Unnecessary diagnostic imaging: a review of the literature on preoperative imaging for boys with undescended testes
Introduction
Cryptorchidism (undescended testis) is one of the most common pediatric disorders of the male endocrine glands and the most common genital disorder identified at birth. Cryptorchidism occurs in 1% to 3% of full-term and up to 45% of preterm male neonates (1). On physical exam, undescended testes may be palpable or non-palpable. When a testis is nonpalpable, the testis may be intra-abdominal or a “peeping” testis at the internal ring (25% to 50%), absent, a testicular nubbin (“vanishing” testis, 15% to 40%), or present but not appreciated on exam in the office due to body habitus, testicular size, and/or limited physical exam secondary to patient cooperation (10% to 30%) (2).
Progressive germ and Leydig cell loss is associated with longer lengths of time a testis remains undescended (3), and cryptorchidism is associated with a 2.7 times greater risk of testicular cancer (4). In order to decrease the risk of impaired fertility and testicular cancer, the recently published American Urological Association (AUA) guidelines recommend that surgery should be performed within the next year if testicular descent does not occur by 6 months (corrected for gestational age) (5). The operative approach for cryptorchidism is based upon the palpability of the testis at the time of examination under anesthesia. However, preoperative imaging is often used to identify cryptorchid or absent testes. The purpose of this article is to review the clinical utility of diagnostic imaging in the preoperative evaluation of boys with cryptorchidism, with respect to its benefits, limitations, and cost.
Clinical evaluation
Gestational history is critical to determine the proper time to refer a child with cryptorchidism. Testicular descent occurs in two phases: transabdominal descent at approximately 22-25 weeks gestational age, and inguinoscrotal migration at 25-30 weeks gestational age (6,7). As migration through the inguinal canal occurs relatively late in gestational development, cryptorchidism is accordingly higher in premature boys in the first few months of life. Testes may descend after birth; however, spontaneous testicular descent is unlikely in boys older than 6 months, corrected for gestational age (8,9). Boys older than 6 months with cryptorchidism should be referred to a surgical specialist for evaluation (5).
Depending on provider preference and age of the child, boys may be examined in a frog legged supine position, sitting with legs crossed, or standing. Assistance with keeping the child still facilitates the exam. Abducting the thigh also helps to inhibit the cremasteric reflex and thus limits testicular elevation from elicitation of this reflex (2). There are several possible findings on physical examination when a testis is not descended to the correct position. An undescended testis may be located in the abdomen, the inguinal canal, the superficial inguinal pouch, the upper scrotum, or another ectopic position such as perineum, contralateral scrotum, or femoral region. The palpability of the undescended testis is the most critical aspect of the evaluation of a boy with cryptorchidism as palpability determines the surgical approach. When the testis is palpable, an inguinal or prescrotal ochiopexy is the preferred approach as the location of the testis is known to be distal to the internal ring. If the testis remains nonpalpable under anesthesia, laparoscopy is the preferred diagnostic and therapeutic approach. Diagnostic laparoscopy or inguinal exploration will identify a viable testis or confirm an absent testis by revealing blind-ending spermatic vessels or a nonviable nubbin (10-13). Laparoscopy has nearly 100% sensitivity and specificity to localize a testis or confirm its absence and this has become the criterion standard against which diagnostic imaging studies are measured (14-19). Depending on access to equipment and surgical expertise, open surgery remains an alternative to laparoscopy.
Preoperative diagnostic imaging
Diagnostic imaging offers the theoretic possibility of sparing a child an operation if a non-palpable testis is confirmed to be absent with 100% certainty (20). In a national cross-sectional survey of pediatricians practicing in the United States conducted by Tasian et al., 67% of respondents reported ordering imaging during the preoperative evaluation of boys with cryptorchidism (21). Of the respondents who ordered imaging, 86% reported doing so because they believe imaging reveals the presence or absence of a nonpalpable testis. Fifty percent of respondents believed imaging reassures the family, and 47% reported imaging assists the surgeon in planning the operative approach.
