
Male genital sensation after spinal cord injury: a review
Introduction
Spinal cord injury (SCI) is a severe threat to human health: it degrades the quality of patients’ lives, deprives them of their ability to work, and even causes death. According to the World Health Organization (WHO), there are about 250,000 to 500,000 new cases of SCI in the world each year (1). The proportion of males and females is about 2:1, and the age of onset is mostly concentrated in young adults, followed by old age. The National Spinal Cord Injury Statistical Center (NSCISC) reported that the incidence of SCI in the United States is 54 cases/million, of which 78% are male, with nearly half (47.6%) of the injuries occurring between the ages of 16 and 30 (2). This demographic is characterized by low mortality after 24 hours of injury and a strong desire for fertility and sexual function (3).
Erectile and ejaculation dysfunction and the abnormal semen quality are the main pathological factors leading to the decline of male fertility and sexual function and have received extensive attention in recent studies (4,5). With the advancement of fertility treatments, improving sexual satisfaction, and maintaining harmonious sexual relations are in greater demand from SCI patients (4,5). Sex is the fundamental right of humanity, and the need for intimacy and sexual pleasure is the primary motivation for SCI patients’ sexual activities (6). Genital sensation (GS) is a central aspect of sexual pleasure and is directly associated with the quality of one’s sex life. GS impairment caused by SCI is a ubiquitous problem in these patients but has not received proportionate attention. This narrative review will address the role of GS in male sexual function, discuss the impact of genital sensation disturbance (GSD) after SCI, and summarize the current diagnosis and treatment of GSD in the male patients.
Innervation of male GS
Male GS includes the somatic sensation of the scrotum and penis. The scrotal skin is innervated by the ilioinguinal nerve and the genital branch of the genitofemoral nerve (GFN). The ilioinguinal nerve passes obliquely across the internal oblique muscle and then enters the inguinal canal medial to the anterior superior iliac spine. Accompanying the spermatic cord, some of the cutaneous branches descend from the inguinal canal into the scrotum. The GFN supplies sensation to the anterior scrotal area. It descends over the surface of the psoas muscle and then separates into the femoral and genital branches. The latter enters the inguinal canal to innervate the cremaster and scrotal skin. Stimulation from the scrotal skin can increase the sensitivity of the penis (7).
The penile sensation is the most sensitive and dominant part of GS, which consists of vibration, cold and thermal sensation, haptic perception, and the related sensation of pain. Also, a variety of receptors are distributed throughout the skin of the penis and include Meissner’s corpuscles, genital corpuscles, and free nerve endings (8-10). The pudendal nerve innervates most of the perineal area. It originates from the of S2–S4 spinal cord segments and ultimately divides into 3 terminal branches: the inferior rectal nerve, the perineal nerve, and the dorsal nerve of the penis (DNP) (11). The sensory fibers of the perineal nerve are distributed across the scrotal and penile ventral area (12,13), while the DNP innervates the penis glans and most of the penile skin (14). The ilioinguinal nerve also supplies sensation to the penis root.
The DNP usually contains 2–6 main branches, which travel along both sides of the penis and are accompanied by the dorsal penile vessels (15). These branch fibers course laterally under the skin, penetrate the tunica albuginea of the penis and are distributed throughout the corpus spongiosum, corpora cavernosa, and urethra. They can receive the signal for micturition and ejaculation and facilitate the bulbocavernosus reflex (BCR). Furthermore, the DNP is extensively associated with the cavernous nerve, especially at the penile hilum and cavernous-spongiosal junction (16,17). The number of nNOS-positive fibers conveyed by the cavernous nerve in DNP is closely correlated with erectile function (18). These features make the DNP not only a somatosensory afferent nerve, but also an integrated nerve that is extensively involved in erection, ejaculation, and urination.
GS in erection and ejaculation
Penile erection is a complex neurovascular event facilitated by the innervations of the sympathetic, parasympathetic, and somatic nervous systems, and is co-regulated by the spinal and cerebral center. The supraspinal center of erection is mainly located in the medial preoptic area of the hypothalamus (MPOA) and paraventricular nucleus (PVN), while the spinal erectile center consists of the thoracolumbar sympathetic neurons in the intermediolateral cell column (IML) and dorsal gray commissure, the sacral parasympathetic nucleus (SPN), and the pudendal motoneurons of Onuf’s nucleus (19-21). The axons of the SPN run in the pelvic nerve to the pelvic plexus, with the postganglionic parasympathetic fibers reaching the penis via the cavernous nerve. The parasympathetic nerve endings release nitric oxide (NO) to relax the penile smooth muscle and blood vessels and eventually cause an erection, while the sympathetic nerves inhibit it. The pudendal motor neurons enhance erection through the motor fibers of the pudendal nerve that innervate the ischiocavernosus muscle (22,23).
According to its origin, the male erection can be divided into psychogenic erections and reflex erections. Non-genital stimulation such as visual and auditory sensations can induce a psychogenic erection, and the direct sensation of genitals can induce a reflex erection. However, they do not occur in mutual exclusion under natural conditions and usually promote each other (22), with GS playing a substantial role in both processes. For one, it serves as an initial signal for the reflective erection that induces and maintains the penile erection. For another, the stimulation of the external genitalia is one of the most influential factors that cause sexual arousal, which in turn promotes the erection and makes it durable enough to last throughout the entirety of sexual intercourse (24).
