
Genitourinary manifestations of hereditary cancer predisposition syndromes in children
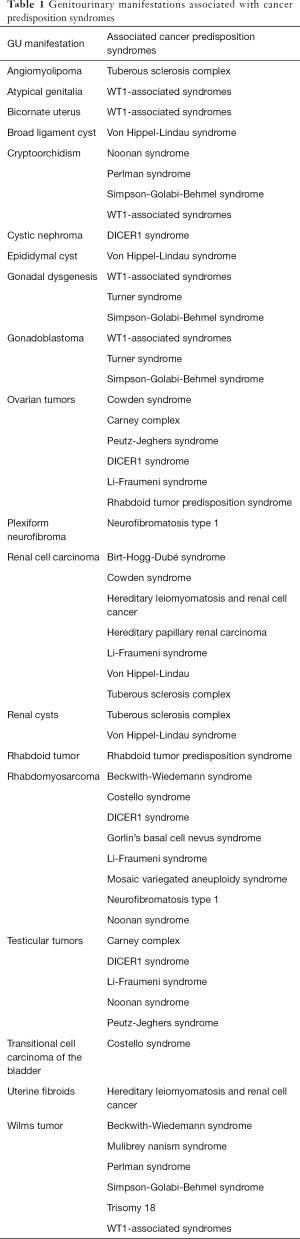
It has been suggested that up to 10% of all pediatric cancers are associated with a germline mutation in a cancer predisposition gene (1-3). A number of hereditary cancer predisposition syndromes have been described in children (4). Some manifest with primary urologic complications, such as syndromes associated with Wilms tumor. Others present with a number of benign and malignant tumors outside of the genitourinary (GU) system, but the GU manifestation may be the critical finding that leads to the overarching diagnosis. As such, knowledge of these syndromes and their associated GU findings is an important role for a pediatric urologist. The goal of this article is to review GU manifestations of cancer predisposition syndromes impacting children. While not comprehensive, it provides an introduction to the subject and strategies for diagnosis and management. The GU manifestations are summarized in Table 1 along with associated syndromes. Some readers may choose to review Table 1 to focus on syndromes of interest based on a presenting symptom. Table 2 provides a list of cancer predisposition syndromes with their genetic cause and manifestations.
Full table

Full table
Predispostion to Wilms tumor
Wilms tumor, or nephroblastoma, represents ~90% of all pediatric renal tumors (5) and affects 1:10,000 children less than 15 years of age (6). Wilms tumor is an embryonal tumor that arises in a kidney cell with pluripotent differentiation potential. Most cases are unilateral (95%), sporadic, and curable with nephrectomy. Chemotherapy and radiation are also utilized in certain situations. In contrast, Wilms tumor associated with a cancer predisposition syndrome tends to occur at an earlier age and is more often associated with bilateral disease. The median age of diagnosis of patients with a Wilms tumor is 38 months, while bilateral Wilms tumors (~5%) generally arise in patients at a younger age (<24 months), often in association with nephroblastomatosis (7). Nephroblastomatosis represents persistence of multiple nephrogenic rests (metanephric blastema) beyond the embryonic period. It can be very challenging to differentiate Wilms tumor from a nephrogenic rest based on radiographic appearance, and it is often difficult to make a distinction even after biopsy. Close observation is warranted as nephroblastomatosis is recognized as a precursor lesion to Wilms tumor. Bilateral Wilms tumor presents a unique challenge in treatment as it requires preservation of tissue for adequate renal function while removing the underlying disease. Complications can include end stage renal failure, which occurs in 11% of cases of bilateral Wilms tumor, as compared to 0.6% of cases of unilateral Wilms tumor (8).
Broadly speaking, predisposition to Wilms tumor is often associated with a germline mutation in the WT1 gene or arises in the setting of an overgrowth syndrome. A number of these syndromes are outlined below. Surveillance guidelines are similar for all syndromes and are reviewed at the end of this section.
WT1-associated cancer predisposition syndromes
The WT1 gene is important for urogenital cellular growth and differentiation. The translational product of the WT1 gene is a zinc finger protein involved in transcription regulation that acts as a tumor suppressor through its role in metanephric stem cell differentiation and genitourinary development (9,10). WT1-associated syndromes are inherited in an autosomal dominant fashion, and most cases occur de novo (11).
Several syndromes have been associated with the WT1 gene and have significant clinical overlap. Classically, a number of eponymous syndromes have been described with variable penetrance of Wilms tumor, testicular feminization, and renal failure. These are described below with the understanding that the utility of distinguishing among these conditions has diminished as we gain a better understanding of how specific mutations in the WT1 gene impact gene function and clinical manifestations.
WAGR syndrome
WAGR syndrome is a WT1-associated syndrome caused by deletion of the 11p13 region, which includes the WT1 and PAX6 genes. Clinical manifestations classically include Wilms tumor, aniridia, genitourinary abnormalities and intellectual disabilities. Aniridia is caused by loss of the PAX6 gene, which is a paired box gene required for normal eye development. The risk of developing Wilms tumor in patients with WAGR syndrome has been reported to be anywhere from 30–77% (12-14). Among patients who develop Wilms tumor, 90% develop a tumor by 4 years of age and 98% develop a tumor by 7 years of age (15). The most common GU abnormality associated with WAGR syndrome aside from Wilms tumors is cryptorchidism, which presents in 60% of males with WAGR syndrome (13). Internal anomalies such as streak ovaries and bicornate uterus are present in approximately 17% of females with WAGR syndrome (13). Atypical genitalia is present in approximately 10% of all patients (13). Notably the risk of developing renal failure in these patients is >50% (8).
Denys-Drash syndrome
Denys-Drash syndrome is another WT1-associated syndrome. It is caused by missense mutations in exon 8 or 9 of the WT1 gene, which encode the zinc finger region important for DNA binding (16). The clinical presentation of Denys-Drash syndrome is characterized by Wilms tumor, atypical genitalia and early onset of renal failure due to nephropathy (typically mesangial sclerosis). Usually, progression to end stage renal failure is seen in early childhood. In patients with Denys-Drash syndrome, the risk of developing Wilms tumor is estimated to be 90% (12). Patients with a 46XY karyotype and gonadal dysgenesis are at increased risk of gonadoblastoma in late childhood or early adolescence. The risk of developing gonadoblastoma is estimated to be >40% in patients with Denys-Drash or Frasier syndrome who meet the above criteria (17). Detailed surveillance guidelines for patients at high risk of gonadoblastoma have been described (18). Some patients undergo prophylactic gonadectomy (19).
Frasier syndrome
Frasier syndrome is another WT1-associated syndrome and shares some overlap with Denys-Drash syndrome. The primary mutation is at the intron 9 donor splice site in the WT1 gene (20). Clinical manifestations can include gonadal dysgenesis and steroid resistant nephrotic syndrome; additionally, it can also be associated with gonadoblastoma and less frequently, Wilms tumor (21). Renal failure tends to develop in adolescence due to focal segmental glomerulosclerosis. As with Denys-Drash syndrome, a gonadectomy may be appropriate (22).