Ultrasound
Ultrasound is the most imaging modality most commonly used to evaluate boys with undescended testes (21). Ultrasound is noninvasive and does not use ionizing radiation, which makes it an attractive imaging study for children. However, the most important limitation of ultrasound is its ability to accurately localize undescended testes. Even for palpable testes, Elder et al. reported poor concordance between physical exam and ultrasound findings; only 12 of the 33 testes palpable either in the scrotum or in the inguinal canal on physical examination could be identified by ultrasound (22). Ultrasound is not necessary in the evaluation of boys with palpable testes because, most importantly, testicular position is already known by physical exam and, secondly, ultrasound may provide misleading information. These testes can be approached through either an inguinal or pre-scrotal orchiopexy. For non-palpable testes, which is the clinical scenario for which ultrasound is most frequently ordered, the sensitivity and specificity of ultrasound is poor. In 1985, Weiss et al. found that ultrasound falsely identified 10% of gubernacular structures as undescended testis (23). Although there have been many advances in ultrasound technology over the past several decades, 20 years later, in 2007, Nijs et al. reported that ultrasound failed to identify all 14 of viable intraabdominal testes when using 5 to 12 MHz and 7 to 10 MHz transducers (24). Thus, even with current technology, ultrasound cannot reliably localize non-palpable testes, which comprise 20% of all undescended testes (25).
Tasian and Copp (26) recently performed a systematic review and meta-analysis of English language studies on ultrasound evaluation of nonpalpable undescended testis. They reported that the sensitivity and specificity of ultrasound in correctly identifying a nonpalpable testis was 45% and 78%, respectively. The positive and negative likelihood ratios (LR), which are the increase and decrease in the odds of a test is actually being in the position identified by ultrasound, were 1.48 and 0.79, respectively. When interpreting LR, a positive LR of 1 to 2 or a negative LR of 0.5 to 1 (27), indicate small and clinically insignificant changes to clinical management (18). Using the positive and negative LR for ultrasound evaluation of non-palpable testes, a positive ultrasound increases the probability that a nonpalpable testis is located within the abdomen from 55% to 64%. A negative ultrasound decreases the probability that a nonpalpable testis is located within the abdomen from 55% to 49% (19). Table 1 demonstrates how ultrasound changes the probability of a nonpalpable test is actually being located in the abdomen, assuming different pre-test probabilities (26). Due to the minimal change in the probability of testicular location and the persistent probability that a testis is in the abdomen even if ultrasound does not identify the testis, Tasian and Copp concluded that ultrasound is not useful in determining the surgical management of patients with nonpalpable undescended testes.
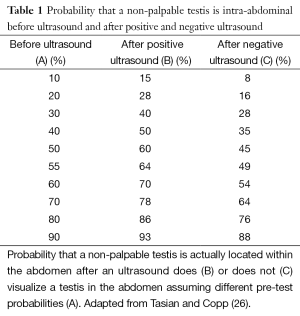
Full table
Reliance on findings of preoperative ultrasound may provide misleading information and can have serious consequences. If a urologist decides not to operate on a child with a nonpalpable testis that was not visualized by ultrasound, there is still, assuming the highest sensitivity and specificity of ultrasound, a 36% probability that the testis is within the abdomen (18). Not operating if this testis is present increases the risk of testicular carcinoma, which, given the intraabdominal location of the testis, potentially places the child at a higher risk of delayed presentation with advanced disease due to the inability to perform routine screening testicular self-examinations (4,28).
Computed tomography (CT)
CT has been used selectively as a diagnostic imaging modality to evaluate undescended testis. In the early 1980s, Lee et al. reported that CT correctly identified 100% of eight undescended testes. However, five of these testes were potentially palpable in the inguinal canal (29,30). Furthermore, there are potential risks of secondary malignancy conferred by medical radiation, which is especially concerning for children given their long life expectancy and greater susceptibility to the effects of ionizing radiation (31). While CT continues to have an important role in staging of testicular cancer for which boys with cryptorchidism are at increased risk, there is no role for routine CT evaluation of boys with undescended testes.