Ejaculation consists of 2 processes: emission and expulsion. During emission, the bladder neck contracts to prevent retrograde spillage of the semen into the bladder. The secretions of the prostate, seminal vesicles, and urethral glands are mixed with sperm in the posterior urethra to form semen. Then, the bladder neck remains closed, the external urinary sphincter relaxes, and the pelvic striated muscles, especially the bulbospongiosus and ischiocavernosus muscles, are forcefully contract to expel semen (25-27). The cerebral control for ejaculation is located in the medial amygdala, bed nucleus of the stria terminalis, MPOA, and PVN areas (28,29). In the spinal cord and its periphery, ejaculation is also innervated by the autonomic somatic nervous system. The parasympathetic nerves innervate the gonads, including the prostate and seminal vesicles, and are associated with glandular secretion (26). The sympathetic nerves contract the vas deferens and smooth glandular muscles through the neurotransmitters of norepinephrine, acetylcholine, and non-adrenergic non-cholinergic transmitters, resulting in emission (25,29). The somatic motor nerves control the coordinated action of the external urinary sphincter and the pelvic striated muscles to produce expulsion. These activities are coordinated by the spinal ejaculation generator (SEG). It receives the stimulating and inhibiting signals from the supraspinal level, and the penile stimulus signal from the periphery (30) contacts and regulates the activities of the autonomic and somatic center of the lumbosacral spinal cord through interneurons, thus ultimately controlling the occurrence of ejaculation (28,31).
The afferent nerves of ejaculation originate from the DNP and the sympathetic afferent fibers in the hypogastric nerve (32), and the GS conducted by the DNP is essential for ejaculation function. Genital stimulation can evoke ejaculation both through the cerebral pathway of the sensory cortex and the spinal pathway of the SEG, and DNP is the common initial route of these 2 processes (33). Furthermore, the anesthetic block of the DNP directly inhibits the reflective ejaculation of SCI patients (34). Although penile vibratory stimulation can induce the ejaculation of men with lesions above T10, the effectiveness is significantly reduced in those with a penile prosthesis. Animal experiments have similar results (35-37). Therefore, the integrity of GS is indispensable for the ejaculation course.
GS in male sexual arousal and orgasm
In 1966, Masters and Johnson first divided the human sexual response into 4 periods: sexual excitement/arousal, plateau, orgasm, and resolution (38). Later, in 1974, Kaplan proposed a three-phase model of desire, arousal, and orgasm (39). These models are collectively referred to as “linear models” and have been widely used to describe male sexual response processes (40). However, there is still no agreement on the exact definition of sexual arousal and orgasm (24,25). As a somatic sensation, GS plays a role not only in the physical reaction but in the psychological pleasure and satisfaction of sex life. Studying the relationship between GS and male sexual arousal and orgasm is of great value for a comprehensive understanding of male sexual function.
Sexual arousal is a complicated sexual response that includes psychological reactions and neurophysiological changes and is usually accompanied by a series of vascular events (41). The current detection and observation of sexual arousal are usually based on the accompanying physiological responses such as heart rate and blood pressure, with penile erection being the primary test indicator in male studies. Nevertheless, sexual arousal and erection are not always synchronous, and it is not advisable to equate evaluation of arousal with the measurement of erection in SCI studies (24,42). The sexual cues of vision, audition, and even fantasy can trigger sexual arousal, but the tactile stimulation of the genitals is still the most potent sensation. After analyzing the erogenous zones of 704 Finns (including 176 men), Nummenmaa et al. (43) found that the genital area is one of the most consistent regions to cause excitement, whether it is in sexual intercourse with a partner or masturbation, and is even more sensitive during the masturbation. The deficiency of GS inhibits sexual arousal and reduces the frequency of sexual activity (44).
The gradual accumulation and release of sexual tension and excitement and the accompanying extremes of pleasure and physiological response are the main features of orgasm (25,45,46). While processing the input information of increased posterior urethral pressure during ejaculation, the brain produces a brief and intense pleasure—orgasm (47,48). It is noticeable, however, that ejaculation is physiologically different from the orgasm, and some patients with SCI can reach orgasm without ejaculation (49,50). As mentioned above, GS is one of the most potent triggers for sexuality. Continuous GS stimulation can effectively accumulate sexual intension and excitement during sexual intercourse and subsequently lead to the climax and sexual pleasure. The accompanying ejaculation induced by GS conversely promotes the orgasmic sensation, thus making the orgasm reach the maximum degree (51).
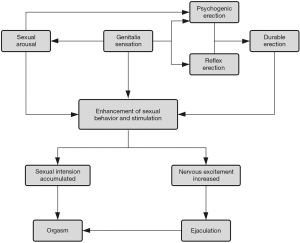
Therefore, GS synergizes erection, ejaculation, sexual arousal, and orgasm courses associated with physiological and psychological responses in male sexual activity. The details of the connection are shown in Figure 1. GS is easily overlooked in male sexual research, while the GSD can seriously affect the sexual function of men. At present, the specific role of GS in various sexual responses is not deeply understood. Further studies are needed to explore the detailed mechanism of GS function in male sexuality.
Male GS after SCI
The disturbance of GS in SCI patients is related to the degree and location of the injury. As the genital afferent nerves are located in the low segments, particularly the sacral level of the DNP, almost all complete suprasacral SCIs and a considerable portion of incomplete SCI patients have varying degrees of GS disturbance, especially the men with conus medullaris injury. According to the 2018 annual report of the National Spinal Cord Injury Statistical Center (NSCISC) (52), about 42.7% of the 33,406 SCI patients recorded had a complete spinal cord injury at discharge, with the percentage of lumbosacral cord injury being 10.7%. This means a considerable proportion of male SCI patients have GSD. Epidemiological studies in other regions have found similar results (53-56). In addition, chronic pain, spasticity, autonomic dysreflexia (AD), and bladder/rectal dysfunction induced by SCI can cause problems in some patients' sexual lives (57). The physical misery and reduced quality of life can also lead to psychological problems in patients. These can reduce the decline of the patient's sexual desire and function and affect the recovery of GS. Some iatrogenic factors, such as the use of vacuum suction devices to treat erectile dysfunction (ED) may cause penile skin damage and pain, which in turn affects GS function (58).
