Adjunctive maneuvers to treat urethral stricture: a review of the world literature
Evidence synthesis for this systematic review
Non-cancerous induced urethral stricture (US)
US disease is one of the oldest known urologic diseases, and continues to be a common and challenging urologic condition. It is defined by the constriction of the urethral lumen due to the fibrosis or scarring of the urethral epithelium and its surrounding tissues. Nowadays, US is increasingly encountered by urologists worldwide and its prevalence can occur at a rate as high as 0.6% in men, resulting in more than 5,000 inpatient visits yearly (1). It can be caused by a wide spectrum of etiologies ranging from pelvic traumas, repetitive instrumentation, traumatic catheterization (CIC), aggressive transurethral resection, localized or diffuse inflammation (balanitis xerotica obliterans), infection (gonorrhea), adult hypospadias, congenital or idiopathic etiologies (1).
Historically, urethral dilation is considered the oldest and simplest form of management of US, which can be performed with a number of different urologic procedures or devices. In 1974, Saches introduced direst vision internal urethrotomy to treat US by either incision or ablation, which has been considered standard therapy for the anterior US. Despite its invasiveness, an open urethral reconstruction is the most successful management option for management of US. These therapeutic options can be carefully selected according to the etiology, site and length of the US as well as patient medical conditions, functional and performance status. A number of clinical reports have examined the role of incision urethrotomy in the management of US over the past 10-20 years. The mean follow-up on these case series was commonly <12 months (range, 3-20 months) with a variety of end-points, stricture locations, and success rates (46-84%) being reported (2,3). Adding to this, a considerable proportion of patients will invariably develop recurrences of the strictures in 6-12 months following their initial treatment and will eventually require surgical repair of higher complexity and morbidity (4).
There is a paucity of published literature regarding adjunctive treatment in the management of non-cancerous induced US. In 2007, a prospective study by Mazdak et al. evaluated 20 patients with anterior USs who underwent submucosal injection of Mitomycin-C (MMC) at site of internal urethrotomy, compared to the matched control group of internal urethrotomy alone. This study shows that US recurred in two patients (10%) in the MMC-treated group, compared to ten patients (50%) in the control group (P=0.006). Mazdak et al. concluded that MMC injection can significantly reduce US recurrence after internal urethrotomy and advocated further investigations to confirm its efficacy and safety (5). Despite this, Mazdak’s study pioneered the utilization of MMC as an adjunctive maneuver in the treatment algorithm of US. There have been no advancements of this innovative technique or validation studies to examine its efficacy, safety and reproducibility in the clinical setting, and the only therapeutic options for US were primarily endoscopic or surgical. Indeed, it is noteworthy to acknowledge that corpora spongiosum is a highly vascular structure that can increase the local diffusion and absorption of the adjunctive agents injected into the US area. Hence, this potential phenomenon can significantly reduce the local effect of these injectable adjunctive agents in the management of US.
Cancerous induced US
With the increased number of patients treated with radical prostatectomy (RP) or radiotherapy for localized prostate cancer, the development of its complications such as US and/or bladder neck contractures (BNC) after these treatment modalities, are expected to rise over the next decade worldwide (6). Traditionally and in contemporary urologic practice these types of complications are usually treated by urologists in both academic and non-academic settings (7). With the recent advent of the surgery and refinements in surgical techniques, the prevalence of BNC following RP has continued to decline over the last two decades. Moreover, the overall prevalence of BNC was lower in the contemporary cohort that underwent robotic assisted laparoscopic prostatectomy (RALP) in comparison to the historical open RP patient population, owing to the advanced robotic surgical techniques such as enhanced magnification and a running bladder anastomosis (7). Despite this, a cohort of patients continued to develop vesicourethral anastomotic stricture and/or BNC that required further specialist referral and subsequent treatment (7-9).