Overgrowth syndromes associated with Wilms tumor
Wilms tumor also arises at an increased rate in the setting of a number of overgrowth syndromes. Examples include Beckwith-Wiedemann, Perlman and Simpson-Golabi-Behmel syndrome, which are reviewed below.
Beckwith-Wiedemann syndrome (BWS)
BWS is associated with inappropriate imprinting at 11p15.5. This can occur by a variety of mechanisms including uniparental disomy of 11p15.5 (~20% of cases), loss (~50%) or gain (~5%) of methylation at defined regions of 11p15.5, discrete DNA translocations/inversions/duplications (~1%), and mutation of the maternal CDKN1C gene (~5%). The cause of BWS is unknown in approximately 20% of cases (23). It is one of the more common cancer predisposition syndromes, with an incidence of at least 1 in 13,700 children (23). Clinical manifestations can include hemihypertrophy, macroglossia, organomegaly, hyperglycemia, omphalocele/umbilical hernia as well as increased risk for embryonal malignancies including Wilms tumor, hepatoblastoma, neuroblastoma and rhabdomyosarcoma. Malignancies in these patients are most commonly seen before 10 years of age. Malignancy is diagnosed in approximately 7.5% of patients, but the risk returns to that of the general population rate after 8 years of age (23). The specific risk of developing Wilms tumor is estimated to be 5% (12), however, patients with evidence of hemihyperplasia, nephromegaly and/or nephrogenic rests are thought to be particularly at risk for developing malignancy (24,25). In addition to serial abdominal ultrasounds to screen for Wilms tumor, alpha-fetoprotein should be obtained every 3 months through age 4 years to screen for hepatoblastoma (17). Neuroblastoma screenings can be implemented for patients with mutations in the CDKN1C gene, which are found in some patients with BWS who have an increased risk of developing neuroblastoma (17,26).
Perlman syndrome
Perlman syndrome is a rare, autosomal recessive congenital overgrowth syndrome, with evidence of linkage to the DIS3L2 gene at 2q37.1. This gene is thought to code for subunits of an RNA exosome. Key features can include nephroblastomatosis, renal hamartomas, facial dysmorphisms, cardiac abnormalities, polyhydramnios, cryptorchidism, macrosomia, developmental delay and renal dysplasia (27). The estimated risk of developing Wilms tumor is 30% and is usually diagnosed in patients before 2 years of age (28). Neonatal mortality rate is high due to risk of respiratory and/or renal complications in patients with Perlman syndrome, and thus the true incidence of Wilms tumors in these patients is likely masked (28).
Simpson-Golabi-Behmel syndrome
Simpson-Golabi-Behmel syndrome is another rare overgrowth syndrome associated with a variety of anomalies due to mutations in the GPC3 gene at Xq26.2. The protein product glypican 3 is found on the outer cell membrane and is involved in diverse extracellular signaling pathways of cell growth, development and survival. The syndrome follows an X-linked inheritance pattern (29). Predominant characteristics can include pre- and post-natal overgrowth, macrocephaly, facial dysmorphisms, predisposition to embryonal malignancies including Wilms tumors and gonadoblastoma, anomalies in various organ systems and intellectual disabilities (29). Males affected by this syndrome have an approximate 10% risk of developing Wilms tumor (30). Females of the carrier state do not require surveillance as they are not at a significantly elevated risk of developing Wilms tumor (30).
Other syndromes associated with Wilms tumor
A number of other syndromes have been associated with Wilms tumor, which have been reviewed elsewhere (17). Examples include Mulibrey nanism, trisomy 18, and others. In general, screening is recommended if the incidence of Wilms tumor is greater than 1% (17).
Screening for Wilms tumor
Screening for patients with a predisposition to Wilms tumor should be initiated after consultation with a clinical geneticist and confirmation with a diagnostic molecular test. Generally, surveillance consists of a renal ultrasound every 3 months through age 7 years, though it can be syndrome-specific. Some authors recommend modulating surveillance recommendations when genotype-phenotype studies suggest a diminished risk (17). For example, a meta-analysis of more than 400 patients with BWS with molecular data at 11p15 revealed that Wilms tumor did not manifest in children with loss of methylation at the imprinting center, which represent approximately 45% of patients (31). In the North America, the guidance is generally to consider surveillance when the risk of developing Wilms tumor is greater than 1% (17).
Predisposition to renal cell carcinoma (RCC)
Von-Hippel-Lindau (VHL) syndrome
The most common syndrome associated with RCC is VHL. It is an autosomal dominant condition that affects approximately 1:36,000 people. Mutations in the VHL gene at 3p25.3 are responsible for VHL syndrome. The protein product of the VHL gene is an E3 ubiquitin ligase that regulates degradation of hypoxia inducible factor (HIF) alpha subunits in the setting of normal oxygen tension. In the absence of the VHL protein, HIF levels increase as seen in the normal physiologic response to hypoxia. As such, there is a commensurate increase in new blood vessel formation, in part regulated by the upregulation of vascular endothelial growth factor receptors (VEGFRs) (32).
VHL is characterized by a variety of benign and malignant tumors of the central nervous system, kidneys, adrenal glands, pancreas, endolymphatic sac tumors, and epididymal and broad ligament cysts. The most common renal manifestations are RCC and cysts. Approximately 70% of individuals will develop RCC, and it is the leading cause of mortality (33). The average age of RCC in patients with VHL is 39 years-old, but it has been reported as young as 16 years-old (34). The RCC is almost always of the clear cell subtype. Renal cysts and tumors are generally followed closely by MRI. Cysts do not generally require intervention, and tumors are unlikely to cause problems when they are <3 cm. The goal is to delay resection for as long as possible in an effort to spare loss of normal tissue since the likelihood of additional lesions arising in the future is high. Among patients with VHL, approximately 80% will have an affected parent and the remainder will have a de novo pathogenic variant.
Surveillance recommendations for patients with VHL syndrome have been published by the VHL Alliance and other groups. The recommendations of the VHL Alliance include annual evaluation of blood pressure and vision starting at 1 year of age, plasma metanephrines annually and hearing assessment every 2–3 years starting at 5 years of age, and abdominal ultrasound annually and MRI abdomen, brain and spine every 2 years starting at 16 years of age. There are not any established strategies to prevent malignant progression in VHL, but there is considerable interest in targeting proteins upregulated by HIFs such as VEGFR. For example, a recent trial showed some potential benefit with pazopanib therapy, a tyrosine kinase inhibitor that inhibits VEGFR-2 among other kinases (35). Further studies will be needed to determine the subset of patients most likely to benefit from therapy.
