Intravesical liposome drug delivery and IC/BPS
Introduction
Intravesical therapies provide a high concentration of drugs to the diseased bladder with minimal or undetectable systemic levels. Evidence to date shows that there is a low risk of systemic side effects (1,2). Intravesical therapy is commonly used to treat superficial bladder cancer; therefore, it seems reasonable to apply these methods to improve the treatment of functional bladder conditions such as interstitial cystitis/bladder pain syndrome (IC/BPS). The urothelium is a highly impermeable surface and many drugs are not stable in the hostile urine environment (2,3). Liposomes (LPs) are lipid vesicles composed of phospholipid bilayers surrounding an aqueous core (4). Empty LPs can protect damaged urothelium and have shown therapeutic benefits for IC/BPS patients (5). In addition, LPs can carry various drugs to penetrate urothelium and modulate afferent neurotransmission (2,6).
Interstitial cystitis/bladder pain syndrome (IC/BPS)
IC/BPS is a chronic disease characterized by suprapubic/bladder discomfort accompanied by urinary frequency, urgency, or nocturia in the absence of infection or other pathological conditions (7,8). The debilitating condition of IC/BPS results in diminished quality of life (9). IC/BPS is not a rare condition, and it occurs more frequently in women than men in a 5:1 ratio. Recent studies have revealed that perhaps over 3 million men and women in the U.S. have symptoms of IC/BPS (10).
One hypothesis of IC/BPS pathology is that dysfunctional epithelium allows the transepithelial migration of toxic solutes, such as potassium, which can depolarize subepithelial afferent nerves and provoke sensory symptoms (8). Dysfunction of the superficial layer of the glycosaminoglycan (GAG) layer, activation of mast cells in the bladder wall, and down-regulation of tight junctional proteins have also been shown to contribute to the pathophysiology of IC/BPS (6,7,11). Pain-sensing C-fibers located within the uroepithelium and submucosa of the bladder can be activated by either a GAG layer deficiency, release of histamine via mast cells, or release of sensory neurotransmitters from urothelium cells. Neurogenic inflammation, primary afferent nerve activation, and central nervous system sensitization may all occur and lead to increasing pain, urinary urgency, and frequency.
Rationale for intravesical treatment of IC/BPS
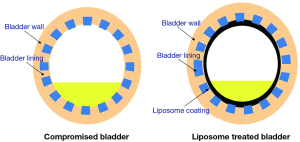
Most of the therapeutic agents for functional bladder disorders are administered orally. These medications may be poorly absorbed and/or metabolized by the liver, and they often fail to have a therapeutic effect at the diseased bladder wall without also producing significant unwanted systemic side effects. Their primary elimination route may not be through the urinary system, which further reduces the amount of drug delivered to the urothelium. The rationale for intravesical treatment for IC/BPS is to apply an effective dose of a therapeutic drug to the diseased organ and only to that organ (Figure 1). The anatomy of the urinary bladder and urethra allows easy access and manipulation with a catheter and allows for increased agent exposure via intravesical therapy (12). The advantages of intravesical treatment include:
- Coating and repair of bladder urothelium;
- High drug concentrations in the bladder;
- Minimal incidence of systemic side effects;
- Modulation of urothelial sensory nerve function and neurotransmission.
Limitations to intravesical therapy
IC/BPS patients may be unable to hold a volume of drug in the bladder long enough for the drug to be efficacious. A reduced drug residence time will most likely attenuate therapeutic effects. Another potential shortcoming of intravesical therapy is the dilution of the instilled drug solution due to the continual flow of urine into the bladder. Patients receiving intravesical therapy are advised to decrease fluid intake and empty their bladder before drug administration.
Despite these limitations, it is likely that intravesical therapy can have a positive effect on many IC/BPS patients, especially those with less severe symptoms. Drug delivery to the urothelium via LPs overcomes traditional disadvantages of intravesical bladder therapy (i.e., lack of drug penetration through the urothelium) by bypassing the protective GAG layer.
