Surgery and hormonal treatment for prostate cancer and sexual function
Introduction
Prostate cancer (PC) is the most common cancer in the aging male population and the second leading cause of cancer death (1). The American Cancer Society estimates 1 in 7 men will be diagnosed with PC in their lifetime, with the average age at 66 years old (1). The risk of PC increases with age. However, because of the slow progression of the cancer, the majority of men diagnosed do not die from the disease. In fact, the ACS quotes the relative 10-year survival rate as 99% (1).
Due to the chronic nature of this cancer, and the extended time from premalignant lesion to “clinically relevant” cancer, treatment should focus not only on survival but also on quality of life and sexual health (2). The goal of treatment should be to minimize the risk-benefit ratio. However, this goal is limited by the lack of complete understanding of the pathophysiology, as well as the heterogeneity, of the disease. Despite this fact, it is widely known that, as whole, androgens promote the growth and progression of PC. Even with a diversity of androgenic interplay among diseased individuals, there appears to be a common side effect among all treatment modalities, as each has a degree of negative impact on male sexual health and function (2). Common alterations to male sexual health include erectile dysfunction (ED), changes in penile length and girth, pain with sexual activity, and dysfunctions of ejaculation and orgasm. Among these, ED is oftentimes cited as the major concern of men following treatment for PC (3). Primary treatment modalities for PC consist of active surveillance, surgical removal of the prostate, radiation, and androgen deprivation therapy (ADT). In this review we will focus on prostatectomy and androgen deprivation, and their effects on male sexual function.
In order to understand the sexual dysfunction resulting from PC treatment, it is necessary to first understand the normal physiology. Normal male sexual function requires the involvement and coordination of multiple regulatory systems and is thus subject to the influence of psychological, hormonal, neurological, vascular, and cavernosal factors. The initial obligatory event required for male sexual activity, the acquisition and maintenance of penile erection, is primarily a vascular phenomenon. The arterial dilatation and venous compression required for erection is triggered by neurologic signals and facilitated only in the presence of an appropriate hormonal milieu and psychological mindset. An alteration in any of these factors may be sufficient to cause sexual dysfunction (4,5).
Radical prostatectomy (RP)
RP is primarily a treatment option for patients with localized PC, and is not indicated for patients with clinical evidence of regional lymph node involvement or distant metastases or when there is tumor fixation to adjacent structures. Moreover, in the recently published Scandinavian Prostate Cancer Group 4 trial it was found that prostatectomy, for localized disease, has significantly lower incidence of death from all causes, death from prostate, distant metastases, and use of ADT compared with those who undergo watchful waiting. The benefits were most pronounced in those less than 65 years of age at diagnosis and in those with intermediate risk disease (6).
However, as with all treatment options, prostatectomy is associated with a number of risks. The most commonly reported postoperative complications include ED and urinary incontinence (2). ED following RP has been reported in 60-70% of men, although definition of ED varies in reported sources (3). The etiology of ED following RP is most likely multifactorial-mechanical or thermal injury intraoperatively or postsurgical inflammation can lead to neuropraxia or permanent damage of the cavernosal neurovascular bundle. Ligation of the accessory internal pudendal arteries also plays a role in postoperative ED, as it decreases arterial inflow leading to subsequent hypoxia and apoptosis (4). Chronic loss of erections in itself contributes to these ischemic changes through decreased blood flow, cavernosal smooth muscle fibrosis, apoptosis, and collagen deposition (3,7). Corporal veno-occlusive dysfunction also may be seen causing clinically evident venogenic impotence (3,7). The nerve-sparing technique for RP, first described by Walsh in 1982, has reported rates of 40-86% positive erectile function following surgery; however still 90% of men will experience some initial decline in sexual function (8).
An alteration of the hypothalamic pituitary axis may also explain the initial ED and urinary incontinence seen following RP, as it has been noted that in the immediate post-operative period there is greater sexual dysfunction and incontinence than there is 15 years out. This can be explained by one prospective study, which enrolled 100 men with clinically localized PC to evaluate the serum levels of testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) following RP. Immediately following surgery, a drastic decline in serum testosterone was observed, along with compensatory rise in LH and FSH. At three months out from surgery, testosterone levels were seen to normalize, however LH and FSH remained elevated. It was postulated by the authors that this could explain the delayed recovery of erectile function and urinary control that is seen following RP (9).