Hereditary Leiomyomatosis and renal cell cancer syndrome (HLRCC)
HLRCC is an autosomal dominant condition caused by a germline mutation in the fumarate hydratase (FH) gene. Symptoms include cutaneous leiomyomas, uterine fibroids, and RCC. The mean age of RCC in patients with HLRCC is approximately 41 years of age, with a reported penetrance of approximately 15% (36). The incidence of patients less than 20 years old developing RCC is estimated at only 1–2%, but rare patients have been described with RCC as young as 10 years of age (36). No clear surveillance guidelines have been established for HLRCC, but the HLRCC Family Alliance (www.hlrccinfo.org) recommends an annual MRI starting at 8 years of age. With regard to treatment, RCC associated with HLRCC tends to be unilateral, solitary, and aggressive. As such, prompt surgical resection with wide margins is advised in contrast to adherence to the “3 cm rule” as described above for VHL (37).
Birt-Hogg-Dubé (BHD) syndrome
BHD syndrome is an autosomal dominant condition associated with increased risk of renal neoplasia as well as pulmonary cysts and spontaneous pneumothoraces. This syndrome is caused by germline mutations in folliculin (FLCN gene), mapped to 17p11.2 (38). The exact function of folliculin is currently unknown. Patients with BHD are at risk of developing bilateral, multifocal renal neoplasms (seen in 12–34% of patients), usually in midlife, but have been reported in the second decade of life (39). BHD tumors are unique in that the most commonly seen tumors on pathology are hybrids, including clear cell RCC, chromophobe RCC and renal oncocytoma (40). Kidneys in these patients are also remarkable for renal oncocytosis, which are microscopic clusters of oncocytic cells that are thought to be precursors to malignancy (41). Periodic MRI or CT imaging of the kidneys has been recommended to assess for renal tumors in the second decade of life, however no clear guidelines have been established (42).
Hereditary papillary renal carcinoma (HPRC)
HPRC is an autosomal dominant syndrome due to mutation in MET on at 7q31.2 (43). It codes for a tyrosine kinase receptor for hepatocyte growth factor and acts as proto-oncogene. Patients with this syndrome have a 90% likelihood of developing RCC within an average lifespan comparable to the general population (44). In a large cohort of patients with HPRC, the median age of RCC was 42 years with a range of 19 to 66 years of age (45). It is associated with bilateral type 1 papillary RCC that can occasionally metastasize (46). Recommended management includes surveillance and nephron sparing surgery for tumors reaching the 3 cm size threshold (45). Most children will not require active surveillance, but it is reasonable to consider in families impacted at younger ages.
Cowden syndrome
Cowden syndrome is a rare autosomal dominant syndrome recognized under the umbrella of Phosphatase and TENsin homolog (PTEN) hamartoma tumor syndrome. It shares some overlap with Bannayan-Riley-Ruvalcaba syndrome. PTEN is mapped to 10q23.31 and is an important tumor suppressor involved in a number of cellular signal transduction pathways. Cowden syndrome was originally characterized by cutaneous lesions, hamartomas and predisposition to malignancies (47). Shared features of PTEN hamartoma tumor syndrome include macrocephaly, gastrointestinal polyps, vascular malformations, and intellectual disabilities/autism spectrum. Although studies of PTEN hamartoma tumor syndrome have been limited in pediatric patients, there have been reports of RCC and granulosa cell tumor of the ovary in patients <17 years old. There are also reports of an association with thyroid carcinoma (48). Generally, surveillance recommendations for the pediatric population include annual thyroid ultrasound and skin examinations, however, new recommendations suggest utility in annual abdominal ultrasound and fecal occult blood tests, as well as a brain MRI, echocardiogram and neurodevelopment screening at time of diagnosis (49).
Other syndromes associated with RCC
In summary, the list of syndromes associated with RCC is long and likely to change over time (50). The current guideline from the American Urological Association is to recommend genetic counseling for all patients ≤46 years of age with renal malignancy, which is likely to capture the majority of affected patients (51). Children with affected family members may benefit from genetic testing/surveillance in select situations.
Predisposition to rhabdoid tumor of the kidney
Rhabdoid tumors are rare, aggressive tumors that can occur in nearly any tissue, but they are most common in the brain and kidney. They arise following loss of the chromatin remodeling genes SMARCB1 or (less commonly) SMARCA4 at 22q11.23 and 19p13.2, respectively. Given their rarity, it is recommended that genetic testing be performed on the tumor as well as germline to establish if the patient has rhabdoid tumor predisposition syndrome. It is estimated that approximately 35% of patients with a rhabdoid tumor will have a mutation in one of the two described genes (52). Most patients will develop tumors before 3 years of age. There are no formalized surveillance guidelines, but it has been recommended to perform a brain MRI and abdominal ultrasound every 3 months through at least 5 years of age (52).
Of note, patients with SMARCA4-associated rhabdoid tumor predisposition syndrome (but not SMARCB1-associated) are also at risk for small cell carcinoma of the ovary of hypercalcemic type (SCCOHT), which is a rare, highly malignant ovarian tumor that impacts young women (53). SCCOHT morphologically resembles malignant rhabdoid tumors, and it has recently been found to harbor mutations in SMARCA4 (53). As such, it has been suggested that SCCOHT be renamed as malignant rhabdoid tumor of the ovary (53).
Predisposition to cancer of the bladder
Children rarely develop bladder cancer, but the most common etiology is rhabdomyosarcoma (54). Approximately 25% of pediatric patients with rhabdomyosarcoma are reported to have a primary site of the bladder or prostate (55). Syndromes predisposing patients to rhabdomyosarcoma include Li-Fraumeni syndrome, Costello syndrome, Noonan syndrome, neurofibromatosis type 1, and others.
Li-Fraumeni syndrome (LFS)
LFS is a rare autosomal disorder caused by a germline mutation in the tumor suppressor gene TP53 at 17p13.1. It increases the risk of developing several neoplasms, including rhabdomyosarcoma, osteosarcoma, adrenocortical carcinoma, premenopausal breast cancer, central nervous system tumors and leukemias. Patients may also develop malignancies in the gonads. The prevalence of rhabdomyosarcoma in patients with LFS is estimated to be 18–27% (56), predominantly in patients less than 5 years of age (57). The diversity of cancers associated with LFS makes surveillance challenging, but a modified Toronto protocol (involving regular physical exams, lab work and imaging) has been proposed (58,59). In particular, use of whole body MR imaging is likely to be particularly helpful for detecting GU malignancies (60). For example, this whole body MR was instrumental in the detection of RCC in a 17-year-old female with LFS (60).
Costello syndrome
Costello syndrome is a rare, autosomal dominant syndrome that is sometimes classified as an overgrowth syndrome. Common features include cardiac abnormalities, characteristic facial features and intellectual disabilities. The etiology of Costello syndrome is secondary to a mutation in HRAS at 11p15.5 (61). Patients have an estimated 10% lifetime risk of developing embryonal rhabdomyosarcoma (62). A variety of other benign and malignant tumors have been reported in patients, including transitional cell carcinoma of the bladder, neuroblastoma and papillomas (63). One proposed screening protocol for patients with Costello syndrome recommends urinalysis starting at age 10 years, urine catecholamine analysis every 6–12 months until the age of 5 years, and abdominal/pelvic ultrasound every 3–6 months until the age of 8–10 years (64).