Magnetic resonance imaging (MRI)
As opposed to CT, MRI does not involved ionizing radiation, which makes it a potential attractive alternative for children in need of cross-sectional imaging. However, MRI has its own limitations, including more limited availability, high cost, and, arguably, most importantly, the need for sedation or anesthesia in order for children to tolerate the scan. In 1999, Yeung et al. (32) reported that the gadolinium-enhanced MRI identified 20 of 21 non-palpable testes of which 4 were intra-abdominal and 8 were intracanicular nubbins. These findings demonstrated that MRI had a sensitivity and specificity of 96% and 100%, respectively. While the authors concluded from this that laparoscopy could have been avoided in 78% of patients who had preoperative identification of inguinal testes or nubbins (31), not identifying a testis does not completely exclude its absence (33,34). This is supported by a recent systematic review by Krishnaswami et al. (35). Krishnaswami reviewed eight observational studies that examined the performance of conventional MRI to identify the presence or absence of testicles and reported that the accuracy of MRI to identify non-palpable testes ranged from 42% to 88%. The false positive rate of conventional MRI was 14% (5 of 35 cases). In those cases, lymph nodes were mistaken for viable testicular tissue. Even more importantly, MIR missed 38% of viable intra-abdominal testes. This high false negative rate makes it unlikely for MRI to reliably replace surgical exploration at this time.
Guidelines for diagnostic imaging use
Guidelines from multiple professional organizations consistently recommend that imaging not be routinely performed in the diagnosis of undescended testes prior to surgical intervention. US Department of Health and Human Services guidelines recommend against ultrasound, CT, and MRI for boys with cryptorchidism as these tests do not add clinically important information to the physical examination (18,22,26,36,37). Grade A recommendations from the National Guideline Clearinghouse (37) state that there is no reliable examination to confirm or rule out an intra-abdominal, inguinal and absent/vanishing testis (non-palpable testis), except for diagnostic laparoscopy. They do, however, recommend an exam under general anesthesia prior to carrying out a laparoscopic assessment, as some, originally non-palpable, testes are palpable under anesthetic conditions.
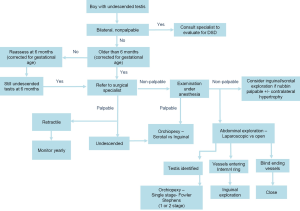
As part of the Choosing Wisely Campaign, a collaborative effort among medical specialities to identify evidence based care that is truly necessary, the AUA stated that ultrasound should not be performed on boys with cryptorchidism (38). Expanding on this, the AUA recently released clinical guidelines that explicitly state that providers should not perform ultrasound or other imaging modalities in the evaluation of boys with cryptorchidism prior to referral, as these studies rarely assist in decision making (5). Figure 1 shows the recently published recommended algorithm for management of boys with cryptorchidism (5).
The European Association of Urology (EAU) guidelines also state that there is no benefit in performing ultrasound, CT, MRI, or angiography (39).
Implications for health care costs
The overuse of imaging by primary care providers in this population likely stems from the erroneous belief that ultrasound is a reliable way to identify nonpalpable testis (21). It is possible that the limitations of imaging in evaluating cryptorchidism are not known to pediatricians. An ancillary hypothesis is that referring providers do not realize that diagnostic imaging would not change the operative approach and surgical decision-making. Knowledge of the nonutility of imaging needs to be disseminated to primary care providers. Younger practitioners and those not practicing in an academic environment may be particularly important populations to target as these were provider characteristics associated with high use of ultrasound (21).
While advances in technology and diagnostic imaging are inevitable and valuable in certain clinical scenarios, it is essential that physicians carefully consider the limitations of diagnostic imaging to determine when imaging does not change clinical decision making, but does unnecessarily increase health care costs. Given the contribution of diagnostic imaging to US health care costs and the rate at which costs are increasing (40,41), it is our hope that the current guideline statements from the AUA and EAU will increase awareness among all physicians involved in the care of children with cryptorchidism and help limit costs from unnecessary diagnostic imaging.
