Association between sodium intake and lower urinary tract symptoms: does less sodium intake have a favorable effect or not?
Introduction
Nutrients from fruit, vegetable and micronutrient are well known antioxidants in that they can affect the cell growth and differentiation of prostate, which may reduce the potential risk of benign prostatic hyperplasia (BPH) with lower urinary tract symptoms (LUTS) (1-3). Although several studies have focused on this issue, their results were conflicting, especially regarding LUTS, and moreover, there has been limited evidence regarding the relationship between sodium preference and LUTS.
Recently, sodium preference has been focused widely on throughout the whole medical field including hypertension (HTN), cardiovascular disease (CVD), and chronic kidney disease (CKD) (4-6). From the view of global health, sodium intake is an important issue because it is directly related with CVD mortality (7,8).
Possible links between sodium intake and BPH/LUTS may be explained by two points: (I) indirect effects from HTN by sodium intake (6,9); (II) direct effects on bladder epithelial sodium channel (10,11). Sodium intake is a major risk factor for developing or aggravating HTN such that HTN patients can be devised by two ways: those who are sensitive to sodium intake or those who are insensitive to sodium intake.
Among BPH/LUTS, urinary storage symptoms were more prevalent in HTN patients than in patients without HTN (5). Indirect effect of sodium intake with LUTS lies in the hyperactivation of the autonomic nerve system, especially innervation of prostate and bladder (12). Moreover, HTN induced by sodium intake could diminish treatment efficacy of alpha blockers (10). Among the nutrients, protein intake was a risk factor for aggravating voiding symptoms, and sodium intake was a risk factor for storage symptoms and for the need of prostatic surgery due to severe BPH (2,13). The direct effects of sodium on LUTS are mostly introduced by experimental studies (6,11). High sodium intake could evoke storage symptoms by the upregulation of epithelial sodium channel.
The main hypothesis of this study is that sodium preference may indirectly impact BPH/LUTS via aggravation of the circulation system including BPH, hyperactivation of adrenergic nerve system, and direct stimulation of the bladder epithelium. We investigated the association between sodium preference and LUTS, and we also investigated possible moderator effect of fruit and vegetable intake. We present the following article in accordance with the SURGE reporting checklist (available at http://dx.doi.org/10.21037/tau-19-808).
Methods
Data and subjects
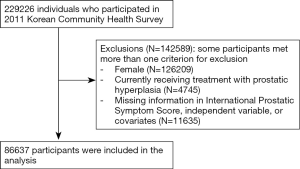
This study used data obtained from the 2011 Korean Community Health Survey (KCHS) for cross-sectional analysis. This study has been approved by Institutional Review Board of Soonchunhyang University Seoul Hospital. The KCHS has been conducted annually since 2008 by the Korean Centers for Disease Control and Prevention in order to produce community-based comparable health statistics for the evaluation of disease prevention programs and community health promotion. The KCHS used multistage sampling design so as to ensure national representativeness. First, a primary sampling unit was extracted through the number of households in each of the smallest governmental administrative units using a probability proportionate to the size sampling method. Next, five sample households on average were extracted in sampling point using systematic sampling methods. Finally, every member of a household who was 19 years or older were interviewed (14). In KCHS, a trained investigator visited the selected households and conducted a face-to-face interview. At least three visits were made to the target household to minimize selection bias. This study excluded 142,589 respondents who were female, who reported currently receiving treatment with prostatic hyperplasia to prevent the bias that may affect on LUTS, who have missing data in International Prostate Symptom Score (IPSS), dietary behavior variables, or covariates. Finally, 86,637 respondents were included in study subjects (Figure 1).
Variables and measurements
This study measured the LUTS of subjects based on responses from the Korean version of International Prostate Symptom Score (IPSS) Questionnaire on KCHS, which is one of the most widely-used tools for evaluating LUTS. Dependent variables were the total sum of IPSS (IPSS total), IPSS grade (mild: IPSS total =0–7, moderate =8–19, severe =20–35), IPSS voiding [sum of IPSS Q1 (incomplete emptying), Q3 (intermittency), Q5 (weak stream), Q6 (straining)], IPSS storage (sum of IPSS Q2 (frequency), Q4 (urgency), Q7 (nocturia)), and nocturia (IPSS Q7). The independent variable of salt intake was measured by self-rated salty taste preference on a five-point Likert scale. It was categorized into (very) salty, neutral, and (very) blandly.