Noonan syndrome (NS)
NS is a heterogenous RASopathy associated with missense mutations in the RAS/MAPK pathway. It has an incidence ranging from 1:1,000 to 1:2,500 live births. Implicated genes in Noonan syndrome include PTPN11, SOS1, KRAS, BRAF, NRAS, MEK1, CBL, SHOC2, and RIT1 (65). Clinical manifestations of NS include short stature, cardiac defects, distinct facial features, cryptorchidism and increased risk of leukemia and myelodysplasia. There have been multiple case reports of embryonal rhabdomyosarcoma in patients with NS as well as testicular cancer (66).
Neurofibromatosis type I (NF1)
NF1 is another syndrome in which patients are at an increased risk of developing rhabdomyosarcoma. NF1 is an autosomal dominant syndrome due to a mutation on the NF1 gene at 17q11.2, which codes for the protein neurofibromin. Neurofibromin is a tumor suppressor that functions by decreasing signaling flux through RAS family members. The incidence is approximately 1:3,000 individuals (67). Clinical manifestations include café au lait spots, Lisch nodules of the iris, axillary or inguinal freckling, neurofibromas, optic pathway gliomas, and tibial dysplasia—however penetrance varies. Prevalence of embryonal rhabdomyosarcoma is elevated in patients with NF1 and has been estimated to occur in up to 1% of patients; tumors most commonly arise in the urogenital system (68,69). However, no routine screening for rhabdomyosarcoma is recommended.
Patients with NF1 are also at risk for transformation of benign plexiform neurofibromas into malignant peripheral nerve sheath tumors (MPNST), which occurs in approximately 10% of cases. There are at least 60 cases in the literature of plexiform neurofibromas invading the bladder in pediatric and adult patients, in addition to several reports of MPNST arising in the bladder (70,71). While benign plexiform neurofibromas lack metastatic potential, they can be locally invasive and require local control via surgery. More recent data support a role for medical therapy with selumetinib, a MEK inhibitor that has recently been approved by the United Stated Food and Drug Administration (72). MPNST, on the other hand, can metastasize and is notoriously difficult to treat. MPNST is not particularly sensitive to chemotherapy and requires surgery or radiation when treated with curative intent.
Other syndromes associated with rhabdomyosarcoma
Rhabdomyosarcoma has been reported in patients with other cancer predisposition syndromes. For example, there are reports of bladder rhabdomyosarcoma in patients with Beckwith-Wiedemann syndrome (discussed previously) (73,74). Other syndromes with a predisposition to rhabdomyosarcoma include mosaic variegated aneuploidy syndrome, Gorlin’s basal cell nevus syndrome, and others (54,75). DICER1 syndrome, which is discussed in the next section, has primarily been associated with uterine rhabdomyosarcoma in females.
Cancer predisposition syndromes associated primarily with nonmalignant GU manifestations
Tuberous sclerosis complex (TSC)
TSC is a neurocutaneous disorder characterized by tumors of the brain, skin, heart, lungs, and kidneys as well as seizures. Angiomyolipomas are benign renal tumors derived from endothelial cells, which are composed of adipose tissue, smooth muscle, and blood vessels (76). Angiomyolipomas are seen in 55–80% of patients with TSC. TSC is caused by mutations in the tumor suppressor genes TSC1 and TSC2 at 9q34.13 and 16p13.3, respectively. The protein products of TSC1 and TSC2 form a complex that inhibits the mechanistic target of rapamycin (mTOR) signaling pathway (77). Unregulated activation of mTOR signaling results in the manifestations of the disease. From a renal perspective, it is recommended to obtain an MRI of the abdomen every 1–3 years throughout the patient’s lifetime and to assess renal function and blood pressure at least annually (77). Angiomyolipomas are generally managed conservatively until they reach a size of 3 cm, at which point the risk of hemorrhage is increased. Treatment with a mTOR inhibitor is then preferred with selective embolization and kidney-sparing resection reserved for refractory cases (78,79).
In addition to angiolipomas, approximately 35% of patients with TSC will develop simple renal cysts and 5% will develop polycystic kidney disease (77). Renal cysts do not generally cause a sharp deterioration in renal function, but they cause issues when multiple in number. This is especially true in the 3% of patients with TSC that have a contiguous gene deletion impacting TSC2 and the autosomal polycystic kidney disease gene, PKD1 (79). Lastly, 1–3% of patients with TSC will develop RCC as adults (78). It is unclear what role the widespread use of mTOR inhibitors will have on this cancer risk.
DICER1 syndrome
DICER1 syndrome is an autosomal dominant cancer predisposition syndrome characterized by a variety of benign and malignant tumors. The pathognomonic neoplasm for DICER1 syndrome is pleuropulmonary blastoma (PPB), a rare, primitive tumor of the lung. The incidence of DICER1 syndrome in the general population has been estimated to be 1:10,600 (80), though it has variable expressivity and incomplete penetrance. DICER1, mapped to 14q32.13, plays a critical role in microRNA processing. Approximately 80–90% of cases of DICER1 syndrome are inherited, while the remainder arise de novo. Unlike most cancer predisposition syndromes, the wild type allele rarely undergoes somatic deletion. Rather, the tumor-specific mutation is more often a missense mutation in the RNase IIIb domain (81). From a GU perspective, the most common manifestations include cystic nephroma, ovarian and testicular stromal tumors, and gynandroblastoma. Cystic nephroma is a rare, benign renal neoplasm. It is generally managed with resection alone via partial nephrectomy with the goal of preventing progression to anaplastic sarcoma of the kidney (82). Sertoli-Leydig cell tumor of the ovary is the most common tumor of the reproductive tract impacting female patients with DICER1 syndrome. Localized disease can be treated with surgery alone, while advanced stage disease requires platinum-based chemotherapy (82). There is also an apparent increased risk of embryonal rhabdomyosarcoma (ERMS) in patients with DICER1 syndrome. Notably, all 6 cases of ERMS captured in a large population of patients with DICER1 syndrome occurred along the gynecologic tract, suggesting the need for heightened suspicion for DICER1 syndrome in newly diagnosed cases (83).
Screening recommendations for DICER1 syndrome include a chest X-ray at birth for all children at risk of a DICER1 variant. Genetic testing should be performed by 3 months of age. For known carriers, a chest CT is recommended by 9 months of age. If negative, then a second chest CT is recommended at 2.5 years of age. In the setting of negative chest CT imaging, then routine surveillance is recommended with an X-ray every 6 months through 7 years of age, then annually thereafter until 12 years of age (82). Surveillance for cystic nephroma consists of an abdominal ultrasound at the time of first chest CT (goal is prior to 9 months of age) and every 6 to 12 months until at least 8 years of age (82). Routine surveillance is also recommended with an ultrasound to evaluate for risk of thyroid nodules and thyroid cancer, ophthalmologic examination to evaluate for risk of ciliary body medulloepithelioma, and physical exam to assess for nasal chondromesenchymal hamartomas (82). Surveillance for Sertoli-Leydig cell tumor of the ovary is a subject of debate, but current guidance is to perform pelvic ultrasound in female pediatric patients in conjunction with abdominal ultrasound every 6–12 months through 8 years of age (82). It is then recommended to continue annual pelvic ultrasounds until at least 40 years of age.