Although numerous patients undergoing initial treatment of BNC can achieve successful early result with satisfactory outcomes, a significant group of patients may develop recurrent, refractory or recalcitrant US and/or BNC (4,10-18). Hence, this group of patients may pose clinical dilemmas and their definitive management can be challenging to reconstructive urologists. Additionally, it is now well known that the type of primary treatment selected for patients with prostate cancer, weather unimodal or bimodal, has detrimental effects on the development of subsequent US and/or BNC. For example, in patients undergoing RP, US and/or BNC often develop because of technical factors related to vesicourethral anastomosis, which can be prevented during RP through a tension-free watertight anastomosis with mucosal eversion (19). In a large retrospective study by Gonzalgo et al., the overall prevalence of BNC decreased after robotic RP (0-3%) (20) as compared to historical data after open RP (0.5-32%) (21). Likewise, US and/or BNC can also develop following pelvic radiotherapy, however, by different mechanistic effect as the microvascular effects and progressive obliterative endarteritis usually lead to tissue necorsis of the urethra and/or bladder neck (22). Although BNC can also manifest following conventional transurethral resection of the prostate (TURP), a smaller rate of such complication has been reported with newer technologies such as laser prostatectomy (3-5%) (23).
Specific risks factors associated with the development of US and/or BNC have recently been examined in various studies in an attempt to prevent and better treat these complications (24,25). Nevertheless, the management of refractory or recalcitrant disease remains challenging and non-standardized, due in part to a paucity of long-term clinical data. Traditionally, most contractures are often managed successfully with conservative measures such as serial dilations or transurethral release/incisions of the bladder neck, with overall success rates ranging from 50-87% (4,24-29). However, when conservative measures fail, patients with refractory or recalcitrant BNC will likely require additional treatments modalities with increasing procedural complexity. Over the last decade, there have been novel experimental and clinical reports that utilized for the first time injectable agents with anti-proliferative, anti-scar properties (steroids and MMC) as adjunct to transurethral incision of BNC. Table 1 lists various therapeutic modalities utilized for the management of refectory or recalcitrant US and/or BNC.

Full table
Risk factors for developing US and/or BNC
Numerous risk factors are associated with the development of US and/or BNC following prostate surgery, including surgical technique, surgeon experience, post operative complications, and patient co-morbidities. In a retrospective analysis by Borboroglu et al., a total of 467 patients were studied following RP and the development of BNC was observed in 52 patients (19). This study incorporated a multivariable analysis to assess clinical predictors of BNC, and determined that intra-operative blood loss, increased operative time, positive smoking history, diabetes mellitus and coronary artery disease were significant predictors associated with the development of BNC. Moreover, a systematic analysis of the Cancer of the prostate Strategic Urologic Research Endeavor (CaPSURE) database, determining the incidence of US and/or BNC following primary treatment for clinically localized prostate cancer, found that patient age, basic metabolic index (BMI) and primary type of treatment were all prognostic indicators for the development of clinically significant strictures requiring treatment and higher rate of BNC with unfavorable consequences after multimodal radiotherapy, such as brachytherapy plus external beam radiotherapy or salvage prostatectomy (34). This study also highlights that strictures after RP occurred typically within the first 24 months, whereas its onset was delayed after pelvic radiotherapy likely because of progressive radiation-induced fibrosis and tissue necrosis (22).
Methods
A detailed, comprehensive literature review was performed to identify all published peer-reviewed articles describing injectable agents and US and BNC in the urological literature over a 13-year period (2000 to 2013). The search was conducted using the MEDLINE® database, the Cochrane Library® Central Search, and the Web of Science. Initial search terms were injectable agents and US and BNC. Search results were screened for appropriate studies with particular emphasis placed on clinical and experimental studies as well as review articles. Articles referenced were screened to maximize review and inclusion of pertinent data. While English language text was not a specific search parameter, only English language publications were considered. All relevant studies collected were carefully examined to extract relevant data pertained to injectable agents used in US and/or BNC.
