
How to deal with steroids use in the management of metastatic prostate cancer during pandemic
The ongoing COVID-19 outbreak is an unprecedented global challenge especially for patients within the high-risk groups such as those with cancer. Protecting fragile patients from COVID-19 infection and exacerbation is of utmost importance. One of the risks of exacerbation of the systems has been the use of some currently used medications such as non-steroid anti-inflammatory drug (NSAID) and steroids (1). Patients suffering from advanced cancer may be more susceptible to develop severe COVID-19 symptoms due to their potential cancer-related or treatment-related immunosuppressive state. Based on the available literature, the prevalence of COVID-19 infection seems higher in cancer patients compared to the general population (2%) resulting in a higher risk for severe respiratory events (39% vs. 8%) (2,3). Moreover, COVID-19 patients with immune deficit should be at higher risk for developing severe respiratory diseases (4).
In the past decade, several new compounds targeting the androgen axis such as abiraterone acetate have been approved and recommended for the treatment of metastatic castration-sensitive prostate cancer (mCSPC) and metastatic castration-resistant prostate cancer (mCRPC) (5). Abiraterone acetate is a CYP17 inhibitor which is prescribed with prednisone (daily dose of 5 or 10 mg) in order to prevent drug induced hyperaldosteronism (5-7). However, the non-essential use of steroids has come under scrutiny during COVID-19 pandemic due to its potential immunosuppressive effect which could predispose patients to more severe infection. Thus, several panels of experts such as the Guidelines Office Rapid Reaction Group of the European Association of Urology (EAU) or the National Comprehensive Cancer Network (NCCN) have warned against the use of abiraterone acetate during the COVID-19 period and recommended alternative approved treatment options with the same level of evidence and approved for the same indications, such as enzalutamide (8,9). There has, unfortunately, not been any data presented to support this deviation from pre-COVID19 standard recommendations without precision about the rational and the different situations in metastatic prostate cancer (mPCa).
Therefore, to understand rational for the recommendations we evaluate the data underlying it and to provide simple recommendations for the stewardships use of androgen receptor targeting agents in the management of mPCa during the COVID-19 pandemic.
Long-term steroids therapy and COVID-19
Steroids are commonly used in the management of chronic diseases such as cancers and rheumatologic diseases. They are also used to treat select symptoms of COVID-19. While the benefit of high-dose steroids during the inflammatory response in patients infected by SaRs-CoV-2 is still debated (10), it is thought that the long-term use of steroids can negatively alter the natural history of COVID-19. Until now, only one case report has been published reporting a familial cluster of COVID-19. A 47-year-old woman with long-term use of prednisone (7.5 mg daily) had atypical primary symptoms followed by a prolonged duration of viral shedding (11). Hence, the authors hypothesized that the long-term use of steroids might cause atypical infections with delay in symptoms (longer pre-symptomatic infectability), a longer period of virus shedding (longer overall infectability) leading to potential prolonged risk of COVID-19 transmission. Controversially, a Chinese retrospective study reported that low-dose steroid therapy does not delay viral clearance in patients with COVID-19 (12). However, to date, the relationship between viral clearance, viral infectability, and the occurrence of serious complications remains unclear.
Only limited data is available on the disease course in patients taking immunosuppressive therapies during previous coronavirus epidemics, such as the 2002–2003 SARS-CoV and the MERS-CoV epidemics. This limits any inferences from these previous similar pathogen outbreaks (13).
Steroids exposure has rarely been addressed as a potential risk factor for pneumonia in the general population. Nevertheless, in a recent UK cohort study which included 275,072 adults the risk of pulmonary infection was significantly higher in patients who had an oral steroids intake for ≥15 days, as compared to those who were not exposed to steroids (OR: 5.84; 95% CI: 5.61–6.08; P<0.001) (14). The extrapolation of this data to SARS-CoV-2 infections is challenging for two reasons. First, the pulmonary manifestations during the COVID-19 pandemic are not caused by bacterial infection but by the virus himself and by an inappropriate immune response. Second, steroids could have a potentially protective effect by mitigating the detrimental inflammatory response to COVID-19 infection.
Among all the factors influencing the adverse effects due to steroids, dose and treatment duration are the most important ones for infection. Severe adverse effects secondary to steroids can occur with “supra-physiologic” doses (such as a dose over 10 mg equivalent dose of prednisone) (15). On the one hand, the long period of exposure, even to low-dose steroid in patients treated for mCSPC and mCRPC might have an immunosuppressive effect and increase the risk of symptomatic infection. On the other hand, the abrupt cessation of long-term steroid therapy, even taken at low doses, can be dangerous and tapering is suggested over a period lasting week. Moreover, the time to recover of a normal immunity and to achieving complete steroid weaning remains unclear, especially in cancer patients with cancer and potentially other comorbidities.
Management of mPCa patients
The recommendations regarding the rational restriction of the use of chemotherapy when possible during the pandemic period seem widely accepted (8,9). In mCSPC and mCRPC, docetaxel combined with androgen deprivation therapy (ADT) and prednisone should be avoided based on the risk of neutropenia and frequent hospital visits during the pandemic. If absolutely needed, the risk of infection has to be minimized by reducing the dose, the number and time between cycles, in addition to the systematic use of granulocyte colony-stimulating factors (G-CSF) and a limited exposure to steroids (16).