Syndromes associated with tumors of the reproductive organs
Syndromes associated with gonadoblastoma
Gonadoblastoma is a neoplasm composed of germ cell and sex cord derived stromal components. It is frequently associated with streak gonads in the setting of gonadal dysgenesis. It is considered a premalignant tumor, as there is a high likelihood of differentiating into malignancies such as dysgerminoma and other germ cell malignancies (84). Clinical findings raising concern for gonadoblastoma include atypical genitalia, hypospadias, cryptorchidism and/or amenorrhea. In rare occasions, a mass may be palpable if large enough. Gonadoblastoma is typically managed with bilateral gonadectomy due to malignant potential (84).
As noted previously, gonadoblastoma has been reported in various Wilms tumor predisposition syndromes, particularly Denys-Drash and Frasier syndromes (85). It has also been described in Turner syndrome, which is due to total or partial X chromosome monosomy and has an estimated incidence of 1:2,500 (86). Although usually sporadic, there have been cases of familial Turner syndrome (87,88). Key features of this syndrome include gonadal dysgenesis, short/webbed neck, widely spaced nipples, congenial heart disease, premature ovarian failure and short stature. Mosaicism in Turner syndrome with presence of Y chromosome material—estimated to occur in 6–9% of patients with Turner syndrome (89)—increases the risk of developing gonadoblastoma and dysgerminoma (90). The overall risk of developing gonadoblastoma is estimated to be 15–33% in patients with these Turner syndrome features (91). Gonadoblastoma tends to be diagnosed early in life in such patients, so it is recommended that patients with 45X/46XY karyotypes undergo prophylactic gonadectomy (92).
Syndromes associated with testicular/ovarian cancer
Peutz-Jeghers syndrome (PJS)
PJS is a rare autosomal dominant syndrome due to mutation of the tumor suppressor gene STK11/LKB1 at 19p13.3 that codes for a serine/threonine kinase (93). The syndrome is characterized by a variety of benign and malignant tumors including ovarian sex cord stromal tumors, gastrointestinal hamartomous polyps, mucocutaneous melanin spots, gastrointestinal, breast, and lung malignancies as well as recurrent intussusception. Most ovarian tumors are seen in the fourth and fifth decade of life with an estimated lifetime risk of 21% (94), however, there are case reports of such tumors in pediatric patients (95). The most common ovarian tumor seen in PJS is sex cord tumor with annular tubules (SCTAT). The estimated risk of developing SCTAT is 5% (96). These tumors are usually bilateral and benign; presenting symptoms can include heavy menstruation and/or precocious puberty (97). Other types of ovarian tumors have been reported, such as mucinous ovarian tumors (97). Surveillance for ovarian neoplasms in patients with PJS includes a transvaginal ultrasound, CA-125 measurements and pelvic exams annually starting at 18–20 years of age (98). Male patients are at risk for developing large cell calcifying Sertoli cell tumor of testes (LCCSCT).
Carney complex (CNC)
CNC is a rare autosomal dominant condition caused by a mutation in the PRKAR1A gene at 17q24.2-24.3. The PRKAR1A gene codes of a regulatory subunit of protein kinase A (99). Clinical manifestations classically include cutaneous pigmentation, myxomas (cutaneous, cardiac and breast), psammomatous melanotic schwannomas, primary pigmented nodular adrenocortical disease (PPNAD), acromegaly, breast ductal adenoma, blue nevus osteochondromyxoma and endocrine malignancies including testicular tumors, ovarian lesions and thyroid tumors. Definitive diagnosis requires at least 2 of the above clinical manifestations verified by additional testing or an affected first degree relative. While peak penetrance tends to occur in early adulthood, manifestations can first be reported during childhood- and abnormal pigmentation may even be seen at birth. The most common endocrine manifestation in these patients is PPNAD, which causes an ACTH independent Cushing’s disease. This may require bilateral adrenalectomy. LCCSCT is diagnosed in over 50% of male patients with CNC, and may even arise during puberty (100). LCCSCT is often an asymptomatic tumor, but it can lead to gynecomastia in young patients if the tumor is functional, obstruct seminiferous tubules (and thus potentially impact fertility) and/or induce precocious puberty. Patients with CNC may also develop fertility issues related to defective sperm and/or low sperm count (101). Ovarian lesions most commonly include cysts and tumors of the ovarian surface epithelium; ovarian carcinomas are rare. Current surveillance recommendations include annual echocardiograms prior to puberty and post puberty annual echocardiograms, testicular ultrasound, serum IGF-1 and urinary free cortisol. At time of diagnosis, female patients with CNC are recommended to undergo a pelvic ultrasound (102).
Other syndromes associated with testicular cancer
Many patients with testicular cancer report a family history of other affected family members, but more studies are required to delineate genetic etiologies (103,104).
Pediatric considerations
It is important to consider the unique challenges of caring for pediatric patients, especially in the context of a cancer predisposition syndrome. Revealing a diagnosis of malignancy or significantly heightened risk of malignancy is always difficult, but it may require a nuanced approach in the pediatric setting. Providers may consider involving a child life specialist to share news in an age-appropriate manner. Oncological manifestations can be particularly distressing to patients and caregivers because of societal connotations of the diagnosis and negative experiences with older family members. Notably, many children with cancer predisposition syndromes may have family members with the same condition who had a poor outcome, and particular attention should be made to that history.
Fertility preservation should be considered in all pediatric patients and offered whenever possible. Providers should discuss risks and benefits of various fertility preservation methods and involve reproductive specialists to further discuss options before surgery, chemotherapy, and/or radiation treatment.
From a practical point of view, if patients require regular surveillance for malignancy risk, then it is important to consider imaging modalities that limit anesthesia and radiation exposure. If patients are being monitored for multiple sites of potential malignancies, and a CT or MR imaging is essential, then it is prudent to confer with other specialists and coordinate care as much as possible.
Finally, several syndromes discussed may involve manifestations of genitalia/reproductive systems, including atypical genitalia and categories such as intersex (which fall under the controversial medical term of “disorders of sexual development”). It is important for providers to disclose information in an age-appropriate, inclusive manner that acknowledges both the patient’s autonomy regarding their body as well as caregivers’ concerns. The younger the patient is and less able to articulate their bodily autonomy, the more attentive providers should be to these issues. This may be the first time a pediatric-patient encounters conversations such as gender versus sex, which requires sensitivity and respect. Providers should also be attentive to current discussions regarding medicalization of certain atypical GU manifestations that have more basis in conforming a patient to a particular sex assignment as opposed to medical benefit for the patient—for example, in the context of atypical appearance of genitalia. Providers, patients and caregivers alike may benefit from inclusion of other experts such as pediatric endocrinologists and child psychologists while navigating these conversations and decisions.