Treatment modalities of US and/or BNC
The Urolume® (AMS, Minnesota, USA) was first introduced by Milroy in 1988 as a novel minimal invasive stent to treat USs (36). Although initial studies regarding the use of the Urolume stents were promising, numerous problems were encountered with urethral stenting, including stent migration, obstruction secondary to tissue in-growth, hematuria, stent encrustation, and the need for repeat surgery (37,38). A prospective study by De Vocht et al. evaluated 15 patient satisfaction surveys 20 years after placement of the Urolume stents and found that only two patients were satisfied with their Urolume stents. Moreover, four patients had their Urolume stents removed (two for intractable pain and two for stent obstruction). Other Urolume complications in De Vocht’s study were urinary incontinence (50%), erectile discomfort and ejaculation disorders (37). Additional study by Hussain et al. evaluated the utilization of Urolume stents in a total of 38 patients who had undergone primary radiation or surgery for the treatment of localized prostate cancer (38). This study showed that 58% had complications, with reoperation rate 45% for obstructing stent hyperplasia (32%), stent obstruction or stricture (25%) and stent encrustation or calcification (17%). Additionally patients experienced post micturition dribbling (32%) and recurrent urinary tract infections (27%). The Urolume stent is no longer commercially available.
More recently, the ability of the Memokath™ (044TW) stent to maintain urethral patency after dilation or internal urethrotomy for recurrent US has been systematically evaluated in a large scale randomized, multi-institutional study (39). The Memokath™ stent is a removable, densely coiled thermoexpandable stent manufactured from nitinol, a biocompatible alloy of nickel and titanium. The stent has a 24 Fr outside diameter and is preloaded on a disposable delivery device. When correctly positioned, the stent is anchored by warm water (55 °C) instillation, which expands the proximal end of the stent from 24 to 42 Fr. The stent was provided in lengths of 30 to 70 mm in 10 mm increments. The Memokath has been previously used to treat prostatic obstruction and detrusor dyssynergy in the posterior urethra and was recently evaluated for use in the anterior urethra. In Jordan’s study, 92 patients were randomized to dilation/incision followed by temporary foley catheter drainage (n=29) versus followed by Memokath stenting (n=63) for recurrent bulbar USs. The primary endpoints were urethral patency, defined as the ability to pass a 16 Fr flexible cystoscopy and reflected in significantly improved uroflowmetry and symptom scores. Urethral patency was 3.5 times longer in the Memkath stented group than the non-stented group (median 292 versus 84 days, P<0.001) and all stents were removed successfully. Durability effect on the US was not assessed. This study concluded that patients with recurrent bulbar USs treated with dilation or urethrotomy followed by a Memokath™ stent maintained urethral patency significantly longer than those treated with dilation or urethrotomy alone (39). Side effects of the Memokath™ stent included urinary tract infections, hematuria, and penile pain. Stent migration occurred in 22% of patients. The ease of placement and removal of the Memokath™ stent may prove useful for recurrent bulbar US in medically unfit patients or patients unable to undergo formal urethral reconstruction. Further clinical study is warranted to validate and reproduce the Memokath™ stent safety and efficacy.
Intermittent or gradual dilation of the bladder neck has been used to treat contractures and prevent recurrent disease. In a large study from the United Kingdom of 510 patients who underwent open RP over a 9-year-period, the prevalence of BNC was reported at 9.4%, which was managed successfully with urethral dilation or clean intermittent self-CIC (29). Although CIC provides a non-surgical approach to BNC management, significant patient tolerance and compliance are necessary for successful results. Adding to this, some patients experience complications of CIC such as recurrent urinary tracts infections, hematuria, false urethral passages and USs.
A more widely practiced approach is cold knife endoscopic incision of BNC. Yurkanin et al. evaluated efficacy of cold knife urethrotomy in first-time diagnosis of BNC following RP (40). The authors reported that cold knife urethrotomy provides safe and effective response for the initial treatment of patients with anastomotic stricture after RP. However, there is limited long-term data for patients undergoing repeat endoscopic procedure for recurrent, refractory or recalcitrant BNC. A recent study by Ramirez et al. from University of Texas Southwestern reported preliminary (3-month follow-up) results on combination of endoscopic balloon dilation with transurethral incision of bladder neck. Short-term success rate were high but longer follow up is needed (24).
