation, dry or sore mouth and drowsiness. It should be remembered that anticholinergics can cause retention of urine and should not be used in patients with glaucoma.
Hydroxyzine hydrochloride
Hydroxyzine hydrochloride is a very effective agent for management of mast cell dysfunction. It is also generally administered at night due to sedative effect. Common side effects are dry mouth, drowsiness and constipation. The usual dose is 25 to 75 mg. In a highly sensitive patient even 10 mg may be effective.
Gabapantin
Gabapantin is drug of choice for neuropathic pain or patients with severe pain. It can decrease neuroinflammation. Up to 2,400 mg can be used in divided dosage. Common side effects are nausea, constipation and drowsiness.
Cyclosporin A
Cyclosporin A is calcineurin inhibitor and is used as immunosuppressant. It has been found more effective than PPS in BPS/IC (28). Cyclosporin is reserved for patients with refractory disease. It is effective but use limited due to severe side effects like hypertension, gum enlargement, trembling, muscle pain, joint pain and renal toxicity.
Diet and self-help
The patient is advised to avoid that food which causes flare. In India some patients get flare up with very spicy and hot food. No diet related cause is found in most of the patients. Normally all patients are advised to drink less fluid and it helps them but some patients can’t tolerate concentrated urine due to less water intake and forced to drink more water. Results of ayurvedic and homeopathic medicines are not encouraging. Yoga, hypnotherapy and acupuncture do not seem to work.
Drugs safe in pregnancy
PPS and intravesical heparin are safe in pregnancy and are recommended (29). In some patients pregnancy has a positive effect on symptoms.
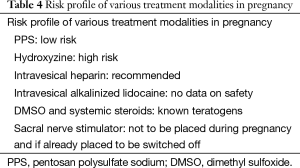
Table 4 serves as a guide for use of various treatment modalities in pregnancy (29).
Subtypes of BPS/IC
There are two subtypes which differ in clinical presentation, age distribution, histopathological and immunological findings and response to treatment (26,30,31). Based on cystoscopic findings, BPS/IC is classified as ulcerative type and non-ulcerative type.
- Ulcerative (Hunner’s lesion) or classic type: it is a rare type accounting for 5-10% of cases (32). It is characterized by Hunner’s lesion during cystoscopy and hydrodistension. It is very important to perform cystoscopy in the early phase of disease to diagnose Hunner’s lesion as they can be successfully treated (25,26,33). This type of disease is associated with more severe disease and more common in elderly patients.
- Non-ulcerative type: this type of IC has pinpoint hemorrhage and ecchymosis on cystoscopy. Some cases will have normal bladder mucosa even on hydrodistension.
Determination of intravesical evaporation of nitric oxide (NO) seems to provide a reliable clue to differentiate the classic Hunner type from other BPS/IC presentations (34). The dramatic increase of luminal NO in BPS/IC of the Hunner type suggests activation of the inducible isoform of nitric oxide synthase (iNOS) (35). A significant advantage is that determination of NO evaporation is easy and simple to use, but disadvantage is that a special device is required (34).
BPS/IC in children
The diagnosis of IC in children is controversial. Twenty-five percent of IC patients report that they had chronic urinary tract problems in childhood (36). Children do indeed present with dysfunctional voiding. There is no theoretical reason why IC cannot exist in children (37) and should be suspected in a child who presents with irritative symptoms and pelvic pain and has no definite diagnosis and has not responded to symptomatic treatment. The youngest patient we have diagnosed is 16 years.
AUA treatment options to avoid (5)
Standard
- Long-term antibiotic administration;
- Intravesical bacillus Calmette-Guerin;
- Intravesical resiniferatoxin.
Recommendation
- High pressure, long duration hydrodistension;
- Systemic (oral) long-term steroid administration.
Author’s criteria for diagnosis of IC in 2015
Patients presenting with lower abdominal/pelvic pain, pressure or urinary discomfort associated with urinary frequency and/or urgency with the following characteristics:
- Nocturia may or may not be present;
- Symptoms present for at least 1 month;
- USG (KUB) is normal, there is no post void residual urine and urine culture is negative;
- No other disease responsible to explain symptoms;
- Under anesthesia, cystoscopy is either normal or shows the presence of pinpoint hemorrhage or ecchymosis or both. Hunner’s lesion may be present (16);
- Diagnosis of IC should also be entertained in following group of patients (16);
- Who has culture positive urinary infection, but in whom the symptoms do not disappear after adequate treatment with a suitable antibiotic;
- Patients of CAP who do not improve with treatment;
- Overactive bladder (OAB) patients not responding to anticholinergics.