Sivarajan et al. prospectively examined sexual function and erectile function in men undergoing open RP over a 10-year period. Men in the study completed a sexual function survey at baseline and at increasing intervals over this time period. The expected initial decline in both measured outcomes was seen, followed by a time-dependent improvement through 2 years post RP. Sexual function appeared to remain stable in the 2-10 year postoperative period. However, younger men and those with pretreatment potency were more likely to actually continue to see improvements in erectile function past 2 years. Despite this, all treatment groups, including RP, radiation treatment and active surveillance, are noted to be subject to time dependent changes of erectile function (10).
RP has also been shown to decrease emission, incontinence during sexual activity, and decrease pleasure with orgasm (10). Loss of penile length and girth is also reported. One study saw up to a 3 cm decrease in stretched penile length at 12 months out from treatment. It has been postulated that parasympathetic damage secondary to cavernosal nerve injury leads to overcompensation of the sympathetic nervous system and release of factors responsible for penile shortening (3). Other changes in penile appearance are seen, including curvature and onset of Peyronie’s disease, all of which negatively impact male sexual health and thus decrease post treatment quality of life.
In a post-operative analysis of men who underwent RP, Dubbleman et al. found that orgasmic function was preserved in 73.4% after a bilateral nerve sparing procedure, in 70.9% after a unilateral nerve-sparing procedure and in 54.0% after non-nerve sparing technique. Indicating that orgasmic dysfunction plays a relatively minor role in post prostatectomy sexual dysfunction (11). However, it has been noted that PC survivors may experience lack of ejaculation at the point of orgasm or urinary incontinence associated with orgasm (climacturia), and the impact of these changes may be challenging for patients and their partners. It has been noted however, that climacturia does not significantly impact sexual satisfaction (12).
Another treatment modality frequently used in localized disease burden is radiotherapy (RT). RT, like RP, is also associated with multiple risks. Commonly observed risks include bowel dysfunction, sexual dysfunction and urinary incontinence. The prevalence of stated risks among patients with localized disease undergoing RP versus those undergoing RT was evaluated in the Prostate Cancer Outcomes Study (PCOS), a cohort comprised of 1,655 men with localized PC. In this study the functional status of patients’ bowel, sexual and bladder functions were assessed at baseline, and at 2, 5, and 15 years following diagnosis. It was found that, at 2 and 5 years post treatment, ED and urinary incontinence were more likely to occur in patients who underwent RP. While bowel urgency was more likely to occur following RT at 2 and 5 years post treatment. However, despite the declines in all functional domains, seen in both treatment groups, during 15 years of follow-up no significant differences in disease-specific functional outcomes were observed. This suggests that both treatment options decrease quality of life in patients with localized disease to an equal extent (13).
Since the median life expectancy following treatment of clinically localized PC is 13.8 years, it is imperative to uncover and address the long-term quality of life outcomes when discussing treatment options with patients. In order to provide the patient with the ability to make the best decision, evidence based prediction models may be used. One such model has been developed for ED by Alemozaffar et al. In their study they examined men within the Prostate Cancer Outcomes and Satisfaction with Treatment Quality Assessment (PROSTQA) cohort with early stage PC who opted to undergo prostatectomy, external RT, or brachytherapy. Pretreatment characteristics of individual patients, quality of life regarding sexual function, and treatment paradigms were included in the prediction model. The primary outcome was erections defined as “firm enough” for intercourse based on the EPIC-26 (Expanded Prostate Cancer Index Composite). Using this data, models predicting erectile function 2 years out were developed. These were validated using a similar community based-cohort. In this cohort, satisfactory erectile function was reported in 177 of 511 [35% (95% CI, 30-39%)] men status post prostatectomy. Younger age, less comorbidities, lower prostate specific antigen (PSA), lower risk PC, erectile function prior to treatment, better sexual quality of life questionnaire scores, and plan for nerve-sparing surgery were associated with greater probability of erectile function 2 years out using univariable analysis. Multivariable analysis, however, only showed younger age, better pretreatment sexual functioning score, and nerve-sparing surgery as factors leading to potency 2 years following surgery (14). By sharing this evidence with patients, and enabling them greater insight into their personal treatment risks, post treatment quality of life may be increased.