In patients with long areas of contractures not amenable to endoscopic procedures, devastating BNC from distraction injuries or those failed numerous endoscopic managements merit consideration for open reconstruction of bladder neck. Open surgical reconstruction may be technically challenging and could cause greater morbidity. Various open surgical approaches have been previously prescribed such as abdomino-perineal, perineal or transpubic. Pfalzgraf et al. examined their single institutional experience with an open retropubic reanastomosis for highly recurrent and complex BNC in 20 patients after RP (41). The reported success rate after reanastomosis was 60%. There was a relatively high risk of new onset urinary incontinence after reconstructive surgery but this was successfully treated with artificial urinary sphincter (AUS) implantation in most patients (41). Mundy et al. recently reported satisfactory results in 21 of 23 (91%) patients who underwent transperineal revision of the vesicourethral anastomosis after RP, with all patients requiring subsequent AUS placement (35). Additional open surgical reconstructive techniques were previously described in a small case series of four patients with vesicourethral anastomotic stricture following RP treated with various surgical approaches: primary excision of bladder neck with end-to-end anastomosis, penile fasciocutaneous flap and free graft urethroplasty (31).
Newer treatment modalities of US and/or BNC
Although conventional approaches to refractory or recalcitrant BNC include endoscopic therapy through incision or dilation or open surgical excision with re-anastomosis, experimental techniques involving resection with the Holmium laser or injection of medications with anti-proliferative properties at the site of the bladder neck incisions have recently been reported in the published literature. With two years of follow up, Eltahawy et al. reported an 83% success rate in BNC treatment in 24 patients undergoing holmium laser incision followed by injection of 80 mg triamcinolone at the contracture site (32).
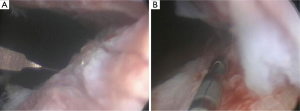
A recent study from the Lahey Clinic described for the first time the management of recurrent BNC with radial urethrotomy combined with intralesional MMC, which resulted in bladder neck patency in 72% of the patients after one procedure and in 89% after two procedures at minimum follow up of 12 months (Figure 1) (33). The MMC drug is an anti-proliferative and anti-scar forming agent, well known as an antitumor antibiotic, was discovered in 1958 and has been used clinically since 1963, when it was first stemmed from pterygium surgery. In in-vitro and animal models MMC has been shown to inhibit fibroblast proliferation, collagen deposition and scar formation (42-44).
Ionizing radiation has been shown to prevent hypertrophic scarring and keloid formation. This prompted Olschewski et al. to evaluate the therapeutic effect of endourethral brachytherapy for prevention of recurrent US after internal urethrotomy (45). In this study, ten patients received internal urethrotomy followed by high-dose-rate (HDR) brachytherapy (12-16 Gy) within 5 hours after internal urethrotomy. There results showed 90% success rate after 15 months follow up. They concluded that endourethral HDR brachytherapy proved to be an effective method that can reduce urethral re-stricture. Similarly, Sun et al. found that an intraurethral brachytherapy (16 Gy) after internal urethrotomy or transurethral resection of scar is a safe and effective treatment for recurrent USs (46). They recruited 17 patients with recurrent US and obtained a 93% stricture-free rate after 20 months follow up. Although initial studies have shown promising results in preventing recurrent US, these results should be interpreted within the limitation of the preliminary results, the small size sample, and the single-arm study design. Whether the endourethral brachytherapy actually helps in the management of recurrent US is yet to be determined, and the actual radiation dose, duration as well as long-term safety and efficacy need to be systematically evaluated as end-points in a large randomized clinical trial.