The covariates considered socio-demographic factors, comorbidities, and dietary behaviors. The socio-demographic variables included age, marital status, education level, household income, and residence. Age was categorized as “19–29”, “30–39”, “40–49”, “50–59”, “60–69”, “70–79”, “80–89”, and “90 or higher”. Marital status was categorized into four categories, corresponding to either “married”, “separated, divorced, or widowed”, or “never married”. Education level was categorized as “elementary school graduate or lower”, “middle school graduate”, “high school graduate”, or “college graduate or higher”. Household income was divided into quartiles. Residence was based on 16 governmental administrative districts and categorized as “capital” (Seoul), “urban” (included Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan), or “rural” (included Gyeonggi, Gangwon, Chungbuk, Chungnam, Chungbuk, Chungnam, Jeonbuk, Jeonnam, Gyeongbuk, Gyeongnam, and Jeju). Comorbidities were comprised of hypertension, diabetes mellitus, and dyslipidemia which showed the highest prevalence rates among adults (15). These were assessed by the physician’s diagnosis and based on responses to questionnaires. Dietary behaviors included breakfast eating, intake of fruit, and intake of vegetable. The breakfast eating was ascertained by the question, “How many days do you eat the breakfast in last week?” Responses were classified into “5–7 days”, “1–4 days”, or “Never eat in last week”. The intake of fruit and vegetable were assessed on a monthly basis. Responses of “3 times/day”, “2 times/day”, “1 time/day”, “less than 1 time/day”, and “never eat in last month” were categorized into “once or more/day”, “less than once/day”, or “never eat in last month”.
Statistical analysis
Descriptive analysis was conducted in order to describe the socio-demographic and LUTS characteristics of the study population. The frequency and percentage by IPSS grade were reported. A Chi-squared test was performed in order to identify the group differences. Considering the characteristics of each dependent variable, a set of multivariable regression models were estimated in order to investigate the relationship between LUTS measured by IPSS and salt intake among Korean male adults. The negative binomial regression (for the IPSS total, IPSS voiding, and IPSS storage symptoms), ordinal logistic regression (for the IPSS grade), and binomial logistic regression (for the IPSS nocturia symptoms) were conducted so as to adjust the independent variables and covariates. The adjusted odds ratio (OR) or coefficient from each model with 95% confidence interval (CI) estimates were reported by applying complex sampling design and benchmark weight from KCHS to ensure the reliability. In order to differentiate the effect between socio-demographics, comorbidity covariates, and dietary behaviors, a two-step approach was used. The first model was adjusted for socio-demographic factors and comorbidities. The second model was additionally adjusted for dietary behaviors. All statistical procedures were carried out using Stata version 14.2 (StataCorp LP, College Station, Texas, USA). The threshold for statistical significance was 0.05 (two-tailed).
Results
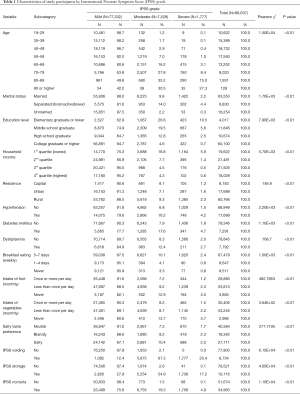
Table 1 summarized the socio-demographic and LUTS characteristics of study subjects. Among 86,637 study subjects, 77,332 (89.3%) were classified as “mild”, 7,525 (8.4%) were “moderate”, and 1,777 (2.1%) were “severe” symptoms according to IPSS grade. Those who being older, separated, divorced, or widowed in marital status, lower education level or household income, residing in rural region, having hypertension, diabetes mellitus, or dyslipidemia, regularly eat breakfast, eat less fruit or vegetables, and prefer salty taste were tending to be have worse IPSS grade condition compared to their counterparts. The distribution of study subjects by IPSS grade showed significant difference in all the independent variables and covariates (P<0.01).
