Clinicopathological features of primary angiosarcoma of the kidney: a review of 62 cases
Introduction
Angiosarcoma (AS) is a rare and aggressive malignant tumor of vascular or lymphatic origin which accounts for <2% of soft tissue sarcomas. Approximately one third of AS occurs in the skin, one third in soft tissues and one third in other sites such as the liver, bone and breast (1-4). This paper reviews the literature for primary AS of the kidney because of its rarity and its overlapping features with other renal tumors which poses diagnostic challenges with implications for management.
Methods
PubMed, Scopus, Google Scholar and Embase (Ovid SP) databases were searched for all articles and case reports of primary AS of the kidney until April 2015. The search strategy combined medical subject heading (MeSH) descriptors and text words such as primary AS of the kidney, primary renal AS and renal hemangiosarcoma. There was no language limitation to the search and a manual search of the reference list of relevant articles was undertaken. Clinical and pathological data were extracted when available and analyzed. The clinical data included age, sex, clinical presentation, presence of metastasis, sites of metastasis, treatment modality and follow up. Pathological data included the maximum tumor dimension measured in centimeters, the laterality of the tumor and immunohistochemical (IHC) expression of the tumor cells. All cases reported prior to Prince et al. (5) in 1942 were excluded from this review because the definition of AS had not been clearly established at that time and this is consistent with the views of Cason et al. (6), Hiratsuka et al. (7) and Leggio et al. (1) in earlier reviews.
Results
Tables 1 and 2 present a summary of the clinical and pathological data of the 62 cases of primary AS of the kidney in the literature.
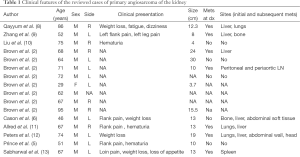
Full table
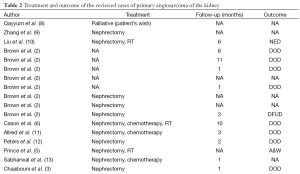
Full table
Discussion
Primary AS of the kidney is rare. Approximately 62 cases have been reported in the literature, mostly as case reports.
Epidemiology
Primary AS of the kidney occurs most frequently in the sixth and seventh decades (2) and this is consistent with the findings of this review with a mean age of 61 years (range, 24-95 years). It has a predilection for the male sex which accounted for 89% (54/61) of the patients in the review. There are seven reports of female patients with primary AS of the kidney in the literature (2,7,37,41,43,53,54). The left kidney was involved in 66.7% (36/54) of the patients in this review. The laterality of the tumor was not documented for eight cases (2,32,33,41,44,48,49,54) and so far, no familial predisposition have been established in spite of the occurrence of the tumor in two brothers aged 52 and 69 years respectively (22).
Etiology
Although the etiology of primary AS of the kidney is not known, the association between some exogenous risk factors and AS of other sites, especially the liver is well documented. The risk factors include thorium dioxide (thorotrast used in the past for angiography), occupational exposure to arsenic in insecticide which is used in agriculture and polyvinyl chloride in synthetic rubber industry (2,24,27,55,56). Radiotherapy and chronic lymphedema of any cause, either due to Milroy’s disease or chronic infections like filariasis is also associated with the development of AS. A classical case in point is the phenomenon known as Stewart-Treves syndrome which describes AS associated with lymphedema that occurs after the treatment of breast cancer (56). However these risk factors have not been proven to have a direct causal relationship with AS of the kidney (1,21).
Clinical features
This review highlights the overlapping features between primary AS of the kidney and other renal tumors. Flank pain was the most common symptom (3,5,6,9,11,18,20-24,28,29,31,34,35,40,46,47,52). Hematuria with or without abdominal pain accounted for 45.6% (21/46) of the clinical presentation (3,5,10,11,14-16,20,22,24,34,35,41,43,45,51,52,54). Other clinical features included weight loss (6,8,12,13,22,26,34), fever (23,26,36), hemoptysis (21,23,37,39), flank or costal swelling (42), malaise and ureteric obstruction (16). One patient had spontaneous rupture with the development of retroperitoneal hematoma (29) and the tumor was an incidental finding in five patients (4,19,27,38,50).
