Presentation, management, and outcomes of complications following prostate cancer therapy
Introduction
Prostate cancer is the most common noncutaneous malignancy and second leading cause of cancer death in American men. Annually, about 240,000 new cases of prostate cancer are diagnosed in the United States and 30,000 men will die from the disease (1). Advancements and increased utilization of prostate specific antigen (PSA) have led to increased and earlier diagnosis of localized disease. Consequently, prostate cancer mortality is decreasing as more men undergo treatment, however treatment-related complications are increasing (2).
Treatment modalities for localized prostate cancer are variable, have various side effects, and depend on patient preference, disease extent, and patient co-morbidities. Treatment may involve active surveillance, radical prostatectomy (RP), or radiation therapies including external beam radiation therapy (EBRT) and brachytherapy (BT). Other interventions include cyroablation, high intensity focused ultrasound (HIFU), and particle beam therapy (3).
As more men are diagnosed with prostate cancer, more will inevitably undergo treatment and develop treatment-related complications. We report commonly observed complications from treatment of prostate cancer and management of these in a contemporary cohort of patients referred to our institution.
Methods
Patient population
After institutional review board approval, data was abstracted from a retrospectively collected single surgeon database from 2006-2010 at a large tertiary care referral-based medical center. Study inclusion criteria were any patient who underwent operative therapy at our institution for sequela or complications from treatment of prostate cancer, regardless of treatment modality. Patients were excluded if their operative intervention did not stem from complications related to prostate cancer treatment.
Data collection
Variables abstracted included age, type of prostate cancer therapy, complication(s) arising from therapy, number of interventions to manage these complications performed at our institution, and types of procedures performed. Complications stemming from initial management of prostate cancer were classified using the Clavien grading system, a validated instrument to characterize postoperative complications (4).
Statistical analysis
We used descriptive statistics to characterize the study population and outcomes. Data was accrued and analyzed using Microsoft Excel.
Results
From June 2006 to June 2010, 890 patients underwent genitourinary reconstructive surgery at the University of California, San Francisco (UCSF) Medical Center by a single surgeon. Of these, 139 patients underwent surgeries to treat complications arising from prostate cancer therapy. The mean age of patients in our study was 72.4 years (range, 72.4±8.3 years). Fifty patients (36%) were referred with complications stemming from RP monotherapy. Thirty one (22%) underwent RP followed by EBRT or BT. In the RP group, 55 were radical retropubic prostatectomies, five radical perineal prostatectomies, five robotic assisted laparoscopic prostatectomies, three laparoscopic prostatectomies, and 18 had unspecified approaches. Fifteen (10%) underwent EBRT only, 11 (8%) BT only, and 23 (17%) underwent combination EBRT and BT. Seven (5%) underwent salvage RP, one (0.5%) underwent high intensity focused ultrasonagraphy and cryoablation each (Table 1).
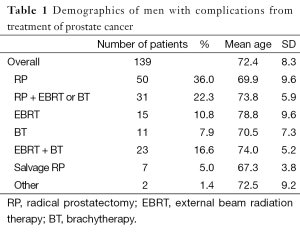
Full table
Complications
Complications managed were classified as Clavien IIIb, given that they required operative intervention with general anesthesia. We noted 59 cases of urinary incontinence (UI), 51 cases of urethral strictures or stenosis, 29 cases of bladder neck contractures, 25 cases of fistulas (vesico-rectal, rectourethral, ileoanal pouch-ureteral in a patient status post proctocolectomy with ileoanal pouch, and recto-prostatic fistula), and nine other complications (radiation cystitis, radiation proctitis, genital edema, bladder/urethral stones) (Table 2). With regards to UI, thirty (50%) occurred in the RP monotherapy group, 20 (34%) occurred in the RP followed by EBRT or BT group, 10 (12%) occurred in the radiation groups (EBRT, BT, or EBRT + BT), and two (3%) occurred in the salvage prostatectomy group. With regards to fistula formation, nine (36%) occurred in the RP monotherapy group, one (4%) in the RP followed by EBRT or BT group, ten (40%) in the radiation groups, and four (16%) in the salvage RP group. With regards to urethral stenosis, six (11%) occurred in the RP monotherapy group, eight (16%) in the RP followed by EBRT or BT group, 35 (69%) in the radiation groups, and one (2%) in the salvage RP cohort. With regards to bladder neck contracture, 14 (48%) occurred in the RP monotherapy group, 11 (38%) occurred in the RP followed by EBRT or BT group, two (7%) occurred in the radiation groups, and two (7%) in the salvage RP group (Table 2).