Histologically, US presents as collagen-rich connective tissue and fibroblasts that replace the corpus spongiosum surrounding the urethra. This suggests that synthesis of collagen and other extracellular matrix proteins are involved in this process. Halofuginone, a plant alkaloid originally isolated from the plant dichroa febrifuga, was identified as an effective antifibrotic agent by inhibiting collagen α-1 gene expression and collagen synthesis in various tissues at extremely low concentrations (47). Nagler et al. administrated halofuginone into a US rat model either orally in concentrations 1 and 5 ppm in the diet or by injection of 0.03% halofuginone dissolved in 2% lignocaine directly into the urethra once a day for 7 days. They found that halofuginone injected into the urethra or orally at 5 ppm normalized the urethrogram and prevented increases in collagen α-1 gene expression and collagen content as well as inhibited the collagen secreted by fibroblasts derived from the rat male urethra (48). Another animal study by Jaidane et al. conducted a two-phase study of Halofuginone on US (49). In the first phase, 20 rabbits of US model induced by electro-coagulation were randomly assigned to two groups of ten each, which received a diet containing halofuginone or a normal diet. Three weeks later, US developed in two study rabbits (20%) versus ten controls (100%). In the second phase, electrocoagulation-induced US was treated with visual internal urethrotomy in halofuginone and a normal-diet group, respectively. US evaluation was done ten weeks thereafter; recurrent stricture was observed in 5 of the 18 study rabbits (27%) versus 14 of the 19 controls (73%). It should be noted that these studies were performed on animal models with limited study size. Halofuginone demonstrated the promising antifibrotic effects; however, the efficacy and safety should be further investigated in clinical settings.
Kim et al. recently described their experience of hyaluronic acid (HA) instillation with visual internal urethrotomy for US (50). They instilled HA via an 18-gauge tube catheter between the urethral lumen and foley catheter after urethrotomy. One year later, 53% of the patients were stricture-free as followed up with retrograde urethrography, uroflowmetry, and post void residual urine. Kim’s study claimed that the success rate of urethrotomy combined with HA instillation was not higher than that observed in the published literature for conventional urethrotomy. The use of HA was further examined by Chung et al. in a randomized fashion in a total of 120 patients who underwent internal urethrotomy for US (51). They recently reported combination use of HA with carboxymethyl cellulose (CMC) in preventing recurrence of US after internal urethrotomy (52). In Chung’s study, patients were randomly divided into two groups: HA/CMC instillation group (n=60) or control-lubricant instillation group (n=60) postoperatively. They found that HA/CMC instillation could effectively decrease the incidence of US formation/recurrence postoperatively as compared with the control group. Extended follow-ups are needed to confirm the long-term effects.
Other therapeutic agents and techniques, including maintaining the temperature of the urethra during TURP, intraurethral use of captopril gel and cyclooxygenase-2 inhibitor are sporadically reported with varying benefits (52-54). However, the safety and efficacy of these agents and techniques will need to be validated in a large randomized study where patient safety, efficacy and outcomes are end-points.
Future directions
Interestingly, newer agents have been validated in treatment of other urologic conditions such as Peyronie’s disease. In a prospective study by Jordan, the efficacy and safety of intralesional clostridial collagenase injection therapy was examined in a total 25 patients with Peyronie’s disease (55). Clostridial collagenase is a chromatographically purified bacterial enzyme that selectively attacks collagen (56), shown to be the primary component of the Peyronie’s disease scar (57). Moreover, Collagenase is currently FDA-approved for debriding chronic dermal ulcers and severely burned tissues (57). Significant decreases from baseline were achieved in the penile deviation angle, plaque length/width and overall patient satisfaction. This study concluded that this novel approach may well have significant benefit in the management of Peyronie’s disease, however a double-blind, placebo-controlled study is warranted to validate its preliminary results and outcomes (57). Given the known anti-collagen properties of clostridial collagenase, this used in combination with transurethral incision of US and/or BNC could improve successful treatment of recurrent, refractory or recalcitrant US and/or BNC.
Animal studies have evaluated the efficacy and effectiveness of biodegradable stents in vitro with promising results, however, clinical studies are required to validate their safety and efficacy in humans (58,59).