Optimal use of abiraterone acetate with prednisone during the pandemic is highly debated. While some studies have shown the safety of abiraterone without prednisone or with small dose (17,18), the risk of adverse events is not negligible and the use of abiraterone without steroids is neither supported by high quality data nor any consensus or guidelines. There are seemingly equally effective approved alternatives to abiraterone acetate for the management of mPCa (5) such as enzalutamide and apalutamide. To enact precautionary measures in the management of high-risk populations such as mPCa during the COVID-19 pandemic because of the availability of treatments with similar efficacy and equivalent profile of tolerability, enzalutamide and apalutamide should be the preferred treatment choices in the management of mCSPC and enzalutamide for mCRPC (Figure 1). Nevertheless, such a statement may be difficult to apply in countries where due to financial or other restrictions, there is no or limit access only to these drugs. As the COVID-19 pandemic evolves, new evidence is being published every day. To date, there is no data to suggest a switch from ongoing abiraterone acetate and steroids therapy to another therapeutic option in patients. It remains to be said that there is no convincing evidence regarding the risks associated with the use of low dose steroids on the COVID-19 infection. It is clear that stopping a steroid therapy can result in undesired adverse events. Moreover, the risk of losing efficacy by stopping an ongoing, well tolerated therapy has to be considered in an overall context of limited health care access during the pandemic. Nevertheless, in case of pursuit of treatment with prednisone, it is utmost of importance to educate patients about symptoms that need to be investigated, and caregivers about the potential consideration of stress-dose steroids in case of advanced COVID-19 symptoms.
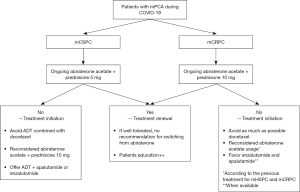
Conclusions
Concerns exist about the potential risk associated with the use of steroids during the COVID-19 pandemic such as a warning of COVID-19 transmission and infection severity. Therefore, for the initiation of mCSPC or mCRPC therapy, one could/should consider the use of an alternative androgen receptor target such as enzalutamide or apalutamide (according to indications) instead of abiraterone acetate with prednisone. The use of docetaxel chemotherapy should also be considered with special care during the pandemic. In case of ongoing abiraterone acetate plus prednisone combination, there is no strong evidence to suggest a treatment switch or early stoppage.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1011). GP reports non-financial support from JANSSEN, ASTELLAS, FERRING, TAKEDA, SANOFI, and IPSEN, outside the submitted work. SFS reports non-financial support from ASTELLAS, ASTRA ZENECA, BAYER, BMS, CEPHEID, FERRING, IPSEN, JANSSEN, LILLY, MSD, OLYMPUS, PFIZER, PIERRE FABRE, RICHARD WOLF, ROCHE, SANOCHEMIA, SANOFI, TAKEDA, and UROGEN, outside the submitted work. PO reports grants from MERCK, and VARIAN, grants and non-financial support from BAYER, and FERRING, non-financial support from ASTELLAS, and JANSSEN, outside the submitted work. MR reports non-financial support from ROCHE, IPSEN, ASTRA ZENECA, GSK, and ASTELLAS, outside the submitted work. BP reports non-financial support from JANSSEN, ASTELLAS, IPSEN, FERRING, and PIERRE FABRE, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pradère B, Ploussard G, Catto JWF, et al. The use of nonsteroidal anti-inflammatory drugs in urological practice in the COVID-19 era: is "safe better than sorry"? Eur Urol 2020;78:134-5. [Crossref] [PubMed]
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335-7. [Crossref] [PubMed]
- Desai A, Sachdeva S, Parekh T, et al. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol 2020;6:557-9. [Crossref] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 2017;71:630-42. [Crossref] [PubMed]
- Auchus RJ, Yu MK, Nguyen S, et al. Use of prednisone with abiraterone acetate in metastatic castration-resistant prostate cancer. Oncologist 2014;19:1231-40. [Crossref] [PubMed]
- Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 2008;26:4563-71. [Crossref] [PubMed]
- Ribal MJ, Cornford P, Briganti A, et al. European Association of Urology Guidelines Office Rapid Reaction Group. An Organisation-wide Collaborative Effort to Adapt the European Association of Urology Guidelines Recommendations to the Coronavirus Disease 2019 Era. Eur Urol 2020;78:21-8. [Crossref] [PubMed]
- Care of prostate cancer patients during the COVID-19 pandemic: recommendations of the NCCN. Available online: (Accessed April 14, 2020).https://www.nccn.org/covid-19/pdf/NCCN_PCa_COVID_guidelines.pdf
- Veronese N, Demurtas J, Yang L, et al. Use of corticosteroids in coronavirus disease 2019 pneumonia: a systematic review of the literature. Front Med (Lausanne) 2020;7:170. [Crossref] [PubMed]
- Han Y, Jiang M, Xia D, et al. COVID-19 in a patient with long-term use of glucocorticoids: a study of a familial cluster. Clin Immunol 2020;214:108413. [Crossref] [PubMed]
- Fang X, Mei Q, Yang T, et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect 2020;81:147-78. [PubMed]
- Guery B, Poissy J, el Mansouf L, et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet 2013;381:2265-72. [Crossref] [PubMed]
- Fardet L, Petersen I, Nazareth I. Common infections in patients prescribed systemic glucocorticoids in primary care: a population-based cohort study. PLoS Med 2016;13:e1002024. [Crossref] [PubMed]
- Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis 1989;11:954-63. [Crossref] [PubMed]
- Mourey L, Falandry C, de Decker L, et al. Taking care of older patients with cancer in the context of COVID-19 pandemic. Lancet Oncol 2020;21:e236. [Crossref] [PubMed]
- McKay RR, Werner L, Jacobus SJ, et al. A phase 2 trial of abiraterone acetate without glucocorticoids for men with metastatic castration-resistant prostate cancer. Cancer 2019;125:524-32. [Crossref] [PubMed]
- Attard G, Merseburger AS, Arlt W, et al. Assessment of the safety of glucocorticoid regimens in combination with abiraterone acetate: a randomized, open-label phase 2 study. JAMA Oncol 2019;5:1159-67. [Crossref] [PubMed]


