
Survival benefit of neoadjuvant chemotherapy in pathologic T2N0 or lower urothelial carcinoma patients: evidence to support the use of neoadjuvant chemotherapy
Introduction
Bladder cancer is one of the most common cancers of the urogenital system (1). About 25% of patients diagnosed with bladder cancer for the first time are diagnosed with muscle invasive bladder cancer (MIBC). Of patients diagnosed with non-muscle invasive bladder cancer (NMIBC), about 20–30% progress to MIBC (2,3). MIBC is an aggressive disease with high risk of metastasis and poor prognosis (4). The standard treatment for MIBC is radical cystectomy with pelvic lymph node dissection (PLND) (5,6). Despite radical cystectomy, 10-year cancer-specific survival (CSS) rate after radical cystectomy for MIBC patients is only about 67% (7). In the case of node positive patients, the 10-year CSS rate is about 17% regardless of the stage of the primary tumor (7). Many patients tend to relapse after surgery probably due to micro-metastasis at or before radical cystectomy (8,9). To improve these results, neoadjuvant chemotherapy (NAC) has been used since the 1980s (10). It has been shown that cisplatin-based NAC can increase overall survival (OS) by 5–8% (11). NAC is recommended for patients with clinical T2, T3, or T4a N0M0 in several guidelines (11,12). In the Southwest Oncology Group (SWOG) Intergroup study, radical cystectomy after NAC improved complete pathologic response by 23% and median OS by 31 months compared with cystectomy alone (5). In a randomized control study for cT2–4N0 patients, NAC and radical cystectomy improved the OS by 16% and complete response by 21.2% compared to radical cystectomy alone (6). However, the role of NAC in patients under T2 is controversial. The purpose of this study was to compare the clinical course differences in patients with pathologic T2N0M0 or lower after NAC and those with pathologic T2N0M0 or less who did not undergo NAC.
Methods
The Institutional Review Board of Seoul National University Hospital approved this retrospective study and waived the requirement to obtain informed consent from patients. All research and related protocols used in this study complied with the principles of the Declaration of Helsinki. We retrospectively reviewed medical records of patients who underwent radical cystectomy with bladder cancer from January 1991 to December 2016. Patients with T2 or less N0M0 were included in this study. All patients underwent radical cystectomy and standard PLND or extended PLND. Only pathologically confirmed patients with urothelial carcinoma (UC) were included. Patients with previous chemotherapy history except NAC were excluded, Patients with previous other cancer treatment or with metastasis were also excluded. Patients were divided into two groups: those who received NAC and those who did not. TNM stage and tumor grade were classified according to the 2010 American Joint Committee on Cancer classification and the 2004 World Health Organization/International Society of Urologic Pathology consensus classifications. We collected information such as patient age, body mass index (BMI), sex, American Society of Anesthesiologists (ASA) physical status, clinical TNM stage, pathological TNM stage, margin positive, number of lymph node (LN) removed, whether to perform NAC, number of NAC cycles, and various oncologic results including recurrence and mortality. In the absence of recurrence or metastasis, most patients underwent a routine test every 3 months for 3 years after surgery and every 6 months for 4 to 5 years. Thereafter, routine test was conducted once a year. Routine test included laboratory test, urine test, urine cytology, and bladder cystoscopy. Patients with neo-bladder included a post-voided residual urine check using ultrasonic bladder scan. Radiological test such as chest computed tomography (CT), abdomen-pelvis CT, and bone scan were performed once a year. NAC used cisplatin and gemcitabine regimen or methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) regimen. The number of NAC cycles was 2 or 3. Continuous variables are expressed as means and standard deviations (SDs) or median values and intermediate ranges (IQRs) while categorical variables are expressed by the frequency of events (%). Primary endpoint was OS. Secondary endpoint was recurrence-free survival (RFS). All survival results were analyzed by Kaplan survival analysis and log-rank test. Cox regression analysis was used to analyze factors associated with various oncologic outcomes. All statistical tests were performed using IBM SPSS Statistics, version 22.0 (IBM, Armonk, NY, USA) and STATA version 14 (StataCorp LP, College Station, TX, USA). A P value of less than 0.05 was considered to indicate statistical significance.
Results
Baseline patient characteristics
A total of 857 patients underwent radical cystectomy with bladder cancer from January 1991 to December 2016 in a tertiary institution. All patients with pathologic findings of UC and less than pT2N0M0 were included in this study. The total number of patients included in the study was 526. Patients were divided into three groups according to the presence or absence of NAC: (I) non-NAC, those who did not receive NAC; (II) partial NAC, those who received less 3 cycles of NAC; and (III) complete NAC, those who received 3 cycles of NAC.