Summary
The development of US and/or BNC is a relatively uncommon but well described condition observed primarily in men. Although the prevalence of US and/or BNC has decreased in recent years, management of this condition remains challenging for clinical urologists. Numerous treatment options exist for this condition that vary in procedural severity, including intermittent self CIC, serial urethral dilation, endoscopic techniques and open reconstructive repairs. Repetitive procedures for this condition may carry increased failure rates and morbidities. For the treatment of refractory or recalcitrant BNC, novel intralesional anti-proliferative, anti-scar agents such as steroids and/or MMC have been used in combination with transurethral bladder neck incisions to augment outcome and long-term effects. Long-term clinical data are lacking and double-blinded randomized clinical trials are needed to validate safety and efficacy where patient safety, efficacy and outcome are end-points.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol 2007;177:1667-74. [PubMed]
- Greenwell TJ, Castle C, Andrich DE, et al. Repeat urethrotomy and dilation for the treatment of urethral stricture are neither clinically effective nor cost-effective. J Urol 2004;172:275-7. [PubMed]
- Mandhani A, Chaudhury H, Kapoor R, et al. Can outcome of internal urethrotomy for short segment bulbar urethral stricture be predicted? J Urol 2005;173:1595-7. [PubMed]
- Buckley JC, Heyns C, Gilling P, et al. SIU/ICUD Consultation on Urethral Strictures: Dilation, internal urethrotomy, and stenting of male anterior urethral strictures. Urology 2014;83:S18-22. [PubMed]
- Mazdak H, Meshki I, Ghassami F. Effect of mitomycin C on anterior urethral stricture recurrence after internal urethrotomy. Eur Urol 2007;51:1089-92. [PubMed]
- Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol 2005;23:8146-51. [PubMed]
- Breyer BN, Davis CB, Cowan JE, et al. Incidence of bladder neck contracture after robot-assisted laparoscopic and open radical prostatectomy. BJU Int 2010;106:1734-8. [PubMed]
- Msezane LP, Reynolds WS, Gofrit ON, et al. Bladder neck contracture after robot-assisted laparoscopic radical prostatectomy: evaluation of incidence and risk factors and impact on urinary function. J Endourol 2008;22:97-104. [PubMed]
- Carlsson S, Nilsson AE, Schumacher MC, et al. Surgery-related complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital, Sweden. Urology 2010;75:1092-7. [PubMed]
- Carr LK, Webster GD. Endoscopic management of the obliterated anastomosis following radical prostatectomy. J Urol 1996;156:70-2. [PubMed]
- Brodak M, Kosina J, Pacovsky J, et al. Bipolar transurethral resection of anastomotic strictures after radical prostatectomy. J Endourol 2010;24:1477-81. [PubMed]
- Giannarini G, Manassero F, Mogorovich A, et al. Cold-knife incision of anastomotic strictures after radical retropubic prostatectomy with bladder neck preservation: efficacy and impact on urinary continence status. Eur Urol 2008;54:647-56. [PubMed]
- Dalkin BL. Endoscopic evaluation and treatment of anastomotic strictures after radical retropubic prostatectomy. J Urol 1996;155:206-8. [PubMed]
- Ramchandani P, Banner MP, Berlin JW, et al. Vesicourethral anastomotic strictures after radical prostatectomy: efficacy of transurethral balloon dilation. Radiology 1994;193:345-9. [PubMed]
- Ramchandani P, Banner MP, Berlin JW, et al. Vesicourethral anastomotic strictures after radical prostatectomy: efficacy of transurethral balloon dilation. Radiology 1994;193:345-9. [PubMed]
- Buckley JC. Complications after radical prostatectomy: anastomotic stricture and rectourethral fistula. Curr Opin Urol 2011;21:461-4. [PubMed]
- Heyns CF, Steenkamp JW, De Kock ML, et al. Treatment of male urethral strictures: is repeated dilation or internal urethrotomy useful? J Urol 1998;160:356-8. [PubMed]