Androgen ablation
The role of androgens in the pathophysiology of PC has been well documented, as it is known that androgen receptor signaling is critical for PC growth and survival (15,16). The first mention of this role was made in 1941 by Huggins and Hodges, with their observation that castration levels of testosterone led to PC regression. This provided the nidus from which ADT developed (17,18). However, a precise understanding of androgenic stimulation and the mechanism by which it effects the initiation and progression of PC has yet to be elucidated, and is likely multimodal and subject to patient specific genetic aberrations (19). Despite this, recent evidence supporting a favorable risk-benefit ratio for ADT in PC is currently limited to men with high-risk or metastatic disease. This is in part because ADT has been associated with a number of constitutional and somatic side effects. Similarly, ADT use has been limited in localized PC due to its association with lower PC-specific survival and no increase in overall survival compared with conservative management (15).
There are still mixed opinions as to whether or not ADT should be used as monotherapy in intermediate and high risk patients. The EUA recommends primary ADT if there is symptomatic locally advanced PC or positive nodal involvement (20). However, the National Institute for Health and Care Excellence (NICE) does not recommend ADT as monotherapy for men with intermediate and high risk localized PC. On the other hand, it has been shown, that for patients undergoing RT, adjunct ADT improves overall patient survival relative to RT alone (21-24).
ADT plays a major role in the treatment of metastatic prostate disease, and is considered mandatory treatment in symptomatic patients, as immediate hormonal therapy may improve cancer-specific survival for men with advanced PC and maximal androgen blockade might increase overall survival at 5 years (21,23,25). The corollary, however, is that ADT has also been associated with increased adverse events and reduced quality of life.
The three most common adverse effects experienced with ADT are hot flashes, bone fractures and impotence with eventual ED. ED is a particularly distressing side effect and may develop in 10-30% men after ADT therapy (26,27). Similarly, in a cohort analysis from a randomized trial, 75% of men reported ED at 5 years following neoadjuvant androgen deprivation plus external beam radiation therapy for localized PC (26).
ADT, accomplished either by surgical or medical means, induces ED via decreased testosterone. The loss of testosterone causes decreased libido and decreased arterial dilatation and flow leading to sexual dysfunction (28). In order to alleviate sexual dysfunction, many modifications of ADT therapy have been looked at. One such alteration is intermittent ADT, which may be used in men with rising PSA after local therapy but no evidence of metastases. This strategy was tested by Cook et al., who randomized patients with rising PSA after either primary or salvage RT to continuous ADT versus intermittent ADT and found no difference in overall survival but significantly better sexual desire in the intermittent group (P<0.001). Unfortunately in men with metastatic disease, intermittent ADT cannot be recommended as a strategy to reduce sexual dysfunction because of the inadequate therapeutic response (21,24).
Another ADT treatment option for patients, which has been shown to decrease sexual side effects, is antiandrogen monotherapy instead of a gonadotropin-releasing hormone (GnRH) agonist. A randomized trial of leuprolide versus bicalutamide 150 mg found less of a decline in sexual interest in the bicalutamide group. However, a decrease in the efficacy of this substitution must be considered (24,29). Nonetheless, exercise is likely the safest means by which sexual side effects may be minimized on ADT. Cormie et al. investigated the effect of a 12-week exercise program on sexual activity in 57 PC patients undergoing ADT and found a significant (P=0.045) adjusted group difference in sexual activity following the 12-week intervention. Following the intervention, the exercise group had a significantly higher percentage of participants reporting a major interest in sex (30).
In conjunction to a direct physiological decrease in libido and erectile function, ADT is also associated with decreased penile length and testicular size which is associated with great regret and likely contributes to the sexual side effects of ADT (21). As such, continued research and verification of current evidence minimizing sexual dysfunction in ADT treated men should continue.
Penile rehabilitation
For PC survivors, sexual dysfunction following PC diagnosis and treatment is common and greatly impacts quality of life. The etiology of sexual dysfunction is multifactorial, but has been tied to orgasmic dysfunction, penile changes, climacturia and a variety of psychological causes.