The urothelium
The structure of the bladder wall, from the luminal to outer surface, consists of the urothelium, detrusor muscle, and adventitia. The urothelium serves as a permeability barrier and prevents urine and waste solute from penetrating into the submucosal layer (13). The urothelium is composed of three different cells: umbrella cells, intermediate cells, and basal cells. Barrier function is established by the arrangement of uroplakins (tight junctional proteins) and is further enhanced by a mucin layer composed of GAG on the luminal surface. The GAG layer is hydrophilic, and forms an aqueous layer on umbrella cells. The GAG layer has been suggested to prevent urine substances from adhering to the bladder lumen. The barrier structure of urothelium restricts the movement of drugs after intravesical administration and restricts the action of the active drug fraction in the urine. Hence, many drugs fail to reach the bladder at desired therapeutic levels and ultimately lack pharmacological effects (14).
Liposomal drug delivery
To overcome the limited permeability of the bladder wall, the intravesical approach is able to modulate the release and absorption characteristics of instilled drugs through coupling them to novel carriers such as LPs. LPs are lipid vesicles composed of synthetic or natural phospholipid bilayers that self-assemble to enclose an aqueous interior. They can incorporate hydrophilic and hydrophobic drug molecules of various sizes and promote cellular drug uptake via endocytosis (4). The nontoxic nature of the lipids improves the delivery of various drugs by altering pharmacokinetics, and they have been widely used as drug carriers for a variety of chemotherapeutic agents (15). There is a long history of pharmaceutical agents with improved safety, and sometimes efficacy, when delivered by LPs (16).
Non-clinical studies of liposome (LP) for IC/BPS
Intravesical delivery of hyaluronic acid (HA), heparin, and chondroitin sulfate (CS) restores the barrier function lost due to epithelial dysfunction in IC/BPS. The same concept can be applied to LPs. LPs may aid in the formation of a lipid film on the luminal surface of the urothelium that protects it from penetration by irritants, stabilize neuromembranes of damaged nerves, and reduces hyperexcitability.
Fraser et al. (17) reported the effect of intravesically administered LPs of L-α-phosphatidylcholine: cholesterol at 2:1 in a rat model of hyperactive bladder induced by protamine sulfate (PS) followed by KCl or acetic acid infusion to mimic the IC state. The cystometrographic results showed that the bladder hyperactivity was partially reversed by treatment with the LP formulation.
Tyagi et al. (18) evaluated the comparative efficacy of LPs against intravesical instillation of dimethyl sulfoxide (DMSO) and pentosan polysulfate (PPS) in chemically induced bladder hyperactivity in rats by sequential infusion of PS and KCl. Intravesical LPs were effective in doubling the intercontractile interval (ICI) compared with PPS, while acute instillation of DMSO failed to produce any protective effect in this animal model.
A recent study showed that LPs carrying a trace amount of near-infrared (NIR) lipophilic fluorescent dye could be tracked microscopically (19). The LPs coating the bladder surface was indicated by blue-colored coating on the bladder luminal surface in NIR light. The study provides evidence to support that LPs form a protective film coating on the injured bladder lumen surface and assist in the repair of leaky and inflamed uroepithelium.
Clinical studies of liposomes (LPs) for IC/BPS
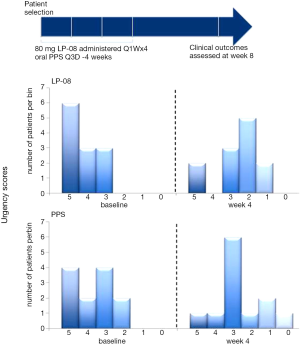
Chuang et al. (5) published the first information on the clinical safety and efficacy of LPs in the urinary bladder in an open-label prospective study of 24 IC/BPS patients. The effect of intravesical LPs (80 mg/40 CC distilled water) once weekly was compared to oral PPS sodium (100 mg) 3 times daily for 4 weeks each. No short- or long-term treatment-related adverse events were reported. Comparable efficacy of significant decreases in urinary frequency and nocturia were observed in each treatment group. Statistically significant decreases in pain, urgency, and the O’Leary-Sant symptom index were observed in the LP group with the effect being most profound on urgency (Figure 2). None of the patients reported urinary incontinence, retention, or infection due to LP instillation.
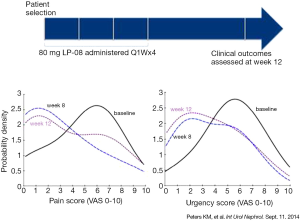
Peters et al. reported the results of 14 symptomatic IC/BPS subjects treated with intravesical LPs once a week for 4 weeks in an open-label study (20). No treatment-related adverse events were found over the course of the study. The most frequently reported pain score reduced by 80% at 8 (P=0.01) and 12 weeks (P=0.29). Urgency scores showed significant improvement (57% reduction) at 8 (P=0.076) and 12 weeks (P=0.084). The multilamellar sphingomyelin LPs used in this study (LP-08) were well-tolerated and their effects were associated with improvement in pain, urinary urgency and overall symptom scores (Figure 3).