Metastatic disease at the time of diagnosis accounted for 44.9% (22/49) of the cases reported and 44.4% (12/27) of the patients with non-metastatic disease at diagnosis subsequently developed metastasis. Approximately half of the patients with metastatic disease had two or more sites involved. The sites of tumor spread included the lung, liver, peritoneum, spleen, abdominal lymph nodes (LNs) and the soft tissues. The liver and the lungs were the most frequent site of metastasis and there were reports of metastatic disease of the bone (4,6,9,14,17,18,20,26,31,39-41,43).
Imaging
Radiological imaging alone cannot ascertain the diagnosis of AS of the kidney. It may appear as a large necrotic renal mass on computed tomography (CT) which is virtually indistinguishable from a renal cell carcinoma (2,15). Contrast-enhanced CT findings (Figure 1) have been variably described as heterogeneous mass with peripheral enhancement (1,7,10,27) or hypo-dense renal mass with areas of enhancement (13,34). CT imaging also helps in delineating metastatic deposits. Given the rarity of AS of the kidney and in the event of small multiple lesions to distant sites such as the lungs and liver, a large single renal mass will be suggestive of a primary AS of the kidney (2).
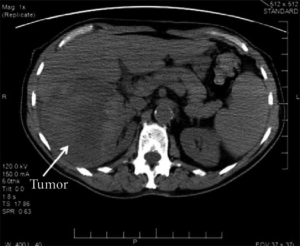
Preoperative diagnosis
Three patients with primary AS of the kidney have been diagnosed with CT guided fine needle aspiration cytology and the fine needle aspiration (FNA) results correlated with the histopathological and immunohistochemistry findings (15,21,36).
Pathologic findings
The histopathological feature of primary AS of the kidney is similar to AS of other sites. The average size of the tumor in this review is 13 cm (range, 3.7-30 cm). Primary AS of the kidney is predominantly solitary lesions. A rare instance of multifocal lesions (three) of the left kidney which measured 0.5-4.5 cm in size has been reported (38). AS of the kidney is mostly a hemorrhagic (1,3,4,9,10,13,19,25,35) ill-defined (7,10) or well circumscribed necrotic renal mass (4,8) (Figure 2). The renal parenchyma may be destroyed with frequent tumor extension to the perinephric fatty tissue (3,4).
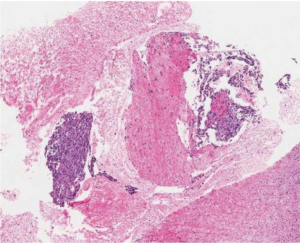
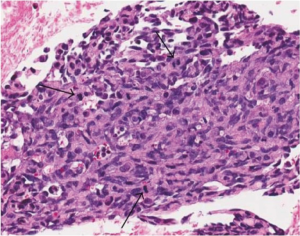
Primary AS of the kidney showed multiple and irregular anastomosing vascular spaces or channels which are lined by discrete and large endothelial cells with variable degrees of cytological pleomorphism, nuclear atypia, mitotic activity (Figure 3) and multilayering (1,4,7,8,10,24,32,34). There was a mix of epithelioid (2,10,15) and spindle cell (4,10,15,28,40) morphological pattern.
IHC studies
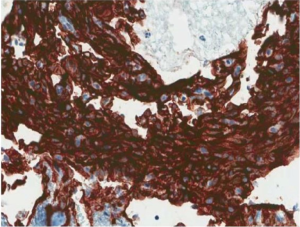
Primary AS of the kidney reacts negatively for epithelial markers like epithelial membrane antigen (EMA), Cam 5.2 and AE1/AE3 (2,4,8). Although AS of the kidney is mostly negative for epithelial markers, positive expression have been infrequently encountered in AS of other sites. A study of 80 cases of AS of the soft tissue demonstrated immunoreactivity to cytokeratins in 35% of the cases (57). This observation may reflect the rarity of primary AS of the kidney and the paucity of comprehensive IHC staining. Some positivity to epithelial markers in AS of the kidney, similar to the soft tissue counterparts may be found if larger numbers of AS of the kidney were studied. This highlights the need for a cocktail of panel of antibodies or markers in the diagnostic workup rather than a single marker. The tumor stained negative for RCC, CK8/18 (8), CD10 (4,8,27), S100, Melan-A and HMB-45 (1,2,4,9,28). Most tumor cells stained positively for endothelial markers (Figure 4) such as CD31, CD34 (1,2,4,8,9,15,24,27,32,34,40), FLI-1 (2,15) and factor 8-related antigen (3,4,7,14,15,27,28,34,40). Co-expression of vimentin may be present (3,10,19,40).