Experience at author’s center
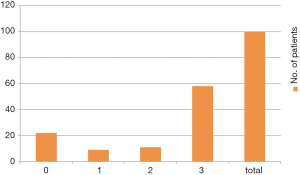
From 1993 to December 2014, we have seen around 900 IC patients and performed cystoscopy in 316 patients (196 women and 120 men). Female to male ratio is 3:2. Recently 133 patients seen from 2001 onwards were contacted and asked about their present disease compared to initial presentation. Mean follow-up was 6.8 years. Four patients had died so we have follow-up details of 129 (79 women and 50 men) patients over a period of 14 years. The response was evaluated using a Global Response Assessment (GRA) scale. A total of 58% had excellent improvement, 22% patients had no improvement or worsened, 9% had mild improvement and 13% moderate improvement (Figure 11). These patients were treated with modalities of treatment which were available at the time of presentation. PPS only became available in India in 2010.
The patients are subjected to rehydrodistension if they have done well for more than a year on previous hydrodistension and other therapies are not working. Experience with botox injection is not good. We have not seen typical Hunner’s lesion in our patients but have seen red patches in three cases. Interstim (neuromodulation) has not been performed in any patient. Around 8% of patients had less than 200 cc capacity bladder on distension under anaesthesia and have been advised surgery. We have done augmentation cystoplasty in five patients with refractory BPS/IC with excellent results.
Intravesical tacrolimus in BPS/IC—pilot study
Intravesical tacrolimus is used at our center in patients with intractable BPS/IC before recommending surgery. A pilot study done in seven patients in 2013 found the drug to be safe. Tacrolimus, a calcineurin inhibitor like cyclosporine A is very toxic drug. Intravesical tacrolimus dissolved in DMSO is instilled in the bladder via catheter and kept for 30 minutes and repeated every 14th day for a total of six instillations. Blood tacrolimus levels post instillation revealed absorption from the bladder. The blood levels of tacrolimus are no-toxic. Patients are checked for complete blood count, blood sugar, renal and hepatic function at the start and completion of the six instillations. The idea is that local tacrolimus will be more effective and less or minimal toxic. Intravesical instillation of tacrolimus has been found safe in this pilot study.
Recently we have used intravesical tacrolimus in four patients offering them just before surgery. Out of four patients, two patients have totally improved. However, more patients need to be studied before any definitive conclusions regarding efficacy and safety can be drawn. This therapy is still in experimental stage and is not recommended for general use.
Conclusions
Even in mid of 2015, no consensus on name, definition, etiopathology and management of BPS/IC exists. Advances in molecular biology point to inflammation as one of the etiologies. It is hoped that these advances will lead to the development of novel therapies and delivery methods to treat BPS/IC. It appears that a lot of research has been covered, but a great deal is still needed to reach the ultimate goal.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Gillenwater JY, Wein AJ. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis, National Institutes of Health, Bethesda, Maryland, August 28-29, 1987. J Urol 1988;140:203-6. [PubMed]
- Hanno P. Interstitial cystitis and related diseases. In: Walsh PC, Wein AJ, Retik AB, et al, editors. Campbell’s Urology. 7th ed. Philadelphia: WB Saunders, 1998:631-62.
- Rosamilia A, Dwyer PL. Pathophysiology of interstitial cystitis. Curr Opin Obstet Gynecol 2000;12:405-10. [PubMed]
- Rovner E, Propert KJ, Brensinger C, et al. Treatments used in women with interstitial cystitis: the interstitial cystitis data base (ICDB) study experience. The Interstitial Cystitis Data Base Study Group. Urology 2000;56:940-5. [PubMed]
- Hanno PM, Burks DA, Clemens JQ, et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 2011;185:2162-70. [PubMed]
- Van de Merwe JP, Nordling J, Bouchelouche P, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol 2008;53:60-7. [PubMed]
- Homma Y, Ueda T, Tomoe H, et al. Clinical guidelines for interstitial cystitis and hypersensitive bladder syndrome. Int J Urol 2009;16:597-615. [PubMed]
- Evans RJ. Treatment approaches for interstitial cystitis: multimodality therapy. Rev Urol 2002;4 Suppl 1:S16-20. [PubMed]
- Parsons CL, Zupkas P, Parsons JK. Intravesical potassium sensitivity in patients with interstitial cystitis and urethral syndrome. Urology 2001;57:428-32; discussion 432-3. [PubMed]
- Hurst RE, Zebrowski R. Identification of proteoglycans present at high density on bovine and human bladder luminal surface. J Urol 1994;152:1641-5. [PubMed]
- Sun Y, Keay S, De Deyne PG, et al. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol 2001;166:1951-6. [PubMed]
- Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology 2001;57:47-55. [PubMed]
- Van De Merwe JP, Arendsen HJ. Interstitial cystitis: a review of immunological aspects of the aetiology and pathogenesis, with a hypothesis. BJU Int 2000;85:995-9. [PubMed]
- Song Y, Zhang W, Xu B, et al. Prevalence and correlates of painful bladder syndrome symptoms in Fuzhou Chinese women. Neurourol Urodyn 2009;28:22-5. [PubMed]
- Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 2011;186:540-4. [PubMed]
- Mishra NN. Interstitial Cystitis/Painful Bladder Syndrome (IC/PBS) Dilemmas in Diagnosis & Treatment. Curr Womens Health Rev 2013;9:122-30.
