Microsurgical denervation of the rat spermatic cord and its connection to the diagnosis and treatment of chronic orchialgia: a bench to bedside experience
Introduction
Chronic orchialgia is a common urologic complaint, however, the diagnosis and subsequent treatment remains challenging for urologists. This condition has been defined as continuous testicular pain lasting for at least three months during which time the patient’s daily activities are compromised prompting them to seek medical attention (1,2). This definition was expanded to include pain originating from the scrotum or epididymis (3). From an even broader prospective, orchialgia can be included in the general category of chronic pelvic pain syndrome (CPPS). Chronic orchialgia can occur in any age group, but the majority of patients present in their mid to late thirties (4).
Etiology
In an attempt to direct treatment, it is important to attempt to identify the underlying pathology in patients who present with chronic testicular pain. Patients may be experiencing orchialgia secondary to a testicular cause, which include: tumor, torsion, varicocele, hydrocele, spermatocele, infection or trauma. It is critical to conduct a thorough history and physical exploring each of these possibilities. Patients also can experience referred pain to the scrotal contents from any organ that share the same nerve supply, such as, the ureter, prostate, lumbar spine, or entrapment of the ilioinguinal or genitofemoral nerves during a surgical procedure, typically inguinal hernia repair (5). Several small series report on the incidence of chronic testicular pain following vasectomy, which may result in an increase in intratubular pressure in the testis and epididymis (6,7). Masarani reported up to 5% of patients who underwent vasectomy reported testicular pain lasting for greater than three months in their studies (8).
Diagnosis
It is critical to perform a careful history and physical when assessing patients with a complaint of chronic orchialgia. The physician must ask patients about past surgeries, such as hernia repairs or vasectomies, any history of trauma, any associated symptoms such as dysuria or incomplete emptying, or any history of previous episodes of testicular pain. Physical exam may be helpful in localizing the pain to the testicle, epididymis, or prostate. In addition, a urinalysis and culture should be performed to exclude the presence of infection. In patients in whom a sexually transmitted infection is suspected, a urethral swab should be performed. With regard to radiologic testing, a scrotal ultrasound should be performed to rule out malignancy or testicular torsion (9).
Medical management
The first line treatment of chronic orchialgia is the use of medical management. If there are clinical, laboratory, or imaging evidence of infection, a combination antibiotic regimen is commonly used with a fluoroquinolone plus doxycycline to cover for the majority of common organisms (10). In the case of idiopathic testicular pain, a stepwise approach of pain management is recommended. Non-steroidal anti-inflammatory drugs (NSAIDs) are the main analgesic used that may be titrated to the maximum recommended dosage for moderate pain (5). However, they should be used only for short-term as their long-term usage can increase the risk of peptic ulcers, platelet dysfunction, and renal dysfunction.
Anti-depressant medications may be used as an adjuvant to NSAID therapy. Tri-cyclic antidepressants such as amitriptyline are most frequently prescribed, but alternatives include serotonin reuptake inhibitors and Gamma-aminobutyric acid (GABA) analogs. It is theorized that these medications address neuropathic pain symptoms as well as any potential major depressive symptoms that might coexist (5,10,11). For persisting or worsening pain, weak opioids such as codeine or tramadol are being used with increasing frequency. In rare circumstances stronger opioids such as morphine or buprenorphine can be tried, although the associated dependence and tolerance to these drugs are concerns (10,12). A trial of alpha-antagonists may be given in pain that is refractory to conservative management in a patient wishes to avoid surgery. Pharmacologic studies have shown a high concentration of α1A-adenoreceptors in the vas deferens. In theory, this can cause a functional obstruction of the vas deferens from muscular spasms that might be relieved by selective α1A-blockade (5). In general, coordination of medical management with a pain specialist and psychiatrist should be considered.