Conclusions
Preoperative imaging does not change surgical management of non-palpable testes because a reliable imaging modality is not yet available that can be used in lieu of the gold standard of laparoscopy to correctly identify the presence and location of a non-palpable undescended testis. Additionally, by ordering preoperative imaging in these cases, one may be delaying evaluation and treatment by a surgical specialist, adding to the costs of our healthcare system, and subjecting a child and family to unnecessary testing.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sijstermans K, Hack WW, Meijer RW, et al. The frequency of undescended testis from birth to adulthood: a review. Int J Androl 2008;31:1-11. [PubMed]
- Barthold JS. Abnormalities of the testis and scrotum and their surgical management. In: Wein AJ, Kavoussi LR, Novick AC, et al. eds. Campbell-Walsh Urology. 10th ed. Philadelphia, PA: Saunders Elsevier, 2011:3557-95.
- Tasian GE, Hittelman AB, Kim GE, et al. Age at orchiopexy and testis palpability predict germ and Leydig cell loss: clinical predictors of adverse histological features of cryptorchidism. J Urol 2009;182:704-9. [PubMed]
- Pettersson A, Richiardi L, Nordenskjold A, et al. Age at surgery for undescended testis and risk of testicular cancer. N Engl J Med 2007;356:1835-41. [PubMed]
- Kolon TF, Herndon CD, Baker LA, et al. Evaluation and treatment of cryptorchidism: AUA guideline. J Urol 2014;192:337-45. [PubMed]
- Hutson JM. A biphasic model for the hormonal control of testicular descent. Lancet 1985;2:419-21. [PubMed]
- Barteczko KJ, Jacob MI. The testicular descent in human. Origin, development and fate of the gubernaculum Hunteri, processus vaginalis peritonei, and gonadal ligaments. Adv Anat Embryol Cell Biol 2000;156:III-IIX. [PubMed]
- Scorer CG. The descent of the testis. Arch Dis Child 1964;39:605-9. [PubMed]
- Berkowitz GS, Lapinski RH, Dolgin SE, et al. Prevalence and natural history of cryptorchidism. Pediatrics 1993;92:44-9. [PubMed]
- Gapany C, Frey P, Cachat F, et al. Management of cryptorchidism in children: guidelines. Swiss Med Wkly 2008;138:492-8. [PubMed]
- Moore RG, Peters CA, Bauer SB, et al. Laparoscopic evaluation of the nonpalpable tests: a prospective assessment of accuracy. J Urol 1994;151:728-31. [PubMed]
- Lotan G, Klin B, Efrati Y, et al. Laparoscopic evaluation and management of nonpalpable testis in children. World J Surg 2001;25:1542-5. [PubMed]
- Snodgrass WT, Yucel S, Ziada A. Scrotal exploration for unilateral nonpalpable testis. J Urol 2007;178:1718-21. [PubMed]
- Ellsworth PI, Cheuck L. The lost testis: Failure of physical examination and diagnostic laparoscopy to identify inguinal undescended testis. J Pediatr Urol 2009;5:321-3. [PubMed]
- Arnbjörnsson E, Mikaelsson C, Lindhagen T, et al. Laparoscopy for nonpalpable testis in childhood: is inguinal exploration necessary when vas and vessels are not seen? Eur J Pediatr Surg 1996;6:7-9. [PubMed]
- Zaccara A, Spagnoli A, Capitanucci ML, et al. Impalpable testis and laparoscopy: when the gonad is not visualized. JSLS 2004;8:39-42. [PubMed]
- Kantarci M, Doganay S, Yalcin A, et al. Diagnostic performance of diffusion-weighted MRI in the detection of nonpalpable undescended testes: comparison with conventional MRI and surgical findings. AJR Am J Roentgenol 2010;195:W268-73. [PubMed]
- Shah A, Shah A. Impalpable testes--is imaging really helpful? Indian Pediatr 2006;43:720-3. [PubMed]