- Berger RE, Miller JE, Rothman I, et al. Bladder petechiae after cystoscopy and hydrodistension in men diagnosed with prostate pain. J Urol 1998;159:83-5. [PubMed]
- Siroky MB, Goldstein I, Krane RJ. Functional voiding disorders in men. J Urol 1981;126:200-4. [PubMed]
- Mayo ME, Ross SO, Krieger JN. Few patients with “chronic prostatitis” have significant bladder outlet obstruction. Urology 1998;52:417-21. [PubMed]
- Tissot WD, Diokno AC, Peters KM. A referral center’s experience with transitional cell carcinoma misdiagnosed as interstitial cystitis. J Urol 2004;172:478-80. [PubMed]
- Mishra N. Are glomerulations typical of interstitial cystitis? AUA 2000; Abstr Poster 270.
- Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol 1998;160:1663-7. [PubMed]
- Denson MA, Griebling TL, Cohen MB, et al. Comparison of cystoscopic and histological findings in patients with suspected interstitial cystitis. J Urol 2000;164:1908-11. [PubMed]
- Messing E, Pauk D, Schaeffer A, et al. Associations among cystoscopic findings and symptoms and physical examination findings in women enrolled in the Interstitial Cystitis Data Base (ICDB) Study. Urology 1997;49:81-5. [PubMed]
- Peeker R, Aldenborg F, Fall M. Complete transurethral resection of ulcers in classic interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct 2000;11:290-5. [PubMed]
- Shanberg AM, Malloy T. Treatment of interstitial cystitis with neodymium:YAG laser. Urology 1987;29:31-3. [PubMed]
- Hanno PM. Bladder pain syndrome (interstitial cystitis) and related disorders. In: Wein AJ, Kavoussi LR, Novick AC, et al, editors. Campbell-Walsh urology. 10th ed. Philadelphia: Elsevier Saunders, 2012:357-401.
- Sairanen J, Tammela TL, Leppilahti M, et al. Cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol 2005;174:2235-8. [PubMed]
- Erickson DR, Propert KJ. Pregnancy and interstitial cystitis/painful bladder syndrome. Urol Clin North Am 2007;34:61-9. [PubMed]
- Fall M, Johansson SL, Aldenborg F. Chronic interstitial cystitis: a heterogeneous syndrome. J Urol 1987;137:35-8. [PubMed]
- Peeker R, Fall M. Toward a precise definition of interstitial cystitis: further evidence of differences in classic and nonulcer disease. J Urol 2002;167:2470-2. [PubMed]
- Sant GR, editor. Interstitial Cystitis. Philadelphia: Lippincott-Raven, 1997.
- Fall M, Peeker R. Methods and incentives for the early diagnosis of bladder pain syndrome/interstitial cystitis. Expert Opin Med Diagn 2013;7:17-24. [PubMed]
- Logadottir YR, Ehren I, Fall M, et al. Intravesical nitric oxide production discriminates between classic and nonulcer interstitial cystitis. J Urol 2004;171:1148-50; discussion 50-1. [PubMed]
- Lirk P, Hoffmann G, Rieder J. Inducible nitric oxide synthase--time for reappraisal. Curr Drug Targets Inflamm Allergy 2002;1:89-108. [PubMed]
- Held PJ, Hanno PM, Wein AJ, et al. Epidemiology of interstitial cystitis. In: Hanno PM, Staskin DR, Krane RJ, et al, editors. Interstitial Cystitis. New York: Springer-Verlag, 1990:29-48.
- Mattox TF. Interstitial cystitis in adolescents and children: a review. J Pediatr Adolesc Gynecol 2004;17:7-11. [PubMed]