Aberrant expression of neuroendocrine markers in AS, which could be a potential diagnostic pitfall was recently documented (58). A case in point was the co-expression of synaptophysin in 5-10% of cells and chromogranin A in >75% of cells in a 29-year-old Afro-American lady with a hemorrhagic 3.7 cm AS of the kidney (2,58). Synaptophysin was positive in >75% of cells in the other two cases of AS reported. This finding suggested the possibility that some AS may show true neuroendocrine differentiation or it was simply a case of anomalous expression of the antigen. The aggressive clinical behavior of AS with aberrant neuroendocrine expression was similar to other AS however it remains unclear if the finding is of any clinical importance (58). The Ki-67 index of 30% (10), 40% (9) and >80% (13) is suggestive of the highly proliferative nature of the tumor.
Erythroblast transformation specific related gene (ERG), an ETS family transcription factor is an IHC marker with high sensitivity and specificity for vascular endothelial tumors. A study of 1,880 tumors which included vascular endothelial, mesenchymal and epithelial tumors that examined the diagnostic utility of ERG, suggested that ERG compares favorably with CD31 as a marker for AS. Ninety-six percent (96/100) AS of different clinicopathological subgroups and sites confirmed as CD31 positive, demonstrated nuclear ERG expression (59).
None of the cases in this review documented IHC study and expression of PAX8 and PAX2 markers. PAX8 and PAX2 belong to the family of paired box gene which encodes for nuclear transcription factors which is important in organogenesis and are excellent markers for tumors of renal, thyroid and mullerian origin (60). The expression of both markers have been documented in primary and metastatic renal epithelial tumors such as chromophobe RCC, oncocytomas, clear cell RCC, renal medullary carcinomas, papillary RCC etc. PAX8 appears to be a more sensitive marker compared to PAX2 (61). To the best of the knowledge of the author, the diagnostic utility of PAX8 and PAX2 in primary AS of the kidney awaits investigation.
Treatment
The rarity of primary AS of the kidney is largely responsible for the lack of standardized therapy. The patients were mostly treated with nephrectomy with varying combinations with chemotherapy (6,11,13,14,20,26,33,35,37,41,43,45,47,51,52), radiotherapy (5,6,10,18,28,35,39,41,43,47,48,50) and recombinant interleukin-2 therapy (40). The best treatment option for AS of the kidney remains controversial. Surgery appears to be the most effective treatment approach (31). Terris et al. (18) suggested that post-operative adjuvant radiotherapy may contribute to local control in a manner similar to AS of other sites, however Martínez-Piñeiro et al. (31) held a contrary view and observed that radiotherapy does not seem to prolong survival and chemotherapy should be added to the treatment. Mordkin et al. (26) suggested that chemotherapy may be used for palliative treatment, although the response is likely to be short. Zenico et al. (19) observed that patients who had the best response to treatment also underwent radiotherapy and chemotherapy with a median survival of 13 months (P>0.05) compared to 7 months in patients who underwent nephrectomy only. Zenico et al. however emphasized that none of the patients in the cohort reviewed who was treated with chemotherapy or radiotherapy had distant metastasis at the time of diagnosis (19).