As mentioned above, the GSD has a severe impact on sexual function and is more complicated in SCI patients. The deficiency of GS makes the tactile stimulation of the penis unable to cause sexual arousal, disturbs the normal processes of erection and ejaculation, and decreases the sexual desire and the satisfaction of an orgasm. Alexander et al. (44) conducted a questionnaire survey of 38 male SCI patients (median age =26) and found that, compared with pre-injury, the frequency of intercourse activity decreased and the interest in alternative sexual activities conversely improved. The decrease in sexual satisfaction was positively correlated with the waning interest in the penile-vaginal activity. Anderson et al. (6) further studied the impacts of GSD on sexual function after SCI. In a questionnaire survey of 199 male SCI patients (in which 42.9% had GS), they found that patients with GS were more likely to have sexual arousal and more frequently expressed confidence and satisfaction with their erectile performance. The association between GS and orgasm is more pronounced. Of the patients who reported having reached the orgasm, 72.8% had also retained genital sensations. GS also has a specific correlation with ejaculation. However, GSD following SCI is usually caused by the disconnection between the high-level spinal cord and the sexual center below the injury plane. The pathway to the cerebral cortex is blocked, but the patient's sensory afferent nerves remain functional. Therefore, the investigative and statistical study cannot fully explain the sexual effects of GS in SCI patients alone, and thus more clinical and experimental research is needed.
The development of non-genitalia erogenous zones after SCI is a notable phenomenon that occurs in the patients of long injury history. Alexander et al. (44) reported that the general arousal area of respondents after SCI (median 37 months postinjury) changed from the genitals to the lips, neck, shoulders, and ears. Unexpectedly, a significant number of patients reported that their genitals were still sexually arousable despite their complete spinal cord injury. Anderson et al. (6) also described this phenomenon. They found that many SCI patients developed new sexual arousal areas at (27.6%) and above (41.7%) their level of lesion, including the head or neck, torso, arms, and shoulders. Moreover, this change had a positive relationship with increasing time postinjury and was more likely to occur in men with penile anesthesia. Borisoff et al. (59) found that with the increase in postinjury time and sexual experience, autonomic hyperactivity and related physiological sensations in SCI patients could be transformed into a pleasant sexual experience and even cause an orgasm. The emergence of these alternative genital sensations may be related to neuroplasticity, showing the enormous potential of SCI patients to restore sexual pleasure and orgasm, and providing valuable clues for treating patients with GSD.
Testing of GS
Self-report
The questionnaire report is a convenient testing method and often does not require specialized equipment. Sexual sensation testing is inevitably subjective in a way and involves patient privacy. Conventional tests are generally more difficult for patients to accept and are susceptible to variability in the detection environment and operation. Therefore, self-reporting from patients based on validated questionnaire tools is an irreplaceable method for detecting GS function (60). Although there are already several standardized questionnaires for evaluating a male sexual function, such as the International Index of Erectile Function (IIEF), the Brief Male Sexual Function Inventory (BMSFI), and the Derogatis Sexual Functioning Inventory (DFSI) (61), few tools are designed to assess sexual sensations, especially GS. At present, the IIEF and the Self-Assessment of Genital Anatomy and Sexual Function, Male questionnaire (SAGASF-M), are the main validated questionnaires in evaluating male genital sensations (61-64).
The IIEF is the most widely used instrument to assess male sexual function. It has been linguistically validated in 32 languages and has been used as the primary endpoint in at least 50 clinical trials (64,65). The questionnaire consists of 15 items and evaluates male sexual function from 5 aspects: erection, ejaculation, libido, sexual satisfaction, and overall satisfaction (66). Furthermore, a simplified version of only 5 items, the IIEF-5 or the Sexual Health Inventory for Men, has been developed. Despite the widespread recognition of IIEF, it is not a specialized scale designed to assess GS, which limits its use in the studies of GS.
The SAGASF-M is a self-evaluation tool proposed by Schober et al. to assess the effects of genital surgery on appearance, sensitivity, and sexual function (60). Unlike traditional questionnaires, SAGASF-M uses many detailed genital pictures and descriptive phrases, which makes self-reporting more accurate and systematic. This questionnaire allows people to assess their genital appearance, overall sexual and pain sensitivity, the intensity of orgasm, and the effort required to reach orgasm by stimulating the penis, scrotum, and specific areas around the anus. The role of other sexually sensitive parts of the body is also involved. The SAGASF-M is more suitable for assessing GS in SCI patients and has been applied in clinical studies (63).
Nevertheless, there is still no validated questionnaire specifically designed for testing male GS function, especially for evaluating the postinjury recovery. Many studies have only used self-made questionnaires or otherwise have modified the validated standardized tools to make them more suitable for their research (59,67,68). The validity and reliability of these self-made instruments remain doubtful.
Quantitative sensory testing (QST)
QST is a standardized and formalized method that captures perception and pain thresholds by applying specific and calibrated stimuli to the tested area (69). Compared with the traditional tests, QST can detect the function of both large and small sensory nerve fibers and has the advantages of being non-invasive and quantitative (70). QST has been used in assessing penile sensory function for several years, including in the detection of penile vibration, spatial perception, stress and touch, temperature perception, and pain sensation (8,9,71).
As a neuropsychological test, the results of QST are highly dependent on the framework and the cooperation of subjects and are susceptible to being influenced by extraneous factors (69,70). Therefore, the reliability and repeatability of the method should be considered when performing QST, and the interpretation of the results should always be based on the patient's clinical presentation (70). The clinical application of penile sensation QST has made initial progress. Yarnitsky et al. (72) detected the cold and thermal sensation threshold of the penis of 35 healthy volunteers using 2 methods and found that the temperature threshold detection had good reproducibility. Bleustein et al. (71) performed an IIEF assessment and a penis QST test, which included an evaluation of vibration, pressure, spatial perception, and temperature sensation in 107 subjects, and found that the change in temperature threshold in all QST items had the best sensitivity and specificity for detecting penile sensation. Lefaucheur et al. reported similar results (73,74). These studies demonstrate that penile thermal threshold detection has the best clinical value in assessing penile sensation, especially in the function of small sensory fibers. Thus, QST is clinically more convenient and practical for testing penile sensation.