Full table
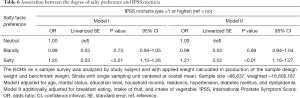
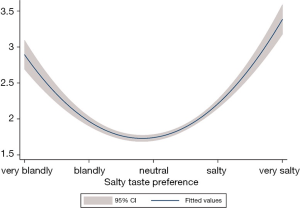
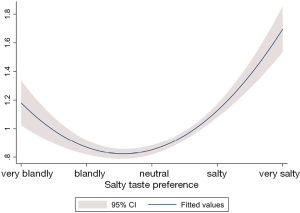
Tables 2-6 were presenting the results from the multivariable analysis on IPSS total score, grade, voiding, storage, and nocturia symptoms respectively. It was identified that subjects those who preferred salty taste were significantly associated with higher IPSS total score (coefficient =0.31; 95% CI: 0.27 to 0.35; P<0.01; Table 2), increased risk of having worst IPSS grade (OR =1.46; 95% CI: 1.35 to 1.57; P<0.01; Table 3), higher IPSS voiding score (coefficient =0.38; 95% CI: 0.32 to 0.44; P<0.01; Table 4), higher IPSS storage score (coefficient =0.25; 95% CI: 0.22 to 0.29; P<0.01; Table 5), and increased risk of having IPSS nocturia symptoms (OR =1.21; 95% CI: 1.16 to 1.27; P<0.01; Table 6) compared to subjects with neutral taste preference group. Subjects who prefer bland taste was also significantly associated with higher IPSS total score (coefficient =0.08; 95% CI: 0.03 to 0.12; P<0.01; Table 2), higher IPSS voiding score (coefficient =0.08; 95% CI: 0.02 to 0.15; P=0.02; Table 4), and higher IPSS storage score (coefficient =0.08; 95% CI: 0.03 to 0.12; P<0.01; Table 5) compared to subjects who did not prefer the too salty or bland taste. However, degree of likelihood was relatively lower than those who prefer salty taste. To look at the association between IPSS score and salt intake, it showed a U-shaped pattern (Figure 2). An elevated IPSS score was observed among aged 50 or higher compared to age under 50 (see unit of y axis in both figures), but trend of “U” curve was persisted in both aged under and over 50 (Figures S1,S2).

Full table

Full table

Full table

Full table
Full table

To examine the impact of breakfast eating, intake of fruit, and intake of vegetable on sodium preference, moderator analysis has been performed. For each dependent variables, model II represented moderator analysis, which showed no significant moderating effect that there was no significant difference between model I and model II.
Discussion
Sodium preference is a crucial issue considering its potential impact on the circulation system, which it is well known that sodium preference aggravates HTN and increases CVD mortality (7,8,16). Considering the close and complex links among HTN, metabolic syndrome, artherosclerosis, fatty liver, and BPH/LUTS, it could be postulated that not only HTN but other circulatory components may influence BPH/LUTS as well.
To date, only limited evidence exists regarding the association between sodium intake or preference and the severity of LUTS. Maserejian et al. (2) reported that sodium intake showed a significant positive association with LUTS in their cross-sectional analysis of random population sampling. Although they reported that men with higher sodium intake were likely to have a higher severity of LUTS (OR =2.25; 95% CI, 1.26–4.03), this linear trend was strong for storage LUTS specifically and there was no consistent association for voiding LUTS. Tavani et al. (13) reported in their case-control study that sodium intake was related with significant high risk (OR =1.30) for the diagnosis of surgically treated BPH.
The expected mechanism of association between sodium intake and LUTS could be explained in two ways: indirect or direct effect. First, it is evident that sodium intake increased HTN, which leads to overactivity of the sympathetic nerve system (17,18). Although LUTS, especially male LUTS, is largely explained by BPH, nowadays other origins include sympathetic nerves hyperinnervation, overproduction of nerve-growth-factor, increased sensitivity of afferent stimulation, changed purinergic system, and oxidative damage (18,19). Not only the indirect effect by HTN for sympathetic nerve activity, but the direct effect of sodium intake for sympathetic nerve activity is also plausible. Sympathetic nerve activity is affected by the types of nutrient of a high protein diet that decreases sympathetic nerve activity, whereas sodium intake increases sympathetic nerve activity (20). Overactivity of the adrenergic nerve system could evoke stimulation of the sympathetic tone of bladder and prostate, which causes c fiber activation (10).