Prognosis
Primary AS of the kidney is a very aggressive tumor with a poor prognosis. The mean follow-up is 7 months (range, 1-30 months) and most patients died of the disease. Data on the treatment outcome was not available for 18 of the patients in the review and that includes the case of a male patient who developed coincidental acute myeloblastic leukemia 7 months post left radical nephrectomy (30). There is evidence to suggest that the size of the tumor seems to be the most important factor for determining the prognosis of AS of the kidney. Tumors <5 cm have a significantly better prognosis compared to larger tumor lesions. Mark et al. (62) in a review of 67 cases of AS reported a 5-year survival of 32% for lesions <5 cm compared to 13% for lesions >5 cm. Consistent with this finding by Mark et al. are the two cases of primary AS of the kidney with the longest post-operative survival in the literature. Akkad et al. reported a patient with AS of the kidney 4.5 cm in diameter who had no evidence of disease 30 months after surgery without adjuvant therapy (27). Hiratsuka et al. also reported a patient with tumor size of 4.5 cm as well and the patient was disease free after 29 months of follow-up (7).
Conclusions
Primary AS of the kidney is a rare malignant tumor with a poor prognosis. It has a propensity for both local recurrence and distant metastasis at the time of diagnosis or shortly afterwards. The pathogenesis remains unclear, it has overlapping features with other renal tumors and imaging does not allow for tumor specific diagnosis. The importance of histopathology and immunohistochemistry cannot be overemphasized in the diagnosis of the tumor. There is no optimal and standard treatment however, current treatment options include a variable combination of surgery, radiotherapy and chemotherapy.
Acknowledgements
Many thanks to the Editor-in-Chief of Case Reports in Pathology for the kind permission to use the images and figures reprinted from Primary renal angiosarcoma with extensive necrosis: a difficult diagnosis.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Leggio L, Addolorato G, Abenavoli L, et al. Primary renal angiosarcoma: a rare malignancy. A case report and review of the literature. Urol Oncol 2006;24:307-12. [PubMed]
- Brown JG, Folpe AL, Rao P, et al. Primary vascular tumors and tumor-like lesions of the kidney: a clinicopathologic analysis of 25 cases. Am J Surg Pathol 2010;34:942-9. [PubMed]
- Chaabouni A, Rebai N, Chabchoub K, et al. Primary renal angiosarcoma: case report and literature review. Can Urol Assoc J 2013;7:E430-2. [PubMed]
- Fukunaga M. Angiosarcoma of the kidney with minute clear cell carcinomas: a case report. Pathol Res Pract 2009;205:347-51. [PubMed]
- Prince CL. Primary angio-endothelioma of the kidney: report of a case and brief review. J Urol 1942;47:787-9.
- Cason JD, Waisman J, Plaine L. Angiosarcoma of the Kidney. Urology 1987;30:281-3. [PubMed]
- Hiratsuka Y, Nishimura H, Kajiwara I, et al. Renal angiosarcoma: a case report. Int J Urol 1997;4:90-3. [PubMed]
- Qayyum S, Parikh JG, Zafar N. Primary renal angiosarcoma with extensive necrosis: a difficult diagnosis. Case Rep Pathol 2014; 2014: 416170.
- Zhang HM, Yan Y, Luo M, et al. Primary angiosarcoma of the kidney: case analysis and literature review. Int J Clin Exp Pathol 2014;7:3555-62. [PubMed]
- Liu H, Huang X, Chen H, et al. Epitheloid angiosarcoma of the kidney: a case report and literature review. Oncol lett 2014;8:1155-8. [PubMed]
- Allred CD, Cathey WJ, McDivitt RW. Primary renal angiosarcoma: a case report. Hum Pathol 1981;12:665-8. [PubMed]
- Peters HJ, Nuri M, Münzenmaier R. Hemangioendothelioma of the kidney: A case report and review of the literature. J Urol 1974;112:723-6. [PubMed]