Neurophysiological examination
The neurophysiological examination is a classic method for detecting peripheral nerve function. The items for testing sensory nerves include nerve conduction velocity and somatosensory evoked potential (SEP). For the penis, the common tests involve the bulbocavernosus reflex (BCR) and penile sympathetic skin responses (pSSRs) (73). BCR and SEP have a specific localization diagnosis for the injury segment of SCI patients. The former can detect the integrity of the spinal reflex arc, while the latter reveals the pathology of the ascending pathway (75). Neurophysiological testing is a more classic and objective method compared with QST, but it is mainly used to detect the function of the large myelinated fibers, and some of the methods are invasive, which limits its use in clinical work (71,75).
Treatments for male GSD after SCI
To cure the GSD in SCI patients is to improve their sexual satisfaction and quality of life. Due to the complexity of SCI and the diversity of its impacts on sexual function, SCI patients often have different levels of sexual requirements. Their mental states, religious beliefs, cultural practices, economic conditions, and social environment also affect their sexual needs. Therefore, developing a reasonable treatment plan not only requires an accurate and comprehensive assessment of the patient's sexual function, but also needs to have good communication with the patient, reasonable treatment goals, and effective therapies. Moreover, the entire process should be coordinated with the patient's health management.
Sexual rehabilitation education and recommendations for SCI
The first step in sexual rehabilitation education for SCI patients is to establish their confidence in restoring their sex life. While patients are willing to communicate with clinicians about their sexual issues, sexual education and counseling have not gained enough attention (58,76). Due to the complications of physical disability, urinary incontinence, and GSD, SCI patients usually lack the self-image and confidence to have sex and may even think that they have permanently lost sexual function. During the treatment, doctors should realize the considerable recovery potential of SCI patients, actively respond to their questions about sexual function, and correctly guide their sexual attitudes and lifestyles.
Sexual education includes teaching patients the necessary knowledge to help them understand their changes in sexual function after injury, broadening their perception of sexual gratification, and encouraging them to engage in self-discovery and adjustment of their sexual lifestyle (58). Professional sexual rehabilitation services can be recommended or provided if patients have requirements. During sexual consultation, the clinician should pay attention to the possible causes of sexual dysfunction, especially any psychological problems, drugs, iatrogenic problems, and the lack of knowledge and awareness of founding a new sexual lifestyle (77). They can then provide further treatments for these causes to more effectively meet the actual needs of the patient.
Learning to reach orgasm via the residual sexual sensitive zone is an effective way to improve sexual sensation (41). If the patient has some remaining degree of GS or sensation of the T11-L2 dermatomes, instructors should recommend that the patient concentrates on these areas during sexual intercourse and masturbation to enhance the sensitivity of these areas (58).
Discovering and developing substitute erogenous zones
Neuroplasticity is a self-repairing mechanism of the nervous system after injury. It plays an essential role in the patient's sensory and motor function recovery and is the basis for the bulk of rehabilitation training and treatment (78,79). After SCI, adaptive and compensatory changes occur in the patient's sexual sensory function due to impaired or missing GS. Residual sexual sensation becomes more sensitive, some secondary sensitive areas (lips, nipples, etc.) are strengthened, and new sexually sensitive areas (neck, ears, etc.) appear above the level of injury (6,43). This makes discovering and developing substitute erogenous zones a feasible way to promote the patients' sexual satisfaction.
Developing a new erogenous area requires the patient’s confidence and persistence, the cooperation and support of the sexual partner, and the guidance of the doctor. Advisors should instruct the patients to pay attention to unconventional excitement points such as ears, neck, and shoulders above their injury levels, encourage them to try a new sexual lifestyle and posture, and repeatedly practice the new sensitive zones. Radios, books, and other helpful resources can also be applied (41,58). Patients should be educated in recognizing the AD that may occur in sex and to understand that the AD symptoms may also be associated with orgasm in SCI. As mentioned above, autonomic responses have the potential to be converted into sexual pleasure, especially for patients with a long history of injury (51,59,80). Some questionnaires, such as those designed by Courtois et al., can also help patients to notice and recognize the role of this autonomic sensation in orgasm (51,80).
Borisoff et al. (59) developed a sensory substitution system to map the hand movements during masturbation onto the tongue. They used a two-dimensional electrotactile stimulator with a square micro-stimulating electrode array to map the hand motion signal onto the tongue in real-time (81). Based on this device, they designed a systematic sensory alternative training and conducted a study of 4 male subjects with SCI (injury history over 1 year with 1 subject departing mid-study). The results were measured by the questionnaires collecting information on orgasm, ejaculation, and sexual sensation (51,82), and indicated that all 3 subjects improved their level of sexual sensation and the sensory satisfaction after training. However, the subjects' evaluation of erectile function and the other 2 dimensions of the orgasm questionnaire did not change markedly, and none of the subjects reached orgasm in training. This study shows the potential effect of GS substitute therapy. Although no one reached orgasm in the study, the sexual pleasure of the subjects was significantly improved. Considering the trial was conducted in a test environment without the help of any other assisted methods, these results are encouraging. Moreover, when asked if they would like to use a similar device at home, all 3 subjects responded affirmatively.
Medication, surgery, and other treatments
Few drugs have been reported for the treatment of GSD after SCI. Midodrine, an alpha-1 receptor agonist, is widely used to treat ejaculation disorders in men with SCI, especially in patients with poor response to penile vibratory stimulation (48,83,84). Its excitatory effects on autonomic nerves can promote ejaculation and improve orgasmic sensation, which is also helpful for patients in adapting to new sexual arousal methods after injury (49,83,85). The main side-effects of midodrine therapy are tingling, spasms, and AD syndrome, which is characterized by headache, sweating, and hot flushes (49,83). During the treatment with midodrine, the blood pressure and heart rate of patients should be monitored regularly for AD detection and management, especially in people with quadriplegia (49).