Yet another contributing factor to sexual dysfunction is decreased penile length. Loss of penile length is a source of patient bother and distress and is seen after RP and ADT. Recent exploration into treatment options has found that decreased flaccid penile length may be reduced and or prevented with treatment with phosphodiesterase-5 (PDE-5) inhibitors after RP. On the other hand, in patients with non-metastatic disease, who undergo short term ADT, it has been shown that men recover supra-castrate testosterone levels. As such these patients should return to pretreatment penile length. The recovery of potency and sexual desire in these patients is dependent upon age and ADT duration (31).
Animal models exhibiting bilateral cavernosal nerve damage have demonstrated corresponding corporal smooth muscle apoptosis and fibrosis. PDE-5 inhibitors in rat models have shown reversal of these changes, with preservation of smooth muscle content and decrease in previous fibrotic deterioration. Despite this evidence seen in animal models, there remains no consensus regarding the clinical effectiveness of PDE-5 inhibitors in preserving the penile morphological changes seen (32). Loss of penile length has been observed in men following bilateral nerve sparing RP (33). The different studies examining this phenomenon had various designs and reported findings in an inconsistent manner (32,33). In addition to this, no studies have shown a “connection between corporal fibrosis and penile size changes” (33).
ED remains the most common cause of sexual dysfunction after RP. The treatment of ED is aimed at penile rehabilitation and attempts to address loss of erections by preventing the post-treatment changes. Such changes lead to ischemia, apoptosis and fibrosis. It is thought that this pathway can be interrupted with therapy to improve erections, with a goal of “natural spontaneous erections” (34). Options available for treatment include PDE-5 inhibitors, intraurethral alprostadil, intracavernosal injections, and vacuum erection devices (VED). Despite evidence available for each, no consensus has been reached on a single protocol for penile rehabilitation following PC treatment.
The REACTT trial (effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing RP) was a randomized controlled trial with the primary outcome of erectile function in men treated with PDE-5 inhibitors following bilateral nerve-sparing RP. The study specifically examined tadalafil daily and on demand versus placebo in men with preoperative normal erectile function and clinically localized PC. At nine months follow-up, recovery of erectile function was significantly higher in the once daily group than in the control group; this was not seen in the on demand group. However, following a drug-free washout period, there were no significant differences in either group compared to placebo. Penile length, evaluated as a secondary study outcome, was significantly less affected in the once daily group compared to placebo; this effect was not observed with on demand dosing. Although the primary objective of the authors was not met, there appears to be a potential role for once daily tadalafil for penile length protection. In a previous study examining vardenafil, on demand and daily dosing resulted in significant improvement in erectile function compared to placebo. Although this seemed to conflict, the authors attributed this to the differing pharmokinetic profiles of each drug (35).
Technological and medicinal advancements have widened ED treatment options. A variety of external penile support devices exist (36). One such external device is the handheld penile vibrator, which is thought to be a good option for penile rehabilitation as it increases the neurotransmitters from the cavernous nerve terminals that are involved in penile erection. Another option is low intensity extracorporeal shockwave (LI-ESW), which is thought to improve erectile function through recruitment of endogenous stem cells (37). A VED is another option for patients; in fact studies show it is the second most commonly used method for penile rehabilitation after RP. VED uses negative pressure to distend the corporal sinusoids and to increase blood inflow to the penis (38).
Despite the wide array of therapeutic options, a study done by Megas et al., which compared penile prosthesis surgery to oral PDE-5 inhibitor administration, in men with ED after nerve-sparing RP, found that currently penile prosthesis provides the most satisfactory option for patients with severe ED (39). However, the study did note that the efficacy and satisfaction results of both treatment types are considered acceptable. As such when notifying patients of the risks of therapeutic options for PC, one should also consider the availability of corrective treatments.