- Sabharwal S, John NT, Kumar RM, et al. Primary renal angiosarcoma. Indian J Urol 2013;29:145-7. [PubMed]
- López Cubillana P, Martínez Barba E, Server Pastor G, et al. Fatal evolution of a renal angiosarcoma. Arch Esp Urol 2004;57:425-6. [PubMed]
- Singh C, Xie L, Schmechel SC, et al. Epithelioid angiosarcoma of the kidney: a diagnostic dilemma in fine-needle aspiration cytology. Diagn Cytopathol 2012;40 Suppl 2:E131-9. [PubMed]
- Askari A, Novick A, Braun W, et al. Late ureteral obstruction and hematuria from de novo angiosarcoma in a renal transplant patient. J Urol 1980;124:717-9. [PubMed]
- Douard A, Pasticier G, Deminière C, et al. Primary Angiosarcoma of the kidney: case report and literature review. Prog Urol 2012;22:438-41. [PubMed]
- Terris D, Plaine L, Steinfeld A. Renal angiosarcoma. Am J Kidney Dis 1986;8:131-3. [PubMed]
- Zenico T, Saccomanni M, Salomone U, et al. Primary renal angiosarcoma: case report and review of world literature. Tumori 2011;97:e6-e9. [PubMed]
- Desai MB, Chess Q, Naidich JB, et al. Primary renal angiosarcoma mimicking a renal cell carcinoma. Urol Radiol 1989;11:30-2. [PubMed]
- Johnson VV, Gaertner EM, Crothers BA. Fine needle aspiration of renal angiosarcoma. Arch Pathol Lab Med 2002;126:478-80. [PubMed]
- Kern SB, Gott L, Faulkner J 2nd. Occurrence of primary renal angiosarcoma in brothers. Arch Pathol Lab Med 1995;119:75-8. [PubMed]
- Adjiman S, Zerbib M, Flam T, et al. Genitourinary tumors and HIV-1 infection. Eur Urol 1990;18:61-3. [PubMed]
- Papadimitriou VD, Stamatiou KN, Takos DM, et al. Angiosarcoma of kidney; a case report and review of literature. Urol J 2009;6:223-5. [PubMed]
- Tsuda N, Chowdhury PR, Hayashi T, et al. Primary renal angiosarcoma: a case report and review of the literature. Pathol Int 1997;47:778-83. [PubMed]
- Mordkin RM, Dahut WL, Lynch JH. Renal angiosarcoma: a rare primary genitourinary malignancy. South Med J 1997;90:1159-60. [PubMed]
- Akkad T, Tsankov A, Pelzer A, et al. Early diagnosis and straightforward surgery of an asymptomatic primary angiosarcoma of the kidney led to long-term survival. Int J Urol 2006;13:1112-4. [PubMed]
- Cerilli LA, Huffman HT, Anand A. Primary renal angiosarcoma: a case report with immunohistochemical, ultrastructural and cytogenetic features and review of the literature. Arch Pathol Lab Med 1998;122:929-35. [PubMed]
- Aksoy Y, Gürsan N, Ozbey I. Spontaneous rupture of a renal angiosarcoma. Urol Int 2002;68:60-2. [PubMed]
- Aydogdu I, Turhan O, Sari R, et al. Coincidental acute myeloblastic leukemia in a patient with renal angiosarcoma. Haematologia (Budap) 1999;29:313-7. [PubMed]
- Martínez-Piñeiro L, Lopez-Ferrer P, Picazo ML, et al. Primary renal angiosarcoma: case report and review of the literature. Scand J Urol Nephrol 1995;29:103-8. [PubMed]
- Lee TY, Lawen J, Gupta R. Renal angiosarcoma: a case report and literature review. Can J Urol 2007;14:3471-6. [PubMed]
- Berretta M, Rupolo M, Buonadonna A, et al. Metastatic angiosarcoma of the kidney: a case report with treatment approach and review of the literature. J Chemother 2006;18:221-4. [PubMed]
- Souza OE, Etchebehere RM, Lima MA, et al. Primary renal angiosarcoma. Int Braz J Urol 2006;32:448-50. [PubMed]
- Costero-Barrios CB, Oros-Ovalle C. Primary renal angiosarcoma. Gac Med Mex 2004;140:463-6. [PubMed]
- Grapsa D, Sakellariou S, Politi E. Fine-needle aspiration cytology of primary renal angiosarcoma with histopathologic and immunocytochemical correlation: a case report. Diagn Cytopathol 2014;42:872-6. [PubMed]
- Carnero López B, Fernández Pérez I, Carrasco Alvarez JA, et al. Renal primary angiosarcoma. Clin Transl Oncol 2007;9:806-10. [PubMed]
- Sesar P, Ulamec M, Šoša S, et al. Primary renal angiosarcoma. Acta Clin Croat 2012;51:182.