The partial NAC group had 40 patients and the complete NAC group had 35 patients. The non-NAC group had 451 patients. Table 1 shows characteristics of these patients. Clinical T stage was cT2 in most patients of the non-NAC group. It was cT3 in more than 60% of patients in the partial NAC group and 65.7% in the complete NAC group (P=0.003). It could be seen that NAC was applied to the group with higher tumor burden. Complete pathologic response was achieved in 21.1% of the non-NAC group, 37.5% of the partial NAC group, and 28.6% of the complete NAC group. Complete pathologic response rate was higher in the NAC group than that in the non-NAC group, although the difference was not statistically significant (Table 1).
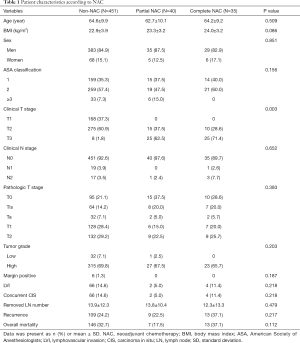
Full table
Survival outcomes according to NAC
There was no difference in the survival results between the NAC group and non-NAC group (Figure 1). Comparison of survival outcome between partial NAC and complete NAC and non-NAC group, the non-NAC group had better (P=0.039) prognosis than the complete NAC group in OS. In CSS, there was no significant difference in prognosis between complete NAC and non-NAC groups. There was no significant difference in OS or CSS between partial NAC and non-NAC groups or between partial NAC and complete NAC groups. In RFS, the non-NAC group had better (P=0.041) prognosis than the complete NAC group. There was no significant difference in prognosis between partial NAC and non-NAC groups or between partial NAC and complete NAC groups (Figure 2).


Multivariate Cox proportional hazards analyses of NAC on survival outcomes
In multivariate Cox proportional hazards analysis, age, pathologic T stage, lymphovascular invasion (LVI), number of removed LNs, and NAC showed significant associations with OS. Age, pathologic T stage, LVI, concurrent carcinoma in situ (CIS), number of removed LNs, and NAC were significant factors associated with CSS. Pathologic T stage, concurrent CIS, and number of removed LNs were significant factors associated with RFS (Table 2).
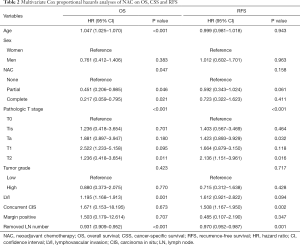
Full table
Univariate and multivariate logistic regression models of predictive factors of pathologic T2N0 or lower after NAC
The difference between NAC cycle and regimen was not related to pT2N0 or lower after NAC. In univariate and multivariate logistic regression analysis, clinical T stage, LVI showed significant associations with pathologic LVI was poor predict factor of pT2N0 or lower after NAC. However, concurrent CIS was a good predict factor (Table 3).
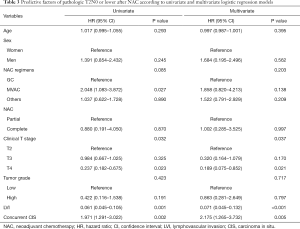
Full table
Discussion
The purpose of NAC before radical cystectomy is to reduce preoperative microinvasion and recurrence (distant recurrence and locoregional recurrence) (13). In several guidelines, NAC is recommended for patients with T2–T4a N0M0 (2,12) because microinvasion is thought to be higher in T3 or T4 with more tumor burden than T2 with smaller tumor burden (14). In the present study, the percentage of high stage above cT3 was higher in the partial and complete NAC groups than that in the non-NAC group. NAC has several advantages. One of them is that NAC affects pathologic status, enabling pT0 or pN0 postoperatively and enabling negative surgical margin (5,13). In clinical T stage, cT2 and cT3 in NAC-administered group was higher than in the non-NAC group. However, in pathologic T stage after surgery, the ratio of T0 in NAC-administered group was similar to or higher than that in the non-NAC group (Table 1).
In OS, the non-NAC group had better (P=0.039) prognosis than the complete NAC group. The complete NAC group had similar or slightly worse prognosis than the non-NAC group. In RFS, the complete NAC group had worse (P=0.041) prognosis than the non-NAC group. In this study, NAC had no significant gain in RFS. However, in OS, there was a gain through NAC. In clinical T stage, the NAC group had higher tumor burden than the non-NAC group. NAC was performed for patients with relatively high tumor burden. Although NAC groups had relatively higher tumor burden, their OS was similar to or slightly worse than the non-NAC group. Proportions of pathologic T0 and negative resection margin were also higher in NAC groups. Results showed that the complete NAC group had worse OS than the non-NAC group. Thus, NAC seemed to be ineffective. However, in the preoperative clinical stage, complete NAC group had higher burden in pathologic status, yet there was no significant difference in OS. This indicated that NAC was effective even for groups with stage below T2 (Figure 1).