Penile vibratory stimulation is the preferred treatment of men with anejaculation after SCI, and it is also effective in relieving spasm symptoms (86). Calabrò et al. (87) recently reported the use of pelvic muscle vibration to treat ED of SCI patients. They performed muscle vibration treatment on 10 ED patients with incomplete SCI and found the amplitude of their pudendal nerve SEP was distinctly increased, and many patients reported an increase in sexual sensation in this study, although this increase was not statistically significant. It has been reported that genital vibration stimulation can also promote the recovery of sexual function and sexual sensation in women (88). Whether pelvic muscle vibration is also helpful for the GS function of male SCI patients requires further research.
Surgical treatments such as penile angioplasty can help patients with penile trauma to recover the shape and partial function of the penis, but these cannot repair the penile sensation damage caused by SCI itself (89). Overgoor et al. (90) reported a penile sensation recovery operation for lumbosacral spina bifida (SB) in 2006, which anastomoses the unilateral penile dorsal nerve with the ilioinguinal nerve, allowing the penile sensation to pass through the afferent fibers of the L1 segment above the lesion. They firstly performed this nerve transposition procedure, or TOMAX surgery, on 3 young L3-L5 male SB patients. Each patient reported a unilateral penile sensation after 15 months of recovery, and their pleasure from masturbation and sex life also improved (90). This procedure was then applied to patients with low-level SCI (91). In a follow-up of 30 patients undergoing repair surgery (including 18 SB and 12 SCI patients), 24 patients (80% of the total) had penile sensation restored 11–24 months after surgery, and 3 SCI patients could not receive the entire operation because of an ilioinguinal nerve lesion. Furthermore, 13 subjects (9 SB, 4 SCI) had only a groin-like sensation, and 11 (8 SB, 3 SCI) had a feeling in the penis glans. The regenerated sensation developed from the cold feeling of the urethral cannula then turned into tactile and painful sensations in the groin, and eventually converted into feeling in the penis glans. Jacobs et al. (92) performed the same procedure on 2 sexually active patients with SB (L5/S1 segment). Both of the patients reported a satisfactory recovery of penile sensation in the 2-year follow-up after surgery. One of the patients even accepted the contralateral surgery because of the encouraging results; similar requests also occurred in Overgoor’s research (91,93,94).
TOMAX surgery is a preferred treatment for GSD in patients with low SCI. This surgical approach to restoring penile sensation through peripheral nerve bypass provides a feasible method to recover GS function. After transposition of the DNP, the patient's penile sensation is conducted through the ilioinguinal to the cerebral cortex, thus causing the initial displacement of the sensation. Some patients undergo correction of the disorder in the recovery period. This correction is related to brain plasticity, but the specific mechanism remains unclear. Kortekaas et al. (95) performed a functional magnetic resonance imaging test on 3 TOMAX-treated SB patients. When stimulating the re-innervated glans penis and the intact contralateral inguinal region, they found that the exciting areas of the primary somatosensory cortex were similar, while the functional networks varied. The middle cingulate cortex and the parietal operculum-insular cortex were found to mediate the novel penis sensation in these patients.
To date, no severe complications have been reported as a result of TOMAX surgery. The sensation in the groin is notably diminished postsurgery but has not generated any discomfort. However, in a recent 43-case report of this procedure (94), 1 patient’s ilioinguinal nerve was accidentally severed during the operation, resulting in delayed recovery; 2 patients developed postoperative hematomas but recovered after repeated microneurography. Owing to the anatomical variations, the ilioinguinal nerve was found to be unilaterally or bilaterally absent in 5 patients, and 4 of them failed the operation. There are also several limitations in Overgoor et al.’s surgery. Firstly, this procedure requires the patient's injury segment to be lower than L1, and the proportion of these SCI patients is only about 5%, thus reducing the applicable scope for SCI (52). Secondly, due to the severance of the DNP, this procedure may impair the patient's residual reflex erection and ejaculation function, which is why the bilateral operation is considered cautiously (93). Furthermore, the treatment effect of this operation is still limited. In the previous reports (91,94), about 80% of subjects had penile sensation restored to varying degrees, with only half of these subjects having feeling in the glans, and the recovery range was unilateral. Given these results, it is necessary for surgeons to fully inform their patients about the treatment effect before the operation.
Conclusion
GS is an essential component of male sexual function. It is one of the most potent triggers for sexual arousal and erection, promotes continuous sexual activity, and induces final ejaculation and orgasm. The GSD caused by SCI, in turn, disrupts the erection and ejaculation, decreases the sexual satisfaction and the quality of orgasm, and changes the sexual arousal areas of the patients.
Despite the massive demand for GS recovery, the studies of diagnosis and treatment of GSD after SCI is still in the early stages. Self-report and electrophysiological tests are the main methods for assessing GS. Penile QST, especially thermal threshold detection, has good clinical application prospects. Sexual rehabilitation education and sexual guidance for patients should be given due attention and be applied in clinical work. Helping patients develop and adapt to new, sexually sensitive areas is an effective way to improve sexual sensory function after SCI. Some new adjuvant therapies and surgical methods have achieved specific results in the treatment of GSD, but the related technologies still need further study and improvement. Future research should continue to explore the role of GS in male sexuality and the impact of GSD on SCI patients' sexual life, focus on how to assess the residual and restored GS function after SCI, help patients develop sexual sensory replacement areas, and improve their degree of genital sensation.