Proposed management algorithm
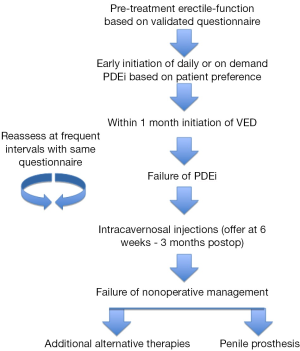
There is no consensus regarding the best rehabilitation program, but based on our clinical practice early initiation of treatment is warranted. After having a discussion with the patient regarding available treatment modalities and the evidence, or lack thereof, supporting the role of each in penile rehabilitation, take an individualized approach to addressing ED in this select patient population. If the patient is willing, it may be beneficial to take an aggressive approach in management. Despite differing evidence, there does appear to be some role of early initiation of rehabilitation in the recovery of erectile function following PC treatment. PDE-5 inhibitors have been shown effective, at least in the short-term, for recovery of erectile function and even in maintaining penile length. Since the psychosocial factor of ED should not be ignored, even a short, if not sustained, benefit could assist in a man’s recovery of sexual function. VED, which are inexpensive and have minimal side effects, should be employed for possible reversal of post-treatment tissue changes. At 6 weeks post-treatment, if PDE-5 inhibitors have failed, offer the patient intracavernosal injections, but no later than 3 months out. If the patient’s function remains suboptimal at a predetermined timeframe, it would be reasonable to offer penile implant, which has excellent satisfaction, or alternate therapies, such as intracavernosal injections, intraurethral therapy, or vibratory stimulation based on patient preference and motivation. Throughout management, erectile function should be assessed with a validated questionnaire at frequent intervals and with the same survey so as to follow response objectively (see Figure 1).
Conclusions
With the advent of the PSA era and the ability to diagnose and treat PC earlier, quality of life is a major consideration when choosing treatment (40). Male sexual health is hampered by the therapies currently available for PC. It is imperative to discuss the risks associated with each respective treatment option with men prior to PC treatment. With the development of clinical prediction models and recent studies examining the expected course of erectile function and overall male sexual health, an individualized discussion with each patient can hopefully be achieved. Options for penile rehabilitation are available; however, no consensus on duration or modality of choice has been defined. The future of clinical research should focus on prospective randomized controlled trials examining optimum treatment aimed at achieving spontaneous erections sooner following treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Cancer Society. What are the key statistics about prostate cancer? Available online: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics. Accessed 2014.
- Gacci M, Baldi E, Tamburrino L, et al. Quality of Life and Sexual Health in the Aging of PCa Survivors. Int J Endocrinol 2014;2014:470592.
- Chung E, Gillman M. Prostate cancer survivorship: a review of erectile dysfunction and penile rehabilitation after prostate cancer therapy. Med J Aust 2014;200:582-5. [PubMed]
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am 2005;32:379-95. v. [PubMed]
- Sáenz de Tejada I, Angulo J, Cellek S, et al. Pathophysiology of erectile dysfunction. J Sex Med 2005;2:26-39. [PubMed]
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014;370:932-42. [PubMed]
- Wang R. Penile rehabilitation after radical prostatectomy: where do we stand and where are we going? J Sex Med 2007;4:1085-97. [PubMed]
- Haliloglu A, Baltaci S, Yaman O. Penile length changes in men treated with androgen suppression plus radiation therapy for local or locally advanced prostate cancer. J Urol 2007;177:128-30. [PubMed]
- Sherer BA, Levine LA. Current management of erectile dysfunction in prostate cancer survivors. Curr Opin Urol 2014;24:401-6. [PubMed]
- Gacci M, Simonato A, Masieri L, et al. Urinary and sexual outcomes in long-term (5+ years) prostate cancer disease free survivors after radical prostatectomy. Health Qual Life Outcomes 2009;7:94. [PubMed]
- Sivarajan G, Prabhu V, Taksler GB, et al. Ten-year outcomes of sexual function after radical prostatectomy: results of a prospective longitudinal study. Eur Urol 2014;65:58-65. [PubMed]
- Dubbelman Y, Wildhagen M, Schröder F, et al. Orgasmic dysfunction after open radical prostatectomy: clinical correlates and prognostic factors. J Sex Med 2010;7:1216-23. [PubMed]
- O'Neil BB, Presson A, Gannon J, et al. Climacturia after definitive treatment of prostate cancer. J Urol 2014;191:159-63. [PubMed]
- Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA 2011;306:1205-14. [PubMed]
- Nelson PS. Targeting the androgen receptor in prostate cancer--a resilient foe. N Engl J Med 2014;371:1067-9. [PubMed]
- Grossmann M, Cheung AS, Zajac JD. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract Res Clin Endocrinol Metab 2013;27:603-16. [PubMed]
- Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 2002;168:9-12. [PubMed]
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin 1972;22:232-40. [PubMed]
- Sissung TM, Price DK, Del Re M, et al. Genetic variation: effect on prostate cancer. Biochim Biophys Acta 2014;1846:446-56.