Other indirect effects include neurotransmitters such as catecholamine which is overexpressed in HTN. Increased sympathetic activation and neurotransmitters stimulate not only the bladder but also the prostate such that they affect smooth muscle tone in prostate, which aggravates the BPH/LUTS (21). Sympathetic hyper-innervation also charges for the pathogenesis of BPH, which could result in ventral prostate hyperplasia (22). Moreover, nerve growth factor is involved in the pathogenesis of BPH in response to sympathetic hyper-innervation (23). In our study, not only storage LUTS but also voiding LUTS was significantly related with sodium intake.
The direct stimulation of sodium intake on bladder epithelium which explains storage symptoms has been introduced by several experimental studies (6,11). Yamamoto et al. (6) reported that high salt intake evokes the upregulation of the sodium channel in bladder epithelium. During stimulation, bioactive substances including neurotransmitters are released from bladder epithelium, which explains the aggravation of storage symptoms by the abnormal activation of bladder afferent pathways (4,24). Interestingly, the upregulation of bladder epithelial sodium channel showed significant correlation with urinary storage symptoms by IPSS (25).
Recently, Matsuo et al. (26) showed in their large cross-sectional study that estimated daily salt intake was positively correlated with daily time frequency and night time frequency. Main mechanism to explain this relationship is salt intake-related polydipsia due to the increased osmotic pressure of blood.
Although we have performed thorough analysis, there are still several limitations remaining. First, cross-sectional study design hampers the establishment of a causal effect of sodium preference on the severity of LUTS. However, designing a randomized controlled trial with this issue is not easy. Second, BMI data is missing in our analysis. Although several studies have showed that obesity is related with the severity of LUTS (27), to date, the association between BMI and severity of LUTS remains controversial (28). Third, the degree of sodium preference has been measured by subjective questionnaire. Measurement of urinary sodium is important to truly quantify the degree of sodium preference, as shown in other studies (8).
Although our study did not measure the direct urine sodium concentrations, several studies already showed the relationship between urine sodium concentrations and self-assessed preference sodium scale. Shim et al. (29) showed significant relationship between self-assessed preference for saltiness and actual sodium intake using 127 item dish frequency questionnaire. In their study, salty taste preference showed positive correlation with daily sodium intake and sodium intake-increasing behaviors. Kim et al. (30) also showed that salty taste thresholds among normal controls and non-dialysis chronic kidney disease patients were related with salty taste thresholds or preferences and urine sodium concentrations.
Fourth, respondents who may have poor nutritional or eating habits were excluded from the study due to missing information in socioeconomic factors. Those who refuse to report characteristics such as household income or education level tend to be have low socioeconomic status which might be associated with bad dietary patterns or nutrition quality. Lastly, the U-shaped distribution between sodium preference and the severity of LUTS could not be fully explained. However, as shown in the similar distribution between sodium preference and CVD mortality, reverse causation could be a possible factor for explaining the association between low sodium preference and aggravation of LUTS, which implies that those patients with HTN or CVD are not willing to intake sodium to prevent future CVD aggravation. However, in our study, the U-shaped distribution was still consistent after adjusting for HTN. Another possible reason for this U-shaped distribution is the activation of the renin-anagiotensin-aldosterone (RAA) system. As is well known, sodium is an essential component for maintaining human physiology and a level of below 3.0 g/day could cause the activation of the RAA system (31-33). Interestingly, the activation of RAA is related with the aggravation of BPH such that angiotensin II peptide in the basal layer of prostate and angiotensin1 receptor on stroma of prostate were expressed, which suggests that angiotensin II may be ted with paracrine functions on hyperplasia of epithelial cells and hypertrophy of smooth muscle of prostate (34).