LP delivery of botulinum toxin
The use of botulinum neurotoxin (BoNT) for the treatment of neurogenic detrusor over-activity (NDO) and idiopathic detrusor over-activity (IDO) has recently been approved by the U.S. FDA. BoNT-A acts by cleaving the soluble N-ethylmaleimide-sensitive fusion attachment protein receptor (SNARE) protein, SNAP-25 (21) and inhibiting release of various neurotransmitters at the presynaptic vesicle by binding to the synaptic vesicle protein, SV2, during neurotransmitter exocytosis. BoNT has been shown to modulate pain and inhibit afferent neurotransmission including substance P, glutamate, nerve growth factor, calcitonin gene related peptide and adenosine triphosphate (22). Given the function of chemical denervation, BoNT has been successfully used to treat overactive bladder (OAB) as well as IC/BPS through a cystoscopically guided injection. However, the method of intravesical injection has the potential for adverse events, such as urinary tract infection, urinary retention, pain, and hematuria.
BoNT serotype A is a neurotoxin with high molecular weight of 150 kDa. BoNT is generally provided in a saline solution. In this form, it cannot gain access to the submucosal nerve plexus without direct injection through the urothelium. Pretreatment of the urothelium with PS was attempted in rats with the goal of improving the permeability to BoNT (23-25). The cationic PS interacts with the anionic GAG layer, leading to a slight increase in permeability of the urothelium (26). Based on LP’s carrier potential and characteristics of adsorption and fusion with cells, the transport of BoNT into urothelium via LPs was studied and confirmed by detection of its unique effect on neurotransmitters and proteolysis of SNAP-25 through western blotting and immunohistochemistry. BoNT encapsulated within LPs is protected from degradation by proteases and proteinases in the urine without compromising efficacy (23). Therefore, instillation of liposomal mediated BoNT (lipo-BoNT) into the bladder is an exciting approach to achieve sustained duration of chemical neuromodulation of afferent neurotransmission underlying IC/BPS and OAB.
Kuo et al. (6) reported a double-blind randomized parallel controlled pilot trial in 24 OAB patients at a single tertiary center. Patients were randomly assigned to intravesical instillation of lipo-BoNT containing 80 mg LPs and 200 U BoNT serotype A or normal saline (N/S). Patients were retreated with lipo-BoNT 1 month later if they failed the first treatment. At 1 month post-treatment, the change of urinary frequency as reported on bladder diaries, which was the primary end point, significantly improved in the lipo-BoNT group (n=12; P=0.008) but not in the N/S group. (n=12; P=0.79). Urgency episodes also showed a significant decrease in the lipo-BoNT group (P=0.01) but not in the N/S group (P=0.2). SV2A and SNAP-25 were expressed in urothelial cells and suburothelial tissues. However, the protein expression did not significantly differ between responders and non-responders at 3 months after treatment. It is possible that the SNAP-25 proteins will have recovered by 3 months after treatment (6).
Chuang et al. (27) reported a two-center, double-blind, randomized, placebo controlled study enrolled patients with OAB inadequately managed with anti-muscarinics. Patients were assigned to intravesical instillation of lipo-BoNT or N/S. At 4 weeks after treatment, the lipo-BoNT instillation was associated with a statistically significant decrease in micturition events per 3 days (−4.64 for lipo-BoNT vs. −0.19 for placebo, P=0.025). The lipo-BoNT instillation was also associated with a statistically significant decrease in urinary urgency events with respect to baseline but not placebo. However, lipo-BoNT instillation was associated with a statistically significant decrease in urgency severity scores compared to placebo (P=0.0181). This study demonstrated that the lipo-BoNT instillation was not accompanied by an increased risk of urinary retention, and none of the patients at either site required intermittent catheterization. Currently, there is an international multicenter prospective double-blind placebo controlled study of lipo-BoNT in IC/BPS that is listed on ClinicalTrials.gov.