Acknowledgments
Funding: This study was supported by grants from the National Natural Science Foundation of China (NSFC) (Grant No. 81671216).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-19-829). HZ and BL report grants from National Natural Science Foundation of China, during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Spinal Cord Injury - Key facts. 2013. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. 2019. Available online: https://www.sci-info-pages.com/wp-content/media/NSCISC-2019-Spinal-Cord-Injury-Facts-and-Figures-at-a-Glance.pdf
- Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 2012;50:365-72. [Crossref] [PubMed]
- Brackett NL, Lynne CM, Ibrahim E, et al. Treatment of infertility in men with spinal cord injury. Nat Rev Urol 2010;7:162-72. [Crossref] [PubMed]
- Ibrahim E, Lynne CM, Brackett NL. Male fertility following spinal cord injury: an update. Andrology 2016;4:13-26. [Crossref] [PubMed]
- Anderson KD, Borisoff JF, Johnson RD, et al. Long-term effects of spinal cord injury on sexual function in men: implications for neuroplasticity. Spinal Cord 2007;45:338-48. [Crossref] [PubMed]
- Halata Z, Munger BL. The neuroanatomical basis for the protopathic sensibility of the human glans penis. Brain Res 1986;371:205-30. [Crossref] [PubMed]
- Bossio JA, Pukall CF, Steele SS. Examining Penile Sensitivity in Neonatally Circumcised and Intact Men Using Quantitative Sensory Testing. J Urol 2016;195:1848-53. [Crossref] [PubMed]
- Morris BJ, Krieger JN. Does male circumcision affect sexual function, sensitivity, or satisfaction?--a systematic review. J Sex Med 2013;10:2644-57. [Crossref] [PubMed]
- Cox G, Krieger JN, Morris BJ. Histological Correlates of Penile Sexual Sensation: Does Circumcision Make a Difference? Sex Med 2015;3:76-85. [Crossref] [PubMed]
- Shafik A, El-Sherif M, Youssef A, et al. Surgical anatomy of the pudendal nerve and its clinical implications. Clin Anat 1995;8:110-5. [Crossref] [PubMed]
- Schraffordt SE, Tjandra JJ, Eizenberg N, et al. Anatomy of the pudendal nerve and its terminal branches: a cadaver study. ANZ J Surg 2004;74:23-6. [Crossref] [PubMed]
- Yucel S, Baskin LS. Neuroanatomy of the male urethra and perineum. BJU Int 2003;92:624-30. [Crossref] [PubMed]
- Yang CC, Bradley WE. Innervation of the human glans penis. J Urol 1999;161:97-102. [Crossref] [PubMed]
- Kozacioglu Z, Kiray A, Ergur I, et al. Anatomy of the dorsal nerve of the penis, clinical implications. Urology 2014;83:121-4. [Crossref] [PubMed]
- Akman Y, Liu W, Li YW, et al. Penile anatomy under the pubic arch: reconstructive implications. J Urol 2001;166:225-30. [Crossref] [PubMed]
- Yucel S, Baskin LS. Identification of communicating branches among the dorsal, perineal and cavernous nerves of the penis. J Urol 2003;170:153-8. [Crossref] [PubMed]
- Chen YL, Chao TT, Wu YN, et al. nNOS-positive minor-branches of the dorsal penile nerves is associated with erectile function in the bilateral cavernous injury model of rats. Sci Rep 2018;8:929. [Crossref] [PubMed]
- Sipski M, Alexander C, Gomez-Marin O, et al. The effects of spinal cord injury on psychogenic sexual arousal in males. J Urol 2007;177:247-51. [Crossref] [PubMed]
- Alexander MS, Marson L. The neurologic control of arousal and orgasm with specific attention to spinal cord lesions: Integrating preclinical and clinical sciences. Auton Neurosci 2018;209:90-9. [Crossref] [PubMed]
- Giuliano F, Rampin O. Central neural regulation of penile erection. Neurosci Biobehav Rev 2000;24:517-33. [Crossref] [PubMed]
- Giuliano F. Neurophysiology of erection and ejaculation. J Sex Med 2011;8 Suppl 4:310-5. [Crossref] [PubMed]
- Giuliano F, Rampin O. Neural control of erection. Physiol Behav 2004;83:189-201. [Crossref] [PubMed]
- Janssen E. Sexual arousal in men: a review and conceptual analysis. Horm Behav 2011;59:708-16. [Crossref] [PubMed]
- Alwaal A, Breyer BN, Lue TF. Normal male sexual function: emphasis on orgasm and ejaculation. Fertil Steril 2015;104:1051-60. [Crossref] [PubMed]
- Clement P, Giuliano F. Anatomy and physiology of genital organs - men. Handb Clin Neurol 2015;130:19-37. [Crossref] [PubMed]
- Gray M, Zillioux J, Khourdaji I, et al. Contemporary management of ejaculatory dysfunction. Transl Androl Urol 2018;7:686-702. [Crossref] [PubMed]
- Coolen LM. Neural control of ejaculation. J Comp Neurol 2005;493:39-45. [Crossref] [PubMed]
- Althof SE, McMahon CG. Contemporary Management of Disorders of Male Orgasm and Ejaculation. Urology 2016;93:9-21. [Crossref] [PubMed]
- Facchinetti P, Giuliano F, Laurin M, et al. Direct brain projections onto the spinal generator of ejaculation in the rat. Neuroscience 2014;272:207-16. [Crossref] [PubMed]
- Chéhensse C, Facchinetti P, Bahrami S, et al. Human spinal ejaculation generator. Ann Neurol 2017;81:35-45. [Crossref] [PubMed]
- Baron R, Janig W. Afferent and sympathetic neurons projecting into lumbar visceral nerves of the male rat. J Comp Neurol 1991;314:429-36. [Crossref] [PubMed]
- Clement P, Giuliano F. Physiology and Pharmacology of Ejaculation. Basic Clin Pharmacol Toxicol 2016;119 Suppl 3:18-25. [Crossref] [PubMed]
- Wieder JA, Brackett NL, Lynne CM, et al. Anesthetic block of the dorsal penile nerve inhibits vibratory-induced ejaculation in men with spinal cord injuries. Urology 2000;55:915-7. [Crossref] [PubMed]
- Larsson K, Sodersten P. Mating in male rats after section of the dorsal penile nerve. Physiol Behav 1973;10:567-71. [Crossref] [PubMed]
- Aronson LR, Cooper ML. Seasonal variation in mating behavior in cats after desensitization of glans penis. Science 1966;152:226-30. [Crossref] [PubMed]
- Aronson LR, Cooper ML. Mating behaviour in sexually inexperienced cats after desensitization of the glans penis. Anim Behav 1969;17:208-12. [Crossref] [PubMed]
- Masters WH, Johnson VE. Human Sexual Response. Little Brown, Boston, 1966.