Aside from the merit of our study in that it includes a large population, another strength is that we also investigated dietary patterns including vegetable, fruit, and breakfast pattern. Although several studies have investigated the association between fruit or vegetable intake and the severity of LUTS, they did not consider sodium intake together. Liu et al. (1) reported that fruit and vegetable intake were significantly associated with reduced IPSS and Rohrmann et al. (3) reported that vegetable intake was inversely associated with BPH, however, fruit intake was not. In our study, vegetable and fruit intake were negatively associated with the severity of LUTS, which was consistent with other studies showing that vegetable and fruit intake was a favorable factor for LUTS.
Conclusions
Sodium preference was associated with the severity of LUTS, which showed a U-shaped distribution. Higher sodium preference and lower sodium preference were both associated with the aggravation of LUTS compared to normal sodium preference. Moreover, sodium preference was closely related to vegetable and fruit intake. More studies are needed to validate this U-shaped distribution of the association between sodium preference and severity of LUTS.
Acknowledgments
Funding: The present research was supported by the research fund of Dankook University in 2019.
Footnote
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at http://dx.doi.org/10.21037/tau-19-808
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-19-808). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The procedures of this study were reviewed and approved by the Institutional Review Board of University Seoul Hospital with a waiver for informed consent (No. 2018-07-017). The KCHS data is openly accessible at the national public repository (http://chs.cdc.go.kr). There are no confidentiality risks to the participants of this study because the survey data were completely anonymized.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu ZM, Wong CK, Chan D, et al. Fruit and Vegetable Intake in Relation to Lower Urinary Tract Symptoms and Erectile Dysfunction Among Southern Chinese Elderly Men: A 4-Year Prospective Study of Mr OS Hong Kong. Medicine (Baltimore) 2016;95:e2557. [Crossref] [PubMed]
- Maserejian NN, Giovannucci EL, McKinlay JB. Dietary macronutrients, cholesterol, and sodium and lower urinary tract symptoms in men. Eur Urol 2009;55:1179-89. [Crossref] [PubMed]
- Rohrmann S, Giovannucci E, Willett WC, et al. Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am J Clin Nutr 2007;85:523-9. [Crossref] [PubMed]
- Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol 2007;4:46-54. [Crossref] [PubMed]
- Sugaya K, Kadekawa K, Ikehara A, et al. Influence of hypertension on lower urinary tract symptoms in benign prostatic hyperplasia. Int J Urol 2003;10:569-74; discussion 575. [Crossref] [PubMed]
- Yamamoto S, Hotta Y, Maeda K, et al. High salt loading induces urinary storage dysfunction via upregulation of epithelial sodium channel alpha in the bladder epithelium in Dahl salt-sensitive rats. J Pharmacol Sci 2017;135:121-5. [Crossref] [PubMed]
- Graudal N, Jurgens G, Baslund B, et al. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens 2014;27:1129-37. [Crossref] [PubMed]
- O'Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014;371:612-23. [Crossref] [PubMed]
- Yoshimura K, Terada N, Matsui Y, et al. Prevalence of and risk factors for nocturia: Analysis of a health screening program. Int J Urol 2004;11:282-7. [Crossref] [PubMed]
- Ito H, Yoshiyasu T, Yamaguchi O, et al. Male Lower Urinary Tract Symptoms: Hypertension as a Risk Factor for Storage Symptoms, but Not Voiding Symptoms. Low Urin Tract Symptoms 2012;4:68-72. [Crossref] [PubMed]
- Kurokawa T, Zha X, Ito H, et al. Underlying mechanisms of urine storage dysfunction in rats with salt-loading hypertension. Life Sci 2015;141:8-12. [Crossref] [PubMed]
- Wyss JM. Pathways by which dietary salt affects blood pressure and the nervous system. Hypertension 2006;47:638-9. [Crossref] [PubMed]