Surgical management: spermatic cord (SC) denervation
Microsurgical denervation
Devine and Schellhammer first described the use of microsurgical denervation for chronic orchialgia in 1978. The procedure aims to transect all nerve fibers that are traveling within the SC (10). Heidenreich et al. reported that 96% (34 of 35) of patients were pain free after microsurgical denervation, and there were no reports of complications such as testicular atrophy or hydrocele (13). Of note, they only performed the procedure on patients who had a positive response of complete pain relief on SC block, which predicted success. Strom et al. reported slightly less efficacy with 71% (67 of 95) patients pain free and 17% (17 of 95) patients with pain reduction, although they only required partial response to the screening nerve block. They had two patients with testicular atrophy and two with hydrocele (14). In general, these studies support the use of microsurgical denervation citing a high likelihood of pain reduction with rare complications of hydrocele and testicular atrophy.
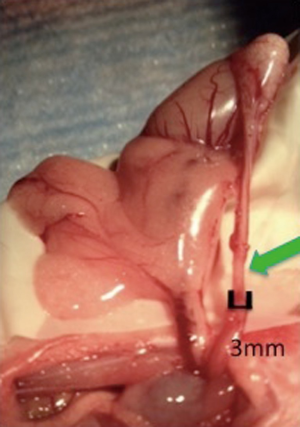
The feasibility of microsurgical denervation of the spermatic cord (MDSC) was recently explored in a rodent model. Laudano et al. compared efficacy rats of microsurgical denervation alone to microsurgical denervation immediately followed by MPM laser ablation (15,16). This study consisted of nine rats that underwent either sham surgery, microsurgical denervation alone, or microsurgical denervation plus immediate laser ablation of residual nerves with multiphoton microscopy (MPM). A denervated segment is shown in Figure 1. The technique of MPM laser ablation is further described below in the recent research section. Compared to sham animals, there was a significant reduction in the median number of nerves remaining around the vas deferens with microsurgical denervation alone (3.5 nerves) and MDSC plus MPM (1.5 nerves). The efficacy of nerve ablation with MDSC alone was 77% and the efficacy of MDSC plus MPM ablation was 90% (16). However, the difference in number of nerves after microsurgical denervation plus MPM compared to microsurgical denervation alone was not statistically significant (P=0.29) (16). At this point, microsurgical denervation is an effective technique to denervate the SC, however, the additional clinical benefit of MPM laser ablation remains unknown.
Robotic denervation
Robotic assisted microsurgical denervation is a relatively new procedure that may be performed from an inguinal, subinguinal, or intra-abdominal approach. Parekattil et al. have investigated the benefit of robotic assisted microsurgical denervation. They report 75% (18 of 24) of patients had complete pain resolution and 17% (4 of 24) had greater than 50% pain reduction. They also report a mean operative time of 41 minutes (17). As such, it appears that robotic assisted denervation procedures can provide similarly high rates of pain resolution as microsurgical denervation with an operating microscope.
Laparoscopic denervation
An alternative to robotic denervation that is less commonly used is transperitoneal laparoscopic testicular denervation. Cadeddu et al. reported a 77% (7 of 9) patient response rate with a mean 69.3% reduction in testicular pain. Additionally, they had no complications such as testicular atrophy in the series. They noted that an advantage of the transperitoneal approach over traditional inguinal microsurgical denervation is the ability to divide the gonadal vessels and ensure that additional nerve fibers running along them are transected. This is possible since the vessels have not yet joined with the vas deferens and collaterals from the deferential artery may continue to provide arterial flow (18). The efficacy of the laparoscopic approach appears similar to the microsurgical and robotic approaches, but there is little data available to add to these findings.
Advances in the field
A promising new technology to assist the accurate identification of nerves around the SC and vas deferens that is currently being performed in animal models is MPM at Weill Cornell Medical College. MPM is an imaging technique wherein, at low laser powers, anatomic structures can be visualized noninvasively. At higher powers, the laser acts as an ablating tool (19). Using MPM, Ramasamy et al. reported finding a median of ten nerve fiber bundles per rat SC measuring between 20 to 50 µm. At high power, they were able to generate a cavitation bubble up to 40 µm and ablate an individual nerve in no more than two minutes. Gross specimen analysis after the procedure showed burns that resemble electrocautery usage. On histological analysis, the vas deferens and vasculature were preserved (19). Therefore, MPM is a technique that may have future applications in the ablation of nerves surrounding the vas deferens.