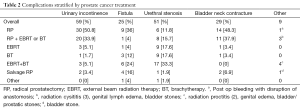
Full table
Interventions
Common interventions performed were direct vision internal urethrotomy (DVIU) in 46 cases, artificial urinary sphincter (AUS) placement in 36 cases, urethral reconstruction in 34 cases, UroLume urethral stent placement in 29 cases, urethral slings in 29 cases, repair of fistulas in 22 cases, and balloon dilation in ten cases (Table 3).
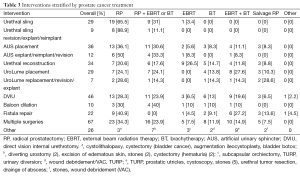
Full table
Other surgical interventions included lithotripsy for bladder, urethral, and prostatic stones; transurethral resection of prostate in the non-operative groups for obstructive urinary symptoms; excision of edematous penile skin in patients with penile and scrotal edema following radiation therapy; wound debridement and incision and drainage for abscesses following operative intervention; augmentation ileocystoplasty; and urinary diversion for concomitant bladder cancer, persistent hematuria in radiation therapy patient, severe urethral strictures following radiation therapy, failed repair of fistula, and severe UI not managed with sphincter.
Sixty seven (48%) patients required multiple operations at our institution. Of the 29 urethral slings placed, nine (31%) required revision or explantation. With regards to the 36 artificial urinary sphincters placed, 12 (33%) required revision or explantation. Reasons a urethral sling or AUS required revision included erosion, chronic pain associated with placement, non-functional prosthesis, or infection of prosthesis.
Fistulas included vesico-rectal fistulas, rectourethral, ileoanal pouch-ureteral in a patient status post proctocolectomy with ileoanal pouch, and recto-prostatic fistula. Repairs were via a transperineal approach with or without a bulbar corpora spongiosum interposition flap, transperineal approach with a dartos interposition flap, transperineal approach with gracilis muscle flap, and transrectal approach with an endorectal advancement flap. Fecal diversion was performed in all patients who had rectal involvement. One patient, who sustained a rectal injury during RRP and had had two prior attempts at repair of a rectourethral fistula with recurrence each time, underwent a urinary diversion via diverting ileostomy.
Number of interventions
The median number of interventions performed at UCSF was two and average was two. This does not include those performed at outside institutions prior to or after referral to UCSF. In the RP monotherapy group, there were an average of 1.9 (±1.2) interventions with median of 2 and range from 1-7. The patient that required seven included a urethroplasty and bladder neck reconstruction, AUS placement then revision, augmentation ileocystoplasty, bulbar cuff sphincter prosthesis implant, radical cystectomy and ileal conduit urinary diversion, and finally an AUS explant. The RP followed by EBRT or BT group required an average of 2.1 (±1.5) interventions, median of 2, and range from 1-7. The patient requiring seven interventions included serial balloon dilations and DVIU. The EBRT group required an average of 1.7 (±1.5) interventions, median 1, ranging from 1-6. The BT group required 2.5 (±1) interventions, median 2, ranging from 1-4. The combined EBRT + BT group required 2.1 (±1.5), median 1.5, ranging from 1-6. The salvage therapy group required 2.4 (±1.51), median 2, ranging from 1-5 (Table 4).
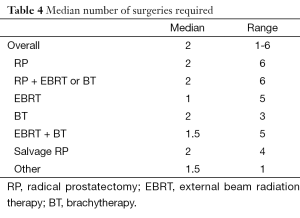
Full table
Discussion
In this retrospective study of a single surgeon’s experience in operatively managing complications stemming from treatment of prostate cancer referred to our practice, we were able to define complications by treatment modality and means of management. Complications we encountered included UI, fistula formation (vesico-rectal fistulas, rectourethral, ileoanal pouch-ureteral, and recto-prostatic), urethral strictures, and bladder neck contractures. UI and bladder neck contractures were more common in patients initially treated with RP. On the other hand, patients initially treated with radiotherapy developed fistulas and urethral strictures more commonly. These were managed with a variety of operative interventions, including urethral sling, artificial urinary sphincter, urethral reconstruction, balloon dilation, and fistula repair. On average patient’s required two surgeries for management of their complication.
Complications following treatment for localized prostate cancer with monotherapy versus multimodal treatments have been well documented. UI is more common following RP than radiotherapy. With RP, 7.9-16% and 1.5-7% of patients will require at least a single pad per day at 12 and 24 months respectively (6,7). In radiotherapy groups, at 24 months, 0-5% of patients will require pads for leak, with a direct correlation to the dose of radiation utilized. Patients treated with multimodal radiotherapy have higher rates of UI (8). Urethral strictures are more common following radiotherapy than surgical therapy. These have been reported to be 1.7-1.8% with monotherapy radiotherapy and 5.2-12% with BT and EBRT combined therapy (9). With high dose rate BT, the rate has been reported at 8% at 41 months, with 92% occurring in the bulbo-membranous urethra (10). However, rates of bladder neck contraction are much more common following RP, ranging from 2.7-25.7%. The highest rates of urethral strictures were seen in patients undergoing surgical and combination radiotherapy (9,11). Rectourethral fistula formation following RP ranges from 0.5-2% (12) versus 1-1.8% from radiotherapy (13). With regards to voiding and bowel symptoms, patients treated with radiotherapy have higher rates of irritative urinary and bowel symptoms versus those treated surgically (14-17).