- Kaplan H. The new sex therapy. Brunner Mazel, New York, 1974.
- Giraldi A, Kristensen E, Sand M. Endorsement of models describing sexual response of men and women with a sexual partner: an online survey in a population sample of Danish adults ages 20-65 years. J Sex Med 2015;12:116-28. [Crossref] [PubMed]
- Krassioukov A, Elliott S. Neural Control and Physiology of Sexual Function: Effect of Spinal Cord Injury. Top Spinal Cord Inj Rehabil 2017;23:1-10. [Crossref] [PubMed]
- Kennedy S, Over R. Psychophysiological assessment of male sexual arousal following spinal cord injury. Arch Sex Behav 1990;19:15-27. [Crossref] [PubMed]
- Nummenmaa L, Suvilehto JT, Glerean E, et al. Topography of Human Erogenous Zones. Arch Sex Behav 2016;45:1207-16. [Crossref] [PubMed]
- Alexander CJ, Sipski ML, Findley TW. Sexual activities, desire, and satisfaction in males pre- and post-spinal cord injury. Arch Sex Behav 1993;22:217-28. [Crossref] [PubMed]
- Gałecki P, Depko A, Jedrzejewska S, et al. Human orgasm from the physiological perspective--part I. Pol Merkur Lekarski 2012;33:48-50. [PubMed]
- Alexander M, Rosen RC. Spinal cord injuries and orgasm: a review. J Sex Marital Ther 2008;34:308-24. [Crossref] [PubMed]
- Rowland D, McMahon CG, Abdo C, et al. Disorders of orgasm and ejaculation in men. J Sex Med 2010;7:1668-86. [Crossref] [PubMed]
- Jenkins LC, Mulhall JP. Delayed orgasm and anorgasmia. Fertil Steril 2015;104:1082-8. [Crossref] [PubMed]
- Soler JM, Previnaire JG, Plante P, et al. Midodrine improves orgasm in spinal cord-injured men: the effects of autonomic stimulation. J Sex Med 2008;5:2935-41. [Crossref] [PubMed]
- Sipski M, Alexander CJ, Gomez-Marin O. Effects of level and degree of spinal cord injury on male orgasm. Spinal Cord 2006;44:798-804. [Crossref] [PubMed]
- Courtois F, Charvier K, Leriche A, et al. Perceived physiological and orgasmic sensations at ejaculation in spinal cord injured men. J Sex Med 2008;5:2419-30. [Crossref] [PubMed]
- National Spinal Cord Injury Statistical Center. 2018 Annual Report – Complete Public Version. 2018. Available online: https://www.sci-info-pages.com/wp-content/media/NSCISC-2018-Annual-Report.pdf
- Chiu WT, Lin HC, Lam C, et al. Review paper: epidemiology of traumatic spinal cord injury: comparisons between developed and developing countries. Asia Pac J Public Health 2010;22:9-18. [Crossref] [PubMed]
- Zhou Y, Wang XB, Kan SL, et al. Traumatic spinal cord injury in Tianjin, China: a single-center report of 354 cases. Spinal Cord 2016;54:670-4. [Crossref] [PubMed]
- Sabre L, Remmer S, Adams A, et al. Impact of fatal cases on the epidemiology of traumatic spinal cord injury in Estonia. Eur J Neurol 2015;22:768-72. [Crossref] [PubMed]
- Bárbara-Bataller E, Mendez-Suarez JL, Aleman-Sanchez C, et al. Change in the profile of traumatic spinal cord injury over 15 years in Spain. Scand J Trauma Resusc Emerg Med 2018;26:27. [Crossref] [PubMed]
- Anderson KD, Borisoff JF, Johnson RD, et al. The impact of spinal cord injury on sexual function: concerns of the general population. Spinal Cord 2007;45:328-37. [Crossref] [PubMed]
- Alexander M, Courtois F, Elliott S, et al. Improving Sexual Satisfaction in Persons with Spinal Cord Injuries: Collective Wisdom. Top Spinal Cord Inj Rehabil 2017;23:57-70. [Crossref] [PubMed]
- Borisoff JF, Elliott SL, Hocaloski S, et al. The development of a sensory substitution system for the sexual rehabilitation of men with chronic spinal cord injury. J Sex Med 2010;7:3647-58. [Crossref] [PubMed]
- Schober JM, Meyer-Bahlburg HF, Dolezal C. Self-ratings of genital anatomy, sexual sensitivity and function in men using the 'Self-Assessment of Genital Anatomy and Sexual Function, Male' questionnaire. BJU Int 2009;103:1096-103. [Crossref] [PubMed]
- Connell KM, Coates R, Wood FM. Sexuality following trauma injury: A literature review. Burns Trauma 2014;2:61-70. [Crossref] [PubMed]
- Masood S, Patel HR, Himpson RC, et al. Penile sensitivity and sexual satisfaction after circumcision: are we informing men correctly? Urol Int 2005;75:62-6. [Crossref] [PubMed]
- Bronselaer GA, Schober JM, Meyer-Bahlburg HF, et al. Male circumcision decreases penile sensitivity as measured in a large cohort. BJU Int 2013;111:820-7. [Crossref] [PubMed]
- Corona G, Rastrelli G, Morgentaler A, et al. Meta-analysis of Results of Testosterone Therapy on Sexual Function Based on International Index of Erectile Function Scores. Eur Urol 2017;72:1000-11. [Crossref] [PubMed]
- Rosen RC, Cappelleri JC, Gendrano NR. The International Index of Erectile Function (IIEF:a state-of-the-science review. Int J Impot Res 2002;14:226-44. [Crossref] [PubMed]
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF:a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. [Crossref] [PubMed]
- Krieger JN, Mehta SD, Bailey RC, et al. Adult male circumcision: effects on sexual function and sexual satisfaction in Kisumu, Kenya. J Sex Med 2008;5:2610-22. [Crossref] [PubMed]