- Baillie CT, Fearns G, Kitteringham L, et al. Management of the impalpable testis: the role of laparoscopy. Arch Dis Child 1998;79:419-22. [PubMed]
- Tasian GE, Copp HL, Baskin LS. Diagnostic imaging in cryptorchidism: utility, indications, and effectiveness. J Pediatr Surg 2011;46:2406-13. [PubMed]
- Tasian GE, Yiee JH, Copp HL. Imaging use and cryptorchidism: determinants of practice patterns. J Urol 2011;185:1882-7. [PubMed]
- Elder JS. Ultrasonography is unnecessary in evaluating boys with a nonpalpable testis. Pediatrics 2002;110:748-51. [PubMed]
- Weiss RM, Carter AR, Rosenfield AT. High resolution real-time ultrasonography in the localization of the undescended testis. J Urol 1986;135:936-8. [PubMed]
- Nijs SM, Eijsbouts SW, Madern GC, et al. Nonpalpable testes: is there a relationship between ultrasonographic and operative findings? Pediatr Radiol 2007;37:374-9. [PubMed]
- Smolko MJ, Kaplan GW, Brock WA. Location and fate of the nonpalpable testis in children. J Urol 1983;129:1204-6. [PubMed]
- Tasian GE, Copp HL. Diagnostic performance of ultrasound in nonpalpable cryptorchidism: a systematic review and meta-analysis. Pediatrics 2011;127:119-28. [PubMed]
- Gallagher EJ. Clinical utility of likelihood ratios. Ann Emerg Med 1998;31:391-7. [PubMed]
- Walsh TJ, Dall’Era MA, Croughan MS, et al. Prepubertal orchiopexy for cryptorchidism may be associated with lower risk of testicular cancer. J Urol 2007;178:1440-6. [PubMed]
- Lee JK, Glazer HS. Computed tomography in the localization of the nonpalpable testis. Urol Clin North Am 1982;9:397-404. [PubMed]
- Lee JK, McClennan BL, Stanley RJ, et al. Utility of computed tomography in the localization of the undescended testis. Radiology 1980;135:121-5. [PubMed]
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009;169:2078-86. [PubMed]
- Yeung CK, Tam YH, Chan YL, et al. A new management algorithm for impalpable undescended testis with gadolinium enhanced magnetic resonance angiography. J Urol 1999;162:998-1002. [PubMed]
- Miyano T, Kobayashi H, Shimomura H, et al. Magnetic resonance imaging for localizing the nonpalpable undescended testis. J Pediatr Surg 1991;26:607-9. [PubMed]
- Maghnie M, Vanzulli A, Paesano P, et al. The accuracy of magnetic resonance imaging and ultrasonography compared with surgical findings in the localization of the undescended testis. Arch Pediatr Adolesc Med 1994;148:699-703. [PubMed]
- Krishnaswami S, Fonnesbeck C, Penson D, et al. Magnetic resonance imaging for locating nonpalpable undescended testicles: a meta-analysis. Pediatrics 2013;131:e1908-16.
- Kanemoto K, Hayashi Y, Kojima Y, et al. Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of non-palpable testis. Int J Urol 2005;12:668-72. [PubMed]
- National Guideline Clearinghouse. Cryptorchidism. In: Guidelines on paediatric urology Rockville MD: Agency for Healthcare Research and Quality (AHRQ). Available online: http://www.guideline.gov/content.aspx?id=14430
- Five Things Physicians and Patients Should Question 2013. Available online: http://www.choosingwisely.org/doctor-patient-lists/american-urological-association
- Tekgül S, Riedmiller H, Hoebeke P, et al. EAU guidelines on vesicoureteral reflux in children. Eur Urol 2012;62:534-42. [PubMed]
- Dinan MA, Curtis LH, Hammill BG, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999-2006. JAMA 2010;303:1625-31. [PubMed]
- Iglehart JK. Health insurers and medical-imaging policy--a work in progress. N Engl J Med 2009;360:1030-7. [PubMed]