Conclusions
A large number of cancer predisposition syndromes have been described with genitourinary manifestations. Pediatric urologists are in a unique position to identify patients with cancer predisposition syndromes, and implementation of a surveillance program has the potential to improve quality of life for affected children. Future studies will undoubtedly uncover additional syndromes, and more work is needed to optimize screening and treatment programs. Knowledge of the underlying gene defect provides a potential therapeutic target, such as the successful implementation of mTOR inhibitors in patients with tuberous sclerosis complex. It is hoped that similar therapies will be developed for other syndromes in the years ahead.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (John Wiener, Jonathan Routh and Nicholas Cost) for the series “Pediatric Urologic Malignancies” published in Translational Andrology and Urology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-2019-pum-09). The series “Pediatric Urologic Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mody RJ, Wu YM, Lonigro RJ, et al. Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA 2015;314:913-25. [Crossref] [PubMed]
- Zhang J, Walsh MF, Wu G, et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N Engl J Med 2015;373:2336-46. [Crossref] [PubMed]
- Parsons DW, Roy A, Yang Y, et al. Diagnostic Yield of Clinical Tumor and Germline Whole-Exome Sequencing for Children With Solid Tumors. JAMA Oncol 2016;2:616-24. [Crossref] [PubMed]
- Brodeur GM, Nichols KE, Plon SE, et al. Pediatric Cancer Predisposition and Surveillance: An Overview, and a Tribute to Alfred G. Knudson Jr. Clin Cancer Res 2017;23:e1-5. [Crossref] [PubMed]
- Breslow N, Olshan A, Beckwith JB, et al. Epidemiology of Wilms tumor. Med Pediatr Oncol 1993;21:172-81. [Crossref] [PubMed]
- Scott RH, Stiller CA, Walker L, et al. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet 2006;43:705-15. [Crossref] [PubMed]
- Charlton J, Irtan S, Bergeron C, et al. Bilateral Wilms tumour: a review of clinical and molecular features. Expert Rev Mol Med 2017;19:e8. [Crossref] [PubMed]
- Breslow NE, Collins AJ, Ritchey ML, et al. End stage renal disease in patients with Wilms tumor: results from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol 2005;174:1972-5. [Crossref] [PubMed]
- Grubb GR, Yun K, Williams BR, et al. Expression of WT1 protein in fetal kidneys and Wilms tumors. Lab Invest 1994;71:472-9. [PubMed]
- Rauscher FJ 3rd, Morris JF, Tournay OE, et al. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science 1990;250:1259-62. [Crossref] [PubMed]
- Knudson AG Jr, Strong LC. Mutation and cancer: a model for Wilms' tumor of the kidney. J Natl Cancer Inst 1972;48:313-24. [PubMed]
- Dome JS, Coppes MJ. Recent advances in Wilms tumor genetics. Curr Opin Pediatr 2002;14:5-11. [Crossref] [PubMed]
- Fischbach BV, Trout KL, Lewis J, et al. WAGR syndrome: a clinical review of 54 cases. Pediatrics 2005;116:984-8. [Crossref] [PubMed]
- Clericuzio C, Hingorani M, Crolla JA, et al. Clinical utility gene card for: WAGR syndrome. Eur J Hum Genet 2011;19. [Crossref] [PubMed]
- Beckwith JB. Nephrogenic rests and the pathogenesis of Wilms tumor: developmental and clinical considerations. Am J Med Genet 1998;79:268-73. [Crossref] [PubMed]
- Mueller RF. The Denys-Drash syndrome. J Med Genet 1994;31:471-7. [Crossref] [PubMed]
- Kalish JM, Doros L, Helman LJ, et al. Surveillance Recommendations for Children with Overgrowth Syndromes and Predisposition to Wilms Tumors and Hepatoblastoma. Clin Cancer Res 2017;23:e115-22. [Crossref] [PubMed]
- McCann-Crosby B, Mansouri R, Dietrich JE, et al. State of the art review in gonadal dysgenesis: challenges in diagnosis and management. Int J Pediatr Endocrinol 2014;2014:4. [Crossref] [PubMed]
- Lipska BS, Ranchin B, Iatropoulos P, et al. Genotype-phenotype associations in WT1 glomerulopathy. Kidney Int 2014;85:1169-78. [Crossref] [PubMed]
- Barbaux S, Niaudet P, Gubler MC, et al. Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet 1997;17:467-70. [Crossref] [PubMed]
- Barbosa AS, Hadjiathanasiou CG, Theodoridis C, et al. The same mutation affecting the splicing of WT1 gene is present on Frasier syndrome patients with or without Wilms' tumor. Hum Mutat 1999;13:146-53. [Crossref] [PubMed]
- Hughes IA, Houk C, Ahmed SF, et al. Consensus statement on management of intersex disorders. J Pediatr Urol 2006;2:148-62. [Crossref] [PubMed]
- Weksberg R, Shuman C, Beckwith JB. Beckwith-Wiedemann syndrome. Eur J Hum Genet 2010;18:8-14. [Crossref] [PubMed]
- DeBaun MR, Siegel MJ, Choyke PL. Nephromegaly in infancy and early childhood: a risk factor for Wilms tumor in Beckwith-Wiedemann syndrome. J Pediatr 1998;132:401-4. [Crossref] [PubMed]
- Tan TY, Amor DJ. Tumour surveillance in Beckwith-Wiedemann syndrome and hemihyperplasia: a critical review of the evidence and suggested guidelines for local practice. J Paediatr Child Health 2006;42:486-90. [Crossref] [PubMed]
- Kamihara J, Bourdeaut F, Foulkes WD, et al. Retinoblastoma and Neuroblastoma Predisposition and Surveillance. Clin Cancer Res 2017;23:e98-e106. [Crossref] [PubMed]
- Morris MR, Astuti D, Maher ER. Perlman Syndrome: Overgrowth, Wilms Tumor Predisposition and DIS3L2. Am J Med Genet C Semin Med Genet 2013;163:106-13. [Crossref] [PubMed]
- Alessandri JL, Cuillier F, Ramful D, et al. Perlman syndrome: report, prenatal findings and review. Am J Med Genet A 2008;146a:2532-7. [Crossref] [PubMed]
- Tenorio J, Arias P, Martínez-Glez V, et al. Simpson-Golabi-Behmel syndrome types I and II. Orphanet Journal of Rare Diseases 2014;9:138. [Crossref] [PubMed]
- Scott RH, Walker L, Olsen OE, et al. Surveillance for Wilms tumour in at-risk children: pragmatic recommendations for best practice. Arch Dis Child 2006;91:995-9. [Crossref] [PubMed]