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2014;65:124-37. [PubMed]
- Parekh A, Chen MH, Hoffman KE, et al. Reduced penile size and treatment regret in men with recurrent prostate cancer after surgery, radiotherapy plus androgen deprivation, or radiotherapy alone. Urology 2013;81:130-4. [PubMed]
- Kumar S, Shelley M, Harrison C, et al. Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev 2006.CD006019. [PubMed]
- Samson DJ, Seidenfeld J, Schmitt B, et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer 2002;95:361-76. [PubMed]
- Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med 2000;132:566-77. [PubMed]
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2014;65:467-79. [PubMed]
- Daly PE, Dunne MT, O'Shea CM, et al. The effect of short term neo-adjuvant androgen deprivation on erectile function in patients treated with external beam radiotherapy for localised prostate cancer: an analysis of the 4- versus 8-month randomised trial (Irish Clinical Oncology Research Group 97-01). Radiother Oncol 2012;104:96-102. [PubMed]
- DiBlasio CJ, Malcolm JB, Derweesh IH, et al. Patterns of sexual and erectile dysfunction and response to treatment in patients receiving androgen deprivation therapy for prostate cancer. BJU Int 2008;102:39-43. [PubMed]
- Higano CS. Sexuality and intimacy after definitive treatment and subsequent androgen deprivation therapy for prostate cancer. J Clin Oncol 2012;30:3720-5. [PubMed]
- Smith MR, Goode M, Zietman AL, et al. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol 2004;22:2546-53. [PubMed]
- Cormie P, Newton RU, Taaffe DR, et al. Exercise maintains sexual activity in men undergoing androgen suppression for prostate cancer: a randomized controlled trial. Prostate Cancer Prostatic Dis 2013;16:170-5. [PubMed]
- Wilke DR, Parker C, Andonowski A, et al. Testosterone and erectile function recovery after radiotherapy and long-term androgen deprivation with luteinizing hormone-releasing hormone agonists. BJU Int 2006;97:963-8. [PubMed]
- Sirad F, Hlaing S, Kovanecz I, et al. Sildenafil promotes smooth muscle preservation and ameliorates fibrosis through modulation of extracellular matrix and tissue growth factor gene expression after bilateral cavernosal nerve resection in the rat. J Sex Med 2011;8:1048-60. [PubMed]
- McCullough A. Penile change following radical prostatectomy: size, smooth muscle atrophy, and curve. Curr Urol Rep 2008;9:492-9. [PubMed]
- Alba F, Wang R. Current status of penile rehabilitation after radical prostatectomy. CML-Urology 2010;16:93-101.
- Montorsi F, Brock G, Stolzenburg JU, et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT). Eur Urol 2014;65:587-96. [PubMed]
- Stein MJ, Lin H, Wang R. New advances in erectile technology. Ther Adv Urol 2014;6:15-24. [PubMed]
- Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med 2013;10:738-46. [PubMed]
- Tal R, Teloken P, Mulhall JP. Erectile function rehabilitation after radical prostatectomy: practice patterns among AUA members. J Sex Med 2011;8:2370-6. [PubMed]
- Megas G, Papadopoulos G, Stathouros G, et al. Comparison of efficacy and satisfaction profile, between penile prosthesis implantation and oral PDE5 inhibitor tadalafil therapy, in men with nerve-sparing radical prostatectomy erectile dysfunction. BJU Int 2013;112:E169-76. [PubMed]
- Cooperberg MR. Implications of the new AUA guidelines on prostate cancer detection in the U.S. Curr Urol Rep 2014;15:420.