In a study of MD Anderson’s clinical risk stratification, patients with pT0–4N0/+ were included. And patients were divided into high- and low-risk groups according to hydroureteronephrosis, LVI, micropapillary or neuroendocrine features, and cT3b–4a stage. The 5-year survival rate for low-risk patients with RC without NAC was 68% (15). In other studies, the 5-year survival rate of low-risk patients who underwent RC without NAC ranged from 77% to 100% (9,16). Based on these studies, the authors recommend ACH after RC rather than NAC in low-risk groups (9,15,16).
However, in Mayo Clinic’s study of 1,931 patients, patients with pT0–4N0/+ were included. And patients were divided into high and low-risk groups according to hydronephrosis, LVI, histologic variant, and cT3–4 stage. NAC patients had better prognosis than non-NAC patients. Five-year CSS rate was 68% for NAC patients and 50% for non-NAC patients (P=0.001) (17). The OS of non-NAC patients was 67.3%, which was lower than that (73.3%) of NAC patients. The proportion of pT0 [OR: 3.05, 95% confidence interval (CI): 1.89–4.93, P<0.001] after RC was significantly higher in the NAC group. The proportion of pT2 or lower (OR: 2.53, 95% CI: 1.64–3.89, P<0.001) after RC was also significantly higher in the NAC group (17). In our study, the CSS rate was 75.4% in the NAC group and 72.4% in the non-NAC group. The NAC group had higher pT0 ratio than the non-NAC group (33.3% vs. 21.1%). At baseline, 66.6% of NAC patients had cT3 or higher stage. However, there was no pT3 or higher patient after NAC and RC. Thus, 66.6% of NAC patients were down-staged to less than T2 after NAC and RC.
Our research has several limitations. First, it was a retrospective study rather than a randomized study. Second, the significant difference may be insufficient for T0 downstaging with various oncological outcomes and survival rate because it is a prime number of NAC group compared to non-NAC group. Third, those who had missing data were included in this study. Due to the nature of this retrospective study, some patients did not receive NAC for preoperative low renal function or had poor performance status. This might lead to some bias for the result of survival outcome. Fourth, we considered NAC without considering previous TURB or intravesical treatment. Fifth, there may be diversity or limitations of potential interoperability with data from a single center.
Conclusions
NAC is eligible for low-risk patients with stage below pT2N0. NAC showed favorable results in T0 down-staging and below pT2 downstaging. A patient with a high tumor burden prior to NAC treatment showed similar prognosis to patients with a low tumor burden after NAC. This will serve as a basis for appropriate treatment selection for MIBC patients.
Acknowledgments
Funding: This work was supported by a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1F1A1050507).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-19-705). JHK serves as an unpaid editorial board member of Translational Andrology and Urology from Jan 2020 to Dec 2021. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of Seoul National University Hospital (H-1811-108-987) and waived the requirement to obtain informed consent from patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Babjuk M, Böhle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Guillaume L, Guy L. Epidemiology of and risk factors for bladder cancer and for urothelial tumors. Rev Prat 2014;64:1372-4, 1378-80. [PubMed]
- Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71:462-75. [Crossref] [PubMed]
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859-66. [Crossref] [PubMed]
- International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011;29:2171-7. [Crossref] [PubMed]
- Stein JP, Skinner DG. Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure. World J Urol 2006;24:296-304. [Crossref] [PubMed]
- Hautmann RE, Gschwend JE, de Petriconi RC, et al. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J Urol 2006;176:486-92; discussion 491-2. [Crossref] [PubMed]
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001;19:666-75. [Crossref] [PubMed]
- David KA, Milowsky MI, Ritchey J, et al. Low incidence of perioperative chemotherapy for stage III bladder cancer 1998 to 2003: a report from the National Cancer Data Base. J Urol 2007;178:451-4. [Crossref] [PubMed]
- Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014;65:778-92. [Crossref] [PubMed]
- Flaig TW, Spiess PE, Agarwal N, et al. NCCN guidelines insights: bladder cancer, version 5.2018. J Natl Compr Canc Netw 2018;16:1041-53. [Crossref] [PubMed]
- Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 2015;67:241-9. [Crossref] [PubMed]
- Sherif A, Holmberg L, Rintala E, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol 2004;45:297-303. [Crossref] [PubMed]
- Culp SH, Dickstein RJ, Grossman HB, et al. Refining patient selection for neoadjuvant chemotherapy before radical cystectomy. J Urol 2014;191:40-7. [Crossref] [PubMed]
- Moschini M, Soria F, Klatte T, et al. Validation of preoperative risk grouping of the selection of patients most likely to benefit from neoadjuvant chemotherapy before radical cystectomy. Clin Genitourin Cancer 2017;15:e267-73. [Crossref] [PubMed]
- Lyon TD, Frank I, Sharma V, et al. A risk-stratified approach to neoadjuvant chemotherapy in muscle-invasive bladder cancer: implications for patients classified with low-risk disease. World J Urol 2019;37:1605-13. [Crossref] [PubMed]