- Steenkamp JW, Heyns CF, de Kock ML. Internal urethrotomy versus dilation as treatment for male urethral strictures: a prospective, randomized comparison. J Urol 1997;157:98-101. [PubMed]
- Borboroglu PG, Sands JP, Roberts JL, et al. Risk factors for vesicourethral anastomotic stricture after radical prostatectomy. Urology 2000;56:96-100. [PubMed]
- Gonzalgo ML, Pavlovich CP, Trock BJ, et al. Classification and trends of perioperative morbidities following laparoscopic radical prostatectomy. J Urol 2005;174:135-9. [PubMed]
- Davidson PJ, van den Ouden D, Schroeder FH. Radical prostatectomy: prospective assessment of mortality and morbidity. Eur Urol 1996;29:168-73. [PubMed]
- Turina M, Mulhall AM, Mahid SS, et al. Frequency and surgical management of chronic complications related to pelvic radiation. Arch Surg 2008;143:46-52. [PubMed]
- Malde S, Rajagopalan A, Patel N, et al. Potassium-titanyl-phosphate laser photoselective vaporization for benign prostatic hyperplasia: 5-year follow-up from a district general hospital. J Endourol 2012;26:878-83. [PubMed]
- Ramirez D, Simhan J, Hudak SJ, et al. Standardized approach for the treatment of refractory bladder neck contractures. Urol Clin North Am 2013;40:371-80. [PubMed]
- Anger JT, Raj GV, Delvecchio FC, et al. Anastomotic contracture and incontinence after radical prostatectomy: a graded approach to management. J Urol 2005;173:1143-6. [PubMed]
- Gillitzer R, Thomas C, Wiesner C, et al. Single center comparison of anastomotic strictures after radical perineal and radical retropubic prostatectomy. Urology 2010;76:417-22. [PubMed]
- Mark S, Pérez LM, Webster GD. Synchronous management of anastomotic contracture and stress urinary incontinence following radical prostatectomy. J Urol 1994;151:1202-4. [PubMed]
- Geary ES, Dendinger TE, Freiha FS, et al. Incontinence and vesical neck strictures following radical retropubic prostatectomy. Urology 1995;45:1000-6. [PubMed]
- Besarani D, Amoroso P, Kirby R. Bladder neck contracture after radical retropubic prostatectomy. BJU Int 2004;94:1245-7. [PubMed]
- Theodoros C, Katsifotis C, Stournaras P, et al. Abdomino-perineal repair of recurrent and complex bladder neck-prostatic urethra contractures. Eur Urol 2000;38:734-40; discusssion 740-1.
- Wessells H, Morey AF, McAninch JW. Obliterative vesicourethral strictures following radical prostatectomy for prostate cancer: reconstructive armamentarium. J Urol 1998;160:1373-5. [PubMed]
- Eltahawy E, Gur U, Virasoro R, et al. Management of recurrent anastomotic stenosis following radical prostatectomy using holmium laser and steroid injection. BJU Int 2008;102:796-8. [PubMed]
- Vanni AJ, Zinman LN, Buckley JC. Radial urethrotomy and intralesional mitomycin C for the management of recurrent bladder neck contractures. J Urol 2011;186:156-60. [PubMed]
- Elliott SP, Meng MV, Elkin EP, et al. Incidence of urethral stricture after primary treatment for prostate cancer: data From CaPSURE. J Urol 2007;178:529-34. [PubMed]
- Mundy AR, Andrich DE. Posterior urethral complications of the treatment of prostate cancer. BJU Int 2012;110:304-25. [PubMed]
- Milroy EJ, Chapple CR, Cooper JE, et al. A new treatment for urethral strictures. Lancet 1988;1:1424-7. [PubMed]
- De Vocht TF, van Venrooij GE, Boon TA. Self-expanding stent insertion for urethral strictures: a 10-year follow-up. BJU Int 2003;91:627-30. [PubMed]
- Hussain M, Greenwell TJ, Shah J, et al. Long-term results of a self-expanding wallstent in the treatment of urethral stricture. BJU Int 2004;94:1037-9. [PubMed]
- Jordan GH, Wessells H, Secrest C, et al. Effect of a temporary thermo-expandable stent on urethral patency after dilation or internal urethrotomy for recurrent bulbar urethral stricture: results from a 1-year randomized trial. J Urol 2013;190:130-6. [PubMed]