Findings from our study are consistent with published data. Surgical groups had higher rates of UI and bladder neck contractures. Likewise urethral strictures and fistulas were more common in radiotherapy groups.
Limitations of our study include that it is a single surgeon’s experience at a single large academic medical center. Consequently, patient population may not be representative of other practice centers and practitioners. Our data is limited to interventions performed at our institution. We did not have original operative reports, interventions performed at outside facilities, and dosing of radiation therapy. Likewise, follow up is limited to care at UCSF and interventions afterwards are not included in our analysis. With regards to study groups, there were more patients referred to our practice after operative intervention versus radiotherapy. Data regarding initial disease, PSA, and margins was not always clearly documented. We also did not evaluate erectile dysfunction following treatment of prostate cancer, although this is a major issue for patients following prostate cancer therapy. Evaluation of outcome was limited and ideally, quantifiable data such as imaging, urodynamics testing, or validated instruments would serve as a superior means to evaluate patient outcome.
As more men undergo treatment for localized disease, more will inevitably have complications stemming from interventions. Consequently treatment of these complications will become increasingly important in counseling patients. Our study evaluated 139 patients with complications from a variety of treatments for prostate cancer, how these were managed, number of treatments required and patient outcome. Given the multitude of choices patients have for localized prostate cancer, counseling with knowledge of potential complications of each is especially important. Additionally, should a patient develop one of these complications, knowing options for management and outcomes are equally important. Our study sheds light on both of these issues. The unique challenges these patients present require innovation and determination.
Future directions of study include correlating patient specific factors including medical and surgical history with outcomes and complications. Additionally, better characterizing patient outcome is paramount as well. Also, as treatment of prostate cancer shifts from open to robotic or laparoscopic approaches, the evolution and frequency of complications would be interesting to evaluate, especially as more urologic surgeons are trained on the robot during residency and thus more proficient once in practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012.
- Cook ED, Nelson AC. Prostate cancer screening. Curr Oncol Rep 2011;13:57-62. [PubMed]
- Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol 2008;53:68-80. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [PubMed]
- Michaelson MD, Cotter SE, Gargollo PC, et al. Management of complications of prostate cancer treatment. CA Cancer J Clin 2008;58:196-213. [PubMed]
- Sacco E, Prayer-Galetti T, Pinto F, et al. Urinary incontinence after radical prostatectomy: Incidence by definition, risk factors and temporal trend in a large series with a long-term follow-up. BJU Int 2006;97:1234-41. [PubMed]
- Parsons BA, Evans S, Wright MP. Prostate cancer and urinary incontinence. Maturitas 2009;63:323-8. [PubMed]
- Mohammed N, Kestin L, Ghilezan M, et al. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:204-12. [PubMed]
- Meeks JJ, Brandes SB, Morey AF, et al. Urethroplasty for radiotherapy induced bulbomembranous strictures: A multi-institutional experience. J Urol 2011;185:1761-5. [PubMed]
- Sullivan L, Williams SG, Tai KH, et al. Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother Oncol 2009;91:232-6. [PubMed]
- Elliott SP, Meng MV, Elkin EP, et al. Incidence of urethral stricture after primary treatment for prostate cancer: Data from CaPSURE. J Urol 2007;178:529-34. [PubMed]
- Buckley JC. Complications after radical prostatectomy: Anastomotic stricture and rectourethral fistula. Curr Opin Urol 2011;21:461-4. [PubMed]
- Elliott SP, McAninch JW, Chi T, et al. Management of severe urethral complications of prostate cancer therapy. J Urol 2006;176:2508-13. [PubMed]
- Ferrer M, Suárez JF, Guedea F, et al. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;72:421-32. [PubMed]
- Moinpour CM, Hayden KA, Unger JM, et al. Health-related quality of life results in pathologic stage C prostate cancer from a southwest oncology group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy. J Clin Oncol 2008;26:112-20. [PubMed]
- Frank SJ, Pisters LL, Davis J, et al. An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy and brachytherapy iodine implantation as monotherapies for localized prostate cancer. J Urol 2007;177:2151-6. [PubMed]
- Wu AK, Cooperberg MR, Sadetsky N, et al. Health related quality of life in patients treated with multimodal therapy for prostate cancer. J Urol 2008;180:2415-22; discussion 2422. [PubMed]