- Pauli JL, Strutton G. Primary renal angiosarcoma. Pathology 2005;37:187-9. [PubMed]
- Yoshida K, Ito F, Nakazawa H, et al. A case of primary renal angiosarcoma. Rare tumors 2009;1:e28. [PubMed]
- Juan CJ, Yu CH, Hsu HH, et al. Visceral and non-visceral angiosarcoma: Imaging features and clinical correlation. Chin J Radiol 2000;25:183-9.
- Xuan Y. Primary renal angiosarcoma: one case report and literatures review. Chin J Clin Oncol 2008;5:229-300.
- Yau T, Leong CH, Chan WK, et al. A case of mixed adult Wilms’ tumor and angiosarcoma responsive to carboplatin, etoposide and vincristine (CEO). Cancer Chemother Pharmacol 2008;61:717-20. [PubMed]
- Garmendia JC, Lopez Garcia JA, Acinas Garcia O, et al. Angiosarcoma of the kidney. Actas Urol Esp 1989;13:223-4. [PubMed]
- Nguyen T, Auquier MA, Renard C, et al. Hemoptysis and spontaneous rupture of a primary renal angiosarcoma: a case report. J Radiol 2010;91:1313-17. [PubMed]
- Limmer S, Wagner T, Leipprand E, et al. Primary renal hemangiosarcoma. Case report and review of the literature. Pathologe 2001;22:343-8. [PubMed]
- Matter LE, Flury R, Hailemariam S, et al. Angiosarcoma of the kidney. Case report and review of the literature. Urologe A 1999;38:65-8. [PubMed]
- Sanyal B, Mehrotra ML, Gupta S, et al. Radiotherapy in renal Angiosarcoma. J Indian Med Assoc 1979;72:85-6. [PubMed]
- Testa G, Talamona G, Tufano A, et al. Primary renal angiosarcoma: a case report. Acta Urologica Italica 1998;12:225-7.
- Yamamoto Y, Izaki H, Harada A, et al. A case of renal capsular hemangiosarcoma. Hinyokika Kiyo 2006;52:215-7. [PubMed]
- Rüb J, Bauer S, Pastor J, et al. Primary renal angiosarcoma: uncommon manifestation of a rare malignancy. Urologe A 2015.
- Celebi F, Pilanci KN, Saglam S, et al. Primary renal Angiosarcoma with progressive clinical course despite surgical and adjuvant treatment: a case report. Oncol Lett 2015;9:1937-9. [PubMed]
- Li N, Li W, Li Z. Primary renal epitheloid angiosarcoma with transitional cell carcinoma in renal pelvis. Zhonghua Wai Ke Za Zhi 1997;35:294-5. [PubMed]
- Witczak W, Szubstarski F, Szymański C, et al. Renal hemangiosarcoma. Pol Tyg Lek 1993;48:483-4. [PubMed]
- Popper H, Thomas LB, Telles NC, et al. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast and arsenic. Am J Pathol 1978;92:349-76. [PubMed]
- Penel N, Marréaud S, Robin YM, et al. Angiosarcoma: state of the art and perspectives. Crit Rev Oncol Hematol 2011;80:257-63. [PubMed]
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol 1998;22:683-97. [PubMed]
- Tessier Cloutier B, Costa FD, Tazelaar HD, et al. Aberrant expression of neuroendocrine markers in Angiosarcoma: a potential diagnostic pitfall. Hum Pathol 2014;45:1618-24. [PubMed]
- Miettinen M, Wang Z, Paetau A, et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol 2011;35:432-41. [PubMed]
- Tong GX, Yu WM, Beaubier NT, et al. Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod Pathol 2009;22:1218-27. [PubMed]
- Ozcan A, de la Roza G, Ro JY, et al. PAX2 and pAX8 expression in primary and metastatic renal tumors: a comprehensive comparison. Arch Pathol Lab Med 2012;136:1541-51. [PubMed]
- Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma: A report of 67 patients and a review of the Literature. Cancer 1996;77:2400-6. [PubMed]