- Rump P, Zeegers MP, van Essen AJ. Tumor risk in Beckwith-Wiedemann syndrome: A review and meta-analysis. Am J Med Genet A 2005;136:95-104. [Crossref] [PubMed]
- Ben-Skowronek I, Kozaczuk S. Von Hippel-Lindau Syndrome. Horm Res Paediatr 2015;84:145-52. [Crossref] [PubMed]
- Maher ER, Yates JR, Harries R, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med 1990;77:1151-63. [Crossref] [PubMed]
- Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet 2003;361:2059-67. [Crossref] [PubMed]
- Jonasch E, McCutcheon IE, Gombos DS, et al. Pazopanib in patients with von Hippel-Lindau disease: a single-arm, single-centre, phase 2 trial. Lancet Oncol 2018;19:1351-9. [Crossref] [PubMed]
- Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer 2014;13:637-44. [Crossref] [PubMed]
- Grubb RL 3rd, Franks ME, Toro J, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J Urol 2007;177:2074-9; discussion 9-80. [Crossref] [PubMed]
- Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell 2002;2:157-64. [Crossref] [PubMed]
- Schmidt LS, Linehan WM. Molecular genetics and clinical features of Birt–Hogg–Dubé syndrome. Nat Rev Urol 2015;12:558-69. [Crossref] [PubMed]
- Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell 2002;2:157-64. [Crossref] [PubMed]
- Pavlovich CP, Walther MM, Eyler RA, et al. Renal tumors in the Birt-Hogg-Dube syndrome. Am J Surg Pathol 2002;26:1542-52. [Crossref] [PubMed]
- Menko FH, van Steensel MAM, Giraud S, et al. Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol 2009;10:1199-206. [Crossref] [PubMed]
- Schmidt L, Duh F-M, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nature Genetics 1997;16:68-73. [Crossref] [PubMed]
- Linehan WM, Bratslavsky G, Pinto PA, et al. Molecular Diagnosis and Therapy of Kidney Cancer. Annu Rev Med 2010;61:329-43. [Crossref] [PubMed]
- Smith AK, Ninos M, Peterson J, et al. 585 Hereditary papillary renal cell carcinoma: a 20-year experience in management of a unique hereditary cancer syndrome. J Urol 2012;187:e239. [Crossref]
- Schmidt LS, Nickerson ML, Angeloni D, et al. Early onset hereditary papillary renal carcinoma: germline missense mutations in the tyrosine kinase domain of the met proto-oncogene. J Urol 2004;172:1256-61. [Crossref] [PubMed]
- Liaw D, Marsh DJ, Li J, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 1997;16:64-7. [Crossref] [PubMed]
- Smpokou P, Fox VL, Tan WH. PTEN hamartoma tumour syndrome: early tumour development in children. Arch Dis Child 2015;100:34-7. [Crossref] [PubMed]
- Ciaccio C, Saletti V, D'Arrigo S, et al. Clinical spectrum of PTEN mutation in pediatric patients. A bicenter experience. Eur J Med Genet 2019;62:103596. [Crossref] [PubMed]
- Carlo MI, Hakimi AA, Stewart GD, et al. Familial Kidney Cancer: Implications of New Syndromes and Molecular Insights. Eur Urol 2019;76:754-64. [Crossref] [PubMed]
- Campbell S, Uzzo RG, Allaf ME, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol 2017;198:520-9. [Crossref] [PubMed]
- Foulkes WD, Kamihara J, Evans DGR, et al. Cancer Surveillance in Gorlin Syndrome and Rhabdoid Tumor Predisposition Syndrome. Clin Cancer Res 2017;23:e62-7. [Crossref] [PubMed]
- Witkowski L, Goudie C, Foulkes WD, et al. Small-Cell Carcinoma of the Ovary of Hypercalcemic Type (Malignant Rhabdoid Tumor of the Ovary): A Review with Recent Developments on Pathogenesis. Surg Pathol Clin 2016;9:215-26. [Crossref] [PubMed]
- Zangari A, Zaini J, Gulìa C. Genetics of Bladder Malignant Tumors in Childhood. Curr Genomics 2016;17:14-32. [Crossref] [PubMed]
- Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol 1995;13:610-30. [Crossref] [PubMed]
- Bougeard G, Renaux-Petel M, Flaman JM, et al. Revisiting Li-Fraumeni Syndrome From TP53 Mutation Carriers. J Clin Oncol 2015;33:2345-52. [Crossref] [PubMed]
- Palmero EI, Achatz MI, Ashton-Prolla P, et al. Tumor protein 53 mutations and inherited cancer: beyond Li-Fraumeni syndrome. Curr Opin Oncol 2010;22:64-9. [Crossref] [PubMed]
- Villani A, Shore A, Wasserman JD, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol 2016;17:1295-305. [Crossref] [PubMed]
- Kratz CP, Achatz MI, Brugieres L, et al. Cancer Screening Recommendations for Individuals with Li-Fraumeni Syndrome. Clin Cancer Res 2017;23:e38-45. [Crossref] [PubMed]
- Ballinger ML, Best A, Mai PL, et al. Baseline Surveillance in Li-Fraumeni Syndrome Using Whole-Body Magnetic Resonance Imaging: A Meta-analysis. JAMA Oncol 2017;3:1634-9. [Crossref] [PubMed]
- Sol-Church K, Stabley DL, Nicholson L, et al. Paternal bias in parental origin of HRAS mutations in Costello syndrome. Hum Mutat 2006;27:736-41. [Crossref] [PubMed]
- Kerr B, Delrue MA, Sigaudy S, et al. Genotype-phenotype correlation in Costello syndrome: HRAS mutation analysis in 43 cases. J Med Genet 2006;43:401-5. [Crossref] [PubMed]
- Gripp KW. Tumor predisposition in Costello syndrome. Am J Med Genet C Semin Med Genet 2005;137C:72-7. [Crossref] [PubMed]
- Gripp KW, Scott CI Jr, Nicholson L, et al. Five additional Costello syndrome patients with rhabdomyosarcoma: proposal for a tumor screening protocol. Am J Med Genet 2002;108:80-7. [Crossref] [PubMed]
- Aoki Y, Niihori T, Banjo T, et al. Gain-of-function mutations in RIT1 cause Noonan syndrome, a RAS/MAPK pathway syndrome. Am J Hum Genet 2013;93:173-80. [Crossref] [PubMed]
- Kratz CP, Rapisuwon S, Reed H, et al. Cancer in Noonan, Costello, cardiofaciocutaneous and LEOPARD syndromes. Am J Med Genet C Semin Med Genet 2011;157C:83-9. [Crossref] [PubMed]
- Ly KI, Blakeley JO. The Diagnosis and Management of Neurofibromatosis Type 1. Med Clin North Am 2019;103:1035-54. [Crossref] [PubMed]