- Nordstrom MP, Westercamp N, Jaoko W, et al. Medical Male Circumcision Is Associated With Improvements in Pain During Intercourse and Sexual Satisfaction in Kenya. J Sex Med 2017;14:601-12. [Crossref] [PubMed]
- Mücke M, Cuhls H, Radbruch L, et al. Quantitative sensory testing (QST). English version. Schmerz 2016. [Crossref] [PubMed]
- Siao P, Cros DP. Quantitative sensory testing. Phys Med Rehabil Clin N Am 2003;14:261-86. [Crossref] [PubMed]
- Bleustein CB, Eckholdt H, Arezzo JC, et al. Quantitative somatosensory testing of the penis: optimizing the clinical neurological examination. J Urol 2003;169:2266-9. [Crossref] [PubMed]
- Yarnitsky D, Sprecher E, Vardi Y. Penile thermal sensation. J Urol 1996;156:391-3. [Crossref] [PubMed]
- Lefaucheur JP, Yiou R, Colombel M, et al. Relationship between penile thermal sensory threshold measurement and electrophysiologic tests to assess neurogenic impotence. Urology 2001;57:306-9. [Crossref] [PubMed]
- Lefaucheur JP, Yiou R, Salomon L, et al. Assessment of penile small nerve fiber damage after transurethral resection of the prostate by measurement of penile thermal sensation. J Urol 2000;164:1416-9. [Crossref] [PubMed]
- Giuliano F, Rowland DL. Standard operating procedures for neurophysiologic assessment of male sexual dysfunction. J Sex Med 2013;10:1205-11. [Crossref] [PubMed]
- Tepper MS. Sexual education in spinal cord injury rehabilitation: Current trends and recommendations. Sex Disabil 1992;10:15-31. [Crossref]
- Korse NS, Nicolai MP, Both S, et al. Discussing sexual health in spinal care. Eur Spine J 2016;25:766-73. [Crossref] [PubMed]
- Onifer SM, Smith GM, Fouad K. Plasticity after spinal cord injury: relevance to recovery and approaches to facilitate it. Neurotherapeutics 2011;8:283-93. [Crossref] [PubMed]
- Dietz V, Fouad K. Restoration of sensorimotor functions after spinal cord injury. Brain 2014;137:654-67. [Crossref] [PubMed]
- Courtois F, Charvier K, Vezina JG, et al. Assessing and conceptualizing orgasm after a spinal cord injury. BJU Int 2011;108:1624-33. [Crossref] [PubMed]
- Danilov Y, Tyler M. Brainport: an alternative input to the brain. J Integr Neurosci 2005;4:537-50. [Crossref] [PubMed]
- Mah K, Binik YM. Do all orgasms feel alike? Evaluating a two-dimensional model of the orgasm experience across gender and sexual context. J Sex Res 2002;39:104-13. [Crossref] [PubMed]
- Courtois FJ, Charvier KF, Leriche A, et al. Blood pressure changes during sexual stimulation, ejaculation and midodrine treatment in men with spinal cord injury. BJU Int 2008;101:331-7. [Crossref] [PubMed]
- Soler JM, Previnaire JG, Plante P, et al. Midodrine improves ejaculation in spinal cord injured men. J Urol 2007;178:2082-6. [Crossref] [PubMed]
- Alexander M, Marson L. Orgasm and SCI: what do we know? Spinal Cord 2018;56:538-47. [Crossref] [PubMed]
- Alaca R, Goktepe AS, Yildiz N, et al. Effect of penile vibratory stimulation on spasticity in men with spinal cord injury. Am J Phys Med Rehabil 2005;84:875-9. [Crossref] [PubMed]
- Calabrò RS, Naro A, Pullia M, et al. Improving Sexual Function by Using Focal Vibrations in Men with Spinal Cord Injury: Encouraging Findings from a Feasibility Study. J Clin Med 2019;8:658. [Crossref] [PubMed]
- Guess MK, Connell KA, Chudnoff S, et al. The Effects of a Genital Vibratory Stimulation Device on Sexual Function and Genital Sensation. Female Pelvic Med Reconstr Surg 2017;23:256-62. [Crossref] [PubMed]
- Selvaggi G, Elander A. Penile reconstruction/formation. Curr Opin Urol 2008;18:589-97. [Crossref] [PubMed]
- Overgoor ML, Kon M, Cohen-Kettenis PT, et al. Neurological bypass for sensory innervation of the penis in patients with spina bifida. J Urol 2006;176:1086-90; discussion 1090. [Crossref] [PubMed]
- Overgoor ML, de Jong TP, Cohen-Kettenis PT, et al. Increased sexual health after restored genital sensation in male patients with spina bifida or a spinal cord injury: the TOMAX procedure. J Urol 2013;189:626-32. [Crossref] [PubMed]
- Jacobs MA, Avellino AM, Shurtleff D, et al. Reinnervating the penis in spina bifida patients in the United States: ilioinguinal-to-dorsal-penile neurorrhaphy in two cases. J Sex Med 2013;10:2593-7. [Crossref] [PubMed]
- Overgoor ML, Braakhekke JP, Kon M, et al. Restoring penis sensation in patients with low spinal cord lesions: the role of the remaining function of the dorsal nerve in a unilateral or bilateral TOMAX procedure. Neurourol Urodyn 2015;34:343-8. [Crossref] [PubMed]
- Overgoor ML, de Jong TP, Kon M. Restoring tactile and erogenous penile sensation in low-spinal-lesion patients: procedural and technical aspects following 43 TOMAX nerve transfer procedures. Plast Reconstr Surg 2014;134:294e-301e. [Crossref] [PubMed]
- Kortekaas R, Nanetti L, Overgoor ML, et al. Central Somatosensory Networks Respond to a De Novo Innervated Penis: A Proof of Concept in Three Spina Bifida Patients. J Sex Med 2015;12:1865-77. [Crossref] [PubMed]