LP delivery of tacrolimus
Tacrolimus is a potent hydrophobic immunosuppressive agent that is involved in the inhibition of IL-2-dependent T-cell activation and has a direct inhibitory effect on cell-mediated immunity. Local treatment with tacrolimus has been shown to be beneficial in an ointment or lotion formulation against inflammatory skin conditions without systemic side effects (28). Tacrolimus has poor aqueous solubility; however, a liposomal formulation of tacrolimus greatly increases its solubility within the bladder, and it increases endocytosis and delivery of the drug. A previous study demonstrated that liposomal tacrolimus significantly inhibited cyclophosphamide-induced inflammatory cystitis through modulating interleukin (IL)-2, prostaglandin (PG) E2, and prostaglandin E receptor 4 (EP) function (29).
Nirmal et al. (30) evaluated the pharmacokinetics of tacrolimus encapsulated in LPs (lipo-tacrolimus). They found the area under the curve of lipo-tacrolimus in serum at 0-24 h was significantly lower than that of tacrolimus instillation or injection, and maximum concentration of lipo-tacrolimus in serum and urine was at 1 and 2 h, respectively. Urine area under the curve after intravesical administration was significantly higher than in the intraperitoneal injection group (P<0.05). Single dose pharmacokinetics revealed that bladder instillation of liposomal tacrolimus significantly decreased systemic exposure to instilled tacrolimus. Taken together, these findings support investigation of local tacrolimus in cases of inflammatory bladder disorders refractory to conventional therapy.
A recent study by Rajaganapathy et al. (31) examined creating a radiative cystitis rat model and observed the effects of lipo-tacrolimus treatment vs. placebo. To generate a radiative cystitis rat model, the animals were attached to a small animal radiation research platform (SARRP). A CT contrast agent was injected to target the SARRP radiation to the rodent bladder. A 40 Gy radiation most reliably produced cystitis symptoms within the bladder, and this level was used for the efficacy study. The radiation significantly lowered inter-micturition interval (IMI) values (P<0.05). Four weeks after the radiation, the rats were treated with a lipo-tacrolimus instillation (saline in placebo). The average IMI 4 weeks post-treatment for the lipo-tacrolimus treatment group returned to baseline levels (P>0.5; baseline vs. treatment) while the saline still showed decreased IMI levels (P<0.5; baseline vs. placebo). Histology showed that the lipo-tacrolimus treated bladder was identical to a healthy bladder with no features of note, whereas the placebo bladder showed degenerative type epithelial changes, urothelial swelling, and evidence of pseudo-carcinomatous epithelial hyperplasia.
Conclusions
Intravesical LPs have shown safety and efficacy in non-clinical and clinical IC/BPS studies. A prospective, double-blind, and placebo-controlled phase two study of using LPs for IC/BPS is currently ongoing. LPs may also improve vesicular trafficking in the urothelium and aid in improving the delivery of agents across the bladder permeability barrier. Encapsulation of botulinum toxin and tacrolimus inside LPs protected them from urinary degradation without compromising the efficacy of the active drug. The safety of intravesical LP therapy in the studies mentioned has been excellent, with no serious adverse events reported. Intravesical LP and liposomal drug delivery may be an exciting new treatment option for IC/BPS and other urology and women’s health disorders.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors are Employees of Lipella Pharmaceuticals, Inc.
References
- Weintraub MD, Li QQ, Agarwal PK. Advances in intravesical therapy for the treatment of non-muscle invasive bladder cancer Mol Clin Oncol 2014;2:656-60. (Review). [PubMed]
- Hsu CC, Chuang YC, Chancellor MB. Intravesical drug delivery for dysfunctional bladder. Int J Urol 2013;20:552-62. [PubMed]
- Apodaca G. The uroepithelium: not just a passive barrier. Traffic 2004;5:117-28. [PubMed]
- Cortesi R, Nastruzzi C. Liposomes, micelles and microemulsions as new delivery systems for cytotoxic alkaloids. Pharm Sci Technolo Today 1999;2:288-98. [PubMed]
- Chuang YC, Lee WC, Lee WC, et al. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. J Urol 2009;182:1393-400. [PubMed]
- Kuo HC, Liu HT, Chuang YC, et al. Pilot study of liposome-encapsulated onabotulinumtoxina for patients with overactive bladder: a single-center study. Eur Urol 2014;65:1117-24. [PubMed]
- Hanno PM, Burks DA, Clemens JQ, et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 2011;185:2162-70. [PubMed]
- Parsons CL, Greene RA, Chung M, et al. Abnormal urinary potassium metabolism in patients with interstitial cystitis. J Urol 2005;173:1182-5. [PubMed]
- Bosch PC, Bosch DC. Treating interstitial cystitis/bladder pain syndrome as a chronic disease. Rev Urol 2014;16:83-7. [PubMed]
- Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 2011;186:540-4. [PubMed]
- Lee JD, Lee MH. Activation of extrinsic apoptotic pathway from bladder biopsy in patients with interstitial cystitis/painful bladder syndrome. Urology 2013;82:1451.e7-11.