- Crucis A, Richer W, Brugieres L, et al. Rhabdomyosarcomas in children with neurofibromatosis type I: A national historical cohort. Pediatr Blood Cancer 2015;62:1733-8. [Crossref] [PubMed]
- Uusitalo E, Rantanen M, Kallionpaa RA, et al. Distinctive Cancer Associations in Patients With Neurofibromatosis Type 1. J Clin Oncol 2016;34:1978-86. [Crossref] [PubMed]
- O'Brien J, Aherne S, Buckley O, et al. Malignant peripheral nerve sheath tumour of the bladder associated with neurofibromatosis I. Can Urol Assoc J 2008;2:637-8. [Crossref] [PubMed]
- Rober PE, Smith JB, Sakr W, et al. Malignant peripheral nerve sheath tumor (malignant schwannoma) of urinary bladder in von Recklinghausen neurofibromatosis. Urology 1991;38:473-6. [Crossref] [PubMed]
- Gross AM, Wolters PL, Dombi E, et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N Engl J Med 2020;382:1430-42. [Crossref] [PubMed]
- Aideyan UO, Kao SC. Case report: Urinary bladder rhabdomyosarcoma associated with Beckwith-Wiedemann syndrome. Clin Radiol 1998;53:457-9. [Crossref] [PubMed]
- Vaughan WG, Sanders DW, Grosfeld JL, et al. Favorable outcome in children with Beckwith-Wiedemann syndrome and intraabdominal malignant tumors. J Pediatr Surg 1995;30:1042-4; discussion 1044-5. [Crossref] [PubMed]
- Kajii T, Ikeuchi T, Yang ZQ, et al. Cancer-prone syndrome of mosaic variegated aneuploidy and total premature chromatid separation: report of five infants. Am J Med Genet 2001;104:57-64. [Crossref] [PubMed]
- Ariceta G, Buj MJ, Furlano M, et al. Recommendations for the management of renal involvement in the tuberous sclerosis complex. Nefrologia 2020;40:142-51. [Crossref] [PubMed]
- Henske EP, Jozwiak S, Kingswood JC, et al. Tuberous sclerosis complex. Nat Rev Dis Primers 2016;2:16035. [Crossref] [PubMed]
- Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013;381:817-24. [Crossref] [PubMed]
- Brakemeier S, Bachmann F, Budde K. Treatment of renal angiomyolipoma in tuberous sclerosis complex (TSC) patients. Pediatr Nephrol 2017;32:1137-44. [Crossref] [PubMed]
- Kim J, Field A, Schultz KAP, et al. The prevalence of DICER1 pathogenic variation in population databases. Int J Cancer 2017;141:2030-6. [Crossref] [PubMed]
- Pugh TJ, Yu W, Yang J, et al. Exome sequencing of pleuropulmonary blastoma reveals frequent biallelic loss of TP53 and two hits in DICER1 resulting in retention of 5p-derived miRNA hairpin loop sequences. Oncogene 2014;33:5295-302. [Crossref] [PubMed]
- Schultz KAP, Williams GM, Kamihara J, et al. DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin Cancer Res 2018;24:2251-61. [Crossref] [PubMed]
- Stewart DR, Best AF, Williams GM, et al. Neoplasm Risk Among Individuals With a Pathogenic Germline Variant in DICER1. J Clin Oncol 2019;37:668-76. [Crossref] [PubMed]
- Coleman JF, MacLennan GT. Gonadoblastoma. J Urol 2006;175:2300. [Crossref] [PubMed]
- Goudie C, Witkowski L, Vairy S, et al. Paediatric ovarian tumours and their associated cancer susceptibility syndromes. J Med Genet 2018;55:1-10. [Crossref] [PubMed]
- Sybert VP, McCauley E. Turner's syndrome. N Engl J Med 2004;351:1227-38. [Crossref] [PubMed]
- Periquito I, Carrusca C, Morgado J, et al. Familial Turner syndrome: the importance of information. J Pediatr Endocrinol Metab 2016;29:617-20. [Crossref] [PubMed]
- Leichtman DA, Schmickel RD, Gelehrter TD, et al. Familial Turner syndrome. Ann Intern Med 1978;89:473-6. [Crossref] [PubMed]
- Lippe B. Turner Syndrome. Endocrinol Metab Clin North Am 1991;20:121-52. [Crossref] [PubMed]
- Brant WO, Rajimwale A, Lovell MA, et al. Gonadoblastoma and Turner Syndrome. J Urol 2006;175:1858-60. [Crossref] [PubMed]
- MacMahon JM, O'Sullivan MJ, McDermott M, et al. Early Bilateral Gonadoblastoma in a Young Child with Mosaicism for Turner Syndrome and Trisomy 18 with Y Chromosome. Horm Res Paediatr 2017;87:130-5. [Crossref] [PubMed]
- Jonson AL, Geller MA, Dickson EL. Gonadal dysgenesis and gynecologic cancer. Obstet Gynecol 2010;116 Suppl 2:550-2. [Crossref] [PubMed]
- Hemminki A, Tomlinson I, Markie D, et al. Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat Genet 1997;15:87-90. [Crossref] [PubMed]
- Giardiello FM, Trimbath JD. Peutz-Jeghers syndrome and management recommendations. Clin Gastroenterol Hepatol 2006;4:408-15. [Crossref] [PubMed]
- Dozois RR, Kempers RD, Dahlin DC, et al. Ovarian tumors associated with the Peutz-Jeghers syndrome. Ann Surg 1970;172:233-8. [Crossref] [PubMed]
- Young RH, Welch WR, Dickersin GR, et al. Ovarian sex cord tumor with annular tubules: review of 74 cases including 27 with Peutz-Jeghers syndrome and four with adenoma malignum of the cervix. Cancer 1982;50:1384-402. [Crossref] [PubMed]
- Meserve EE, Nucci MR. Peutz-Jeghers Syndrome: Pathobiology, Pathologic Manifestations, and Suggestions for Recommending Genetic Testing in Pathology Reports. Surg Pathol Clin 2016;9:243-68. [Crossref] [PubMed]
- Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110:223-62. [Crossref] [PubMed]
- Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet 2000;26:89-92. [Crossref] [PubMed]
- Wilkes D, McDermott DA, Basson CT. Clinical phenotypes and molecular genetic mechanisms of Carney complex. Lancet Oncol 2005;6:501-8. [Crossref] [PubMed]
- Wieacker P, Stratakis CA, Horvath A, et al. Male infertility as a component of Carney complex. Andrologia 2007;39:196-7. [Crossref] [PubMed]
- Kamilaris CDC, Faucz FR, Voutetakis A, et al. Carney Complex. Exp Clin Endocrinol Diabetes 2019;127:156-64. [Crossref] [PubMed]
- Pyle LC, Nathanson KL. Genetic changes associated with testicular cancer susceptibility. Semin Oncol 2016;43:575-81. [Crossref] [PubMed]
- Litchfield K, Loveday C, Levy M, et al. Large-scale Sequencing of Testicular Germ Cell Tumour (TGCT) Cases Excludes Major TGCT Predisposition Gene. Eur Urol 2018;73:828-31. [Crossref] [PubMed]