- Yurkanin JP, Dalkin BL, Cui H. Evaluation of cold knife urethrotomy for the treatment of anastomotic stricture after radical retropubic prostatectomy. J Urol 2001;165:1545-8. [PubMed]
- Pfalzgraf D, Beuke M, Isbarn H, et al. Open retropubic reanastomosis for highly recurrent and complex bladder neck stenosis. J Urol 2011;186:1944-7. [PubMed]
- Ferguson B, Gray SD, Thibeault S. Time and dose effects of mitomycin C on extracellular matrix fibroblasts and proteins. Laryngoscope 2005;115:110-5. [PubMed]
- Simman R, Alani H, Williams F. Effect of mitomycin C on keloid fibroblasts: an in vitro study. Ann Plast Surg 2003;50:71-6. [PubMed]
- Ayyildiz A, Nuhoglu B, Gülerkaya B, et al. Effect of intraurethral Mitomycin-C on healing and fibrosis in rats with experimentally induced urethral stricture. Int J Urol 2004;11:1122-6. [PubMed]
- Olschewski T, Kröpfl D, Seegenschmiedt MH. Endourethral brachytherapy for prevention of recurrent urethral stricture following internal urethrotomy--first clinical experiences and results. Int J Radiat Oncol Biol Phys 2003;57:1400-4. [PubMed]
- Sun YH, Xu CL, Gao X, et al. Intraurethral brachytherapy for prevention of recurrent urethral stricture after internal urethrotomy or transurethral resection of scar. J Endourol 2001;15:859-61. [PubMed]
- Pines M, Nagler A. Halofuginone: a novel antifibrotic therapy. Gen Pharmacol 1998;30:445-50. [PubMed]
- Nagler A, Gofrit O, Ohana M, et al. The effect of halofuginone, an inhibitor of collagen type i synthesis, on urethral stricture formation: in vivo and in vitro study in a rat model. J Urol 2000;164:1776-80. [PubMed]
- Jaidane M, Ali-El-Dein B, Ounaies A, et al. The use of halofuginone in limiting urethral stricture formation and recurrence: an experimental study in rabbits. J Urol 2003;170:2049-52. [PubMed]
- Kim HM, Kang DI, Shim BS, et al. Early experience with hyaluronic Acid instillation to assist with visual internal urethrotomy for urethral stricture. Korean J Urol 2010;51:853-7. [PubMed]
- Chung JH, Kang DH, Choi HY, et al. The effects of hyaluronic acid and carboxymethylcellulose in preventing recurrence of urethral stricture after endoscopic internal urethrotomy: a multicenter, randomized controlled, single-blinded study. J Endourol 2013;27:756-62. [PubMed]
- Shirazi M, Khezri A, Samani SM, et al. Effect of intraurethral captopril gel on the recurrence of urethral stricture after direct vision internal urethrotomy: Phase II clinical trial. Int J Urol 2007;14:203-8. [PubMed]
- Park JK, Lee SK, Han SH, et al. Is warm temperature necessary to prevent urethral stricture in combined transurethral resection and vaporization of prostate? Urology 2009;74:125-9. [PubMed]
- Sciarra A, Salciccia S, Albanesi L, et al. Use of cyclooxygenase-2 inhibitor for prevention of urethral strictures secondary to transurethral resection of the prostate. Urology 2005;66:1218-22. [PubMed]
- Jordan GH. The use of intralesional clostridial collagenase injection therapy for Peyronie’s disease: a prospective, single-center, non-placebo-controlled study. J Sex Med 2008;5:180-7. [PubMed]
- Gelbard MK, James K, Riach P, et al. Collagenase versus placebo in the treatment of Peyronie’s disease: a double-blind study. J Urol 1993;149:56-8. [PubMed]
- Ehrlich HP. Scar contracture: cellular and connective tissue aspects in Peyronie’s disease. J Urol 1997;157:316-9. [PubMed]
- Fu WJ, Zhang X, Zhang BH, et al. Biodegradable urethral stents seeded with autologous urethral epithelial cells in the treatment of post-traumatic urethral stricture: a feasibility study in a rabbit model. BJU Int 2009;104:263-8. [PubMed]
- Kotsar A, Nieminen R, Isotalo T, et al. Preclinical evaluation of new indomethacin-eluting biodegradable urethral stent. J Endourol 2012;26:387-92. [PubMed]