Safety considerations following denervation
A second objective of the study by Laudano et al. was to evaluate the structural and functional changes of microsurgical denervation with or without MPM laser ablation on the testis and vas deferens. Of note, there was a difference between the three groups in terms of spermatogenesis measured with Johnsen score, a marker of impaired spermatogenesis. However, the lowest Johnsen score, indicating impaired spermatogenesis, was seen among the sham rats (16). Given the relatively small sample size, this effect may have been an artifact. Taken together, these observations suggest that neither MDSC nor MPM impairs spermatogenesis. Motile sperm were found distal to the denervation site in all vasa with the exception of one in the sham group and one in the denervation alone group. Vasograms in these cases demonstrated patency. These findings corresponded with testicles that had impaired spermatogenesis rather than evidence of vasal obstruction. The authors conclude that microsurgical denervation and MPM laser ablation did not have visible evidence of deleterious effects on the testis or vas deferens compared to sham (16).
Alternative surgical options
Vasectomy reversal
As previously mentioned, post-vasectomy pain syndrome (PVPS) is a rare complication of vasectomies, but if it does occur management can be challenging. Horovitz et al. reported outcomes in 14 patients who underwent vasovasotomies for chronic orchialgia. In this cohort, 93% of patients (13 of 14 men) reported pain improvement, and 50% (7 of 14) reported complete resolution of pain. However, 15% (2 of 14) had return of testicular pain to baseline (20). Nangia et al. similarly reported in their series of 13 patients with vasectomy reversal that 69% (9 of 13) became completely pain free (5,21). A disadvantage of vasectomy reversal is that it is a difficult procedure to perform and only an option for a small subset of patients. However, results show that it can be a useful modality in patients with post-vasectomy pain refractory to medical management. Brahmbhatt et al. presented a series of robotic-assisted vasectomy reversals performed for PVPS. In this cohort of 24 men, robotic-assisted vasectomy reversal reduced the visual pain score from 6.9 to 1.8 after six months of follow-up. There also was an improvement in the standardized pain impact questionnaire score in 85% (17/20) of patients (22).
Orchiectomy
Orchiectomy is considered a last resort operative intervention. It should be entertained only after medical management and other minimally invasive testes-preserving procedures have been attempted. However, efficacy rates following this procedure varied with Costabile et al. reporting that up to 80% of patients may have unresolved pain (10,23). An additional consideration with orchiectomy is the use of inguinal versus scrotal surgical approach. Davis et al. found that an inguinal orchiectomy had superior outcomes with 73% (11 of 15) of patients reporting pain resolution whereas scrotal orchiectomy had only 55% (5 of 9) of patients with pain resolution (2,5). In general, patients should be counseled that pain might still persist despite removal of the testicle.