- Kaufman J, Tyagi V, Anthony M, et al. State of the art in intravesical therapy for lower urinary tract symptoms. Rev Urol 2010;12:e181-9. [PubMed]
- Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol 2000;278:F867-74. [PubMed]
- Min G, Zhou G, Schapira M, et al. Structural basis of urothelial permeability barrier function as revealed by Cryo-EM studies of the 16 nm uroplakin particle. J Cell Sci 2003;116:4087-94. [PubMed]
- Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol 1995;13:527-37. [PubMed]
- Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine 2012;7:49-60. [PubMed]
- Fraser MO, Chuang YC, Tyagi P, et al. Intravesical liposome administration--a novel treatment for hyperactive bladder in the rat. Urology 2003;61:656-63. [PubMed]
- Tyagi P, Hsieh VC, Yoshimura N, et al. Instillation of liposomes vs dimethyl sulphoxide or pentosan polysulphate for reducing bladder hyperactivity. BJU Int 2009;104:1689-92. [PubMed]
- Tyagi P, Kashyap MP, Kawamorita N, et al. Intravesical liposome and antisense treatment for detrusor overactivity and interstitial cystitis/painful bladder syndrome. ISRN Pharmacol 2014;2014:601653.
- Peters KM, Hasenau D, Killinger KA, et al. Liposomal bladder instillations for IC/BPS: an open-label clinical evaluation. Int Urol Nephrol 2014;46:2291-5. [PubMed]
- Dong M, Yeh F, Tepp WH, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science 2006;312:592-6. [PubMed]
- Chuang YC, Kuo HC, Chancellor MB. Botulinum toxin for the lower urinary tract. BJU Int 2010;105:1046-58. [PubMed]
- Chuang YC, Tyagi P, Huang CC, et al. Urodynamic and immunohistochemical evaluation of intravesical botulinum toxin A delivery using liposomes. J Urol 2009;182:786-92. [PubMed]
- Khera M, Somogyi GT, Salas NA, et al. In vivo effects of botulinum toxin A on visceral sensory function in chronic spinal cord-injured rats. Urology 2005;66:208-12. [PubMed]
- Chuang YC, Yoshimura N, Huang CC, et al. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol 2004;172:1529-32. [PubMed]
- Tzan CJ, Berg JR, Lewis SA. Mammalian urinary bladder permeability is altered by cationic proteins: modulation by divalent cations. Am J Physiol 1994;267:C1013-26. [PubMed]
- Chuang YC, Kaufmann JH, Chancellor DD, et al. Bladder instillation of liposome encapsulated onabotulinumtoxina improves overactive bladder symptoms: a prospective, multicenter, double-blind, randomized trial. J Urol 2014;192:1743-9. [PubMed]
- Migita K, Eguchi K. FK 506-mediated T-cell apoptosis induction. Transplant Proc 2001;33:2292-3. [PubMed]
- Chuang YC, Tyagi P, Huang HY, et al. Intravesical immune suppression by liposomal tacrolimus in cyclophosphamide-induced inflammatory cystitis. Neurourol Urodyn 2011;30:421-7. [PubMed]
- Nirmal J, Tyagi P, Chancellor MB, et al. Development of potential orphan drug therapy of intravesical liposomal tacrolimus for hemorrhagic cystitis due to increased local drug exposure. J Urol 2013;189:1553-8. [PubMed]
- Rajaganapathy BR, Janicki JJ, Levanovich P, et al. Intravesical Liposomal Tacrolimus Protects against Radiation Cystitis Induced by 3-Beam Targeted Bladder Radiation. J Urol 2015;194:578-84. [PubMed]