- Tavani A, Longoni E, Bosetti C, et al. Intake of selected micronutrients and the risk of surgically treated benign prostatic hyperplasia: a case-control study from Italy. Eur Urol 2006;50:549-54. [Crossref] [PubMed]
- Kang YW, Ko YS, Kim YJ, et al. Korea Community Health Survey Data Profiles. Osong Public Health Res Perspect 2015;6:211-7. [Crossref] [PubMed]
- Lim S, Shin H, Song JH, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care 2011;34:1323-8. [Crossref] [PubMed]
- Nerbass FB, Pecoits-Filho R, McIntyre NJ, et al. High sodium intake is associated with important risk factors in a large cohort of chronic kidney disease patients. Eur J Clin Nutr 2015;69:786-90. [Crossref] [PubMed]
- Azadzoi KM, Yalla SV, Siroky MB. Oxidative stress and neurodegeneration in the ischemic overactive bladder. J Urol 2007;178:710-5. [Crossref] [PubMed]
- McVary KT, Rademaker A, Lloyd GL, et al. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 2005;174:1327-433. [Crossref] [PubMed]
- Rohrmann S, Smit E, Giovannucci E, et al. Associations of obesity with lower urinary tract symptoms and noncancer prostate surgery in the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2004;159:390-7. [Crossref] [PubMed]
- Kaufman LN, Young JB, Landsberg L. Effect of protein on sympathetic nervous system activity in the rat. Evidence for nutrient-specific responses. J Clin Invest 1986;77:551-8. [Crossref] [PubMed]
- Michel MC, Heemann U, Schumacher H, et al. Association of hypertension with symptoms of benign prostatic hyperplasia. J Urol 2004;172:1390-3. [Crossref] [PubMed]
- Huang S, Fang X, Meng Y, et al. Sympathetic nervous system overactivity in the Wistar rat with proliferative lesions of ventral prostate induced by chronic stress. Urol Int 2009;83:230-5. [Crossref] [PubMed]
- Huang S, Zhang X, Xu L, et al. Expression of nerve growth factor in the prostate of male rats in response to chronic stress and sympathetic denervation. Exp Ther Med 2014;8:1237-40. [Crossref] [PubMed]
- Gonzalez EJ, Merrill L, Vizzard MA. Bladder sensory physiology: neuroactive compounds and receptors, sensory transducers, and target-derived growth factors as targets to improve function. Am J Physiol Regul Integr Comp Physiol 2014;306:R869-78. [Crossref] [PubMed]
- Araki I, Du S, Kamiyama M, et al. Overexpression of epithelial sodium channels in epithelium of human urinary bladder with outlet obstruction. Urology 2004;64:1255-60. [Crossref] [PubMed]
- Matsuo T, Miyata Y, Sakai H. Effect of salt intake reduction on nocturia in patients with excessive salt intake. Neurourol Urodyn 2019;38:927-33. [Crossref] [PubMed]
- Kim JH, Sun HY, Park SY, et al. Association between obesity and lower urinary tract symptoms: propensity score matching study between healthy controls and obese patients seeking bariatric surgery. Surg Obes Relat Dis 2016;12:1585-93. [Crossref] [PubMed]
- Kim GW, Doo SW, Yang WJ, et al. Effects of obesity on prostate volume and lower urinary tract symptoms in korean men. Korean J Urol 2010;51:344-7. [Crossref] [PubMed]
- Shim E, Yang YJ, Yang YK. Relationship between thresholds and self-assessed preference for saltiness and sodium intake in young women. J Nutr Health 2016;49:88-98. [Crossref]
- Kim TH, Kim YH, Bae NY, et al. Salty taste thresholds and preference in patients with chronic kidney disease according to disease stage: A cross-sectional study. Nutr Diet 2018;75:59-64. [Crossref] [PubMed]
- Brunner HR, Laragh JH, Baer L, et al. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med 1972;286:441-9. [Crossref] [PubMed]
- Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev 2011;11:CD004022. [PubMed]
- Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev 2017;4:CD004022. [PubMed]
- Dinh DT, Frauman AG, Somers GR, et al. Evidence for activation of the renin-angiotensin system in the human prostate: increased angiotensin II and reduced AT(1) receptor expression in benign prostatic hyperplasia. J Pathol 2002;196:213-9. [Crossref] [PubMed]