Conclusions
Chronic orchialgia is a complex condition. Due to its variable presentation, diagnosis can be challenging and requires a careful history and physical examination. The first line treatment is medical management, however, there is no clear algorithm for those who fail. Surgical options range from vasectomy reversal to orchiectomy. Current data supports the use of microsurgical denervation, which can be done through a variety of approaches, including with an operating microscope, robotically, or laparoscopically. New techniques, such as the use of MPM for laser ablation of nerves surrounding the vas deferens are currently underway. Finally, given the young age of men presenting with condition, concerns regarding the effect of treatment on future fertility is warranted. Our pilot animal study suggests that microsurgical denervation and MPM laser ablation can be performed with minimal effects on the vas deferens or testicle in a rat model. Future larger studies are needed to evaluate the safety and efficacy of these procedures going forward.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gray CL, Powell CR, Amling CL. Outcomes for surgical management of orchalgia in patients with identifiable intrascrotal lesions. Eur Urol 2001;39:455-9. [PubMed]
- Davis BE, Noble MJ, Weigel JW, et al. Analysis and management of chronic testicular pain. J Urol 1990;143:936-9. [PubMed]
- Nickel JC, Siemens DR, Nickel KR, et al. The patient with chronic epididymitis: characterization of an enigmatic syndrome. J Urol 2002;167:1701-4. [PubMed]
- Wesselmann U, Burnett AL, Heinberg LJ. The urogenital and rectal pain syndromes. Pain 1997;73:269-94. [PubMed]
- Granitsiotis P, Kirk D. Chronic testicular pain: an overview. Eur Urol 2004;45:430-6. [PubMed]
- Johnson AL, Howards SS. Intratubular hydrostatic pressure in testis and epididymis before and after vasectomy. Am J Physiol 1975;228:556-64. [PubMed]
- Shafik A. Electrovasogram in normal and vasectomized men and patients with obstructive azoospermia and absent vas deferens. Arch Androl 1996;36:67-79. [PubMed]
- Masarani M, Cox R. The aetiology, pathophysiology and management of chronic orchialgia. BJU Int 2003;91:435-7. [PubMed]
- Campbell MF, Wein AJ, Kavoussi LR. Campbell-Walsh urology. In: Wein AJ, Kavoussi LR. eds. 9th ed. Philadelphia: W.B. Saunders, 2007.
- Kumar P, Mehta V, Nargund VH. Clinical management of chronic testicular pain. Urol Int 2010;84:125-31. [PubMed]
- Schover LR. Psychological factors in men with genital pain. Cleve Clin J Med 1990;57:697-700. [PubMed]
- Reid C, Davies A. The World Health Organization three-step analgesic ladder comes of age. Palliat Med 2004;18:175-6. [PubMed]
- Heidenreich A, Olbert P, Engelmann UH. Management of chronic testalgia by microsurgical testicular denervation. Eur Urol 2002;41:392-7. [PubMed]
- Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: long-term results from a single center. J Urol 2008;180:949-53. [PubMed]
- Laudano MA Osterberg EC, Sheth S, et al. Video: Microsurgical Denervation of Rat Spermatic Cord: Safety and Efficacy Data 2013. AUA 2013 San Diego.
- Laudano MA, Osterberg EC, Sheth S, et al. Microsurgical Denervation of Rat Spermatic Cord: Safety and Efficacy Data. BJU Int 2013. [Epub ahead of print]. [PubMed]
- Parekattil SJ, Brahmbhatt JV. Robotic approaches for male infertility and chronic orchialgia microsurgery. Curr Opin Urol 2011;21:493-9. [PubMed]
- Cadeddu JA, Bishoff JT, Chan DY, et al. Laparoscopic testicular denervation for chronic orchalgia. J Urol 1999;162:733-5. [PubMed]
- Ramasamy R, Sterling J, Li PS, et al. Multiphoton imaging and laser ablation of rodent spermatic cord nerves: potential treatment for patients with chronic orchialgia. J Urol 2012;187:733-8. [PubMed]
- Horovitz D, Tjong V, Domes T, et al. Vasectomy reversal provides long-term pain relief for men with the post-vasectomy pain syndrome. J Urol 2012;187:613-7. [PubMed]
- Nangia AK, Myles JL, Thomas AJ JR. Vasectomy reversal for the post-vasectomy pain syndrome: a clinical and histological evaluation. J Urol 2000;164:1939-42. [PubMed]
- Brahmbhatt J, Gudeloglu A, Parekattil S. The efficicacy of robotic-assisted vasectomy reversal for post-vasectomy pain. Fertil Steril 2013;100:S216.
- Costabile RA, Hahn M, McLeod DG. Chronic orchialgia in the pain prone patient: the clinical perspective. J Urol 1991;146:1571-4. [PubMed]


