The role of animal models in the study of varicocele
Introduction
Varicocele is the abnormal enlargement of the testicular pampiniform venous plexus. Varicoceles are present in 15% to 20% of the male population, 35% of men with primary infertility, and in 75% of men with secondary infertility (1,2). It is the most common identifiable and correctible cause of male factor infertility (3). Varicocele has been shown to be associated with abnormal semen analysis parameters (4). Additionally, this pathology has also been shown to be a risk factor for androgen deficiency (5). The pathophysiology of varicocele and its effect of spermatogenesis are poorly understood. The majority of patients with varicocele are asymptomatic and fertile; only 15% to 20% of men with varicocele experience symptoms of discomfort or infertility (6). There continues to be a significant amount of ongoing research to better elucidate this disease pathophysiology (7,8).
Varicocele is almost exclusively found in humans, most likely due to our upright posture, which creates a challenge for opportunities of basic science research. Many animal models have been created, but the most widely used animal model involves the partial occlusion of the left renal vein medial to the insertion of the internal spermatic vein (9). This surgical procedure is able to create a varicocele model because the increased pressure proximal to partial occlusion results in increased pressure in the internal spermatic vein. The increased venous pressure results in dilation of the left internal spermatic vein and the pampiniform venous plexus. This model has been used to successfully create a varicocele that results in increased testicular blood flow, elevated intra-testicular temperature, decreased sperm concentration and motility, and decreased intra-testicular testosterone levels (9-11). While some investigators have had success reproducing the effects of varicocele with this model, other researchers have had varied success with it (12-15).
Partial ligation of the left renal vein
Partial ligation of the left renal vein has been used to model varicocele since its initial description by Saypol et al. (9). The following is a detailed description of the surgical procedure that is used. Most publications have utilized a rodent model, so this description will focus on the surgery in rodents.
The procedure begins with a midline laparotomy incision in order to expose the upper left abdominal quadrant. The left kidney and associated venous vasculature is then exposed by packing the abdominal contents to the right. The left kidney, left renal vein, left adrenal vein, and left spermatic vein should be visualized. Through blunt dissection, the left renal vein is cleared of adherent fat and connective tissue. The left renal vein must be exposed at a position which is medial to the insertion of the left spermatic vein and left adrenal vein (16).
After the left renal vein has been cleared of any tissue, a 4-0 silk suture is used to partially occlude the vein. In order to create a consistent model, the suture is tied down around the vein and an interposed metal wire of 0.85 mm diameter. After the suture has been tied, the metal wire is removed and the vessel will expand as much as the suture will allow. This should result in a consistent external diameter of ~1 mm at the point of partial occlusion. The abdominal contents can then be returned and the rat can be closed. The original lab group reports that about 90% of their surgeries result in the successful formation of a varicocele (16). The varicocele is confirmed by direct measurement of the left spermatic vein at the level of the crossing iliolumbar vein. The spermatic veins in rats are typically 0.15-0.2 mm and reach 1-1.5 mm within 30 days after surgery (11,17).
Rat venous anatomy
Partial occlusion of the left renal vein has produced varying results in creating a varicocele model in rodents. The reason for these inconsistent results is likely due to the variability of the venous anatomy of the rat testis. Failure to identify accessory gonadal veins could lead to unsuccessful creation of a varicocele. Some investigators have observed two separate left gonadal veins, one draining into the left renal vein and another draining into the left common iliac vein (Figure 1) (18,19). In one study, where the vascular anatomy of 31 rats was investigated, it was found that 28 out of the 31 rats had two left gonadal veins as described above (15). In addition, it was found that the left gonadal vein draining into the left common iliac was six times larger in diameter compared to the gonadal vein draining into the left renal vein (3). These findings most likely explain why partial occlusion of left renal vein alone fails to consistently produce a varicocele model.
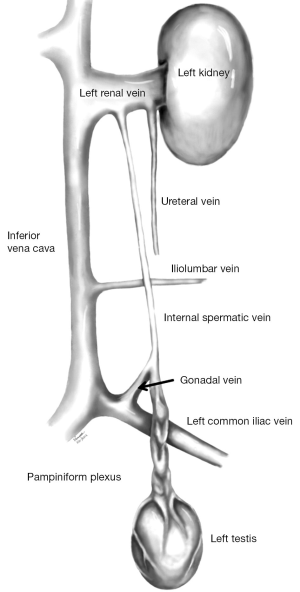
Importance of identifying and ligating collaterals
The use of partial occlusion of the left renal vein to create a rat varicocele model has produced variable results in elucidating the pathophysiology of varicocele. The early findings of this model, which showed that varicocele resulted in increased testicular blood flow, were not reproduced in later experiments (12,13). This partial occlusion model had also shown that varicocele was associated with germ cell apoptosis; however these results were not reproduced consistently in the literature (20,21). These findings, along with the data on the rat venous anatomy, highlight the importance of identifying and ligating the second left gonadal vein which drains into the left common iliac vein.
We have shown that utilizing a microsurgical technique, ligation of the gonadal vein draining into the common iliac vein and partial occlusion of the left renal vein produced a more effective varicocele model in rats compared to partial occlusion alone. Ligation of the gonadal vein draining into the left common iliac vein requires a microsurgical approach because of its small diameter, approximately 0.6 mm, which is significantly smaller than the renal vein measuring approximately 4 mm in diameter. Our results showed that, at five weeks after surgery, the microsurgical approach resulted in a larger spermatic cord diameter in the rats compared to both a sham group and a group where a varicocele was created via partial ligation of the left renal vein alone. At 12 weeks, the microsurgical group had larger spermatic cord diameter, lower sperm motility, worse histology, and lower cauda epididymal sperm concentration compared to both other groups (3). These results are similar to what would be expected to be found clinically in humans with a varicocele. The combined ligation of the left gonadal vein draining into left common iliac vein along with partial occlusion of the left renal vein produced a more effective varicocele model in rats.
Use of a rat model
The use of a rat model has been very important in investigating several hypotheses related to the pathophysiology of varicocele. There are currently several hypotheses related to the impairment of spermatogenesis seen in patients with varicocele, but it appears to be multifactorial with no single disturbance able to completely explain the mechanism of impairment.
One possible cause of impaired spermatogenesis is that varicocele causes apoptosis of germ cells. It has been shown that an experimentally induced varicocele in rats causes apoptosis of germ cells at 7-28 days, and that varicocele repair results in a decrease in the number of apoptotic cells (22-24). It is believed that the apoptosis is due to oxidative stress. One study demonstrated that the rat varicocele model induces reactive oxygen species and apoptosis in the testes (20). It has also been shown that varicocele results in increased expression of the Bax protein, which is a pro-apoptotic protein (25). There continues to be more research to evaluate the role of germ cell apoptosis in rat varicocele model as it appears to play an important role in varicocele pathophysiology (21,26).
Another possible mechanism of impaired spermatogenesis in varicocele is testicular hypoxia. The increased hydrostatic pressure in the venous system is believed to disturb the arterial inflow and cause hypoxia of the testes. The results from the rat varicocele model have been mixed, as it was found that the induced varicocele has resulted in both increased and decreased arterial blood flow (9,13). Additional research into the hypoxia theory has shown that rats with experimentally induced varicocele have increased VEGF expression in the cytoplasm of germ cells (27). The rise in VEGF most likely acts as a compensatory mechanism to increase angiogenesis in response to the hypoxic state (28). Moving forward, the rat model will play an important role in improving our understanding of hypoxia in varicocele.
The most investigated factor related to the pathophysiology of varicocele is oxidative stress. The rat model has been utilized to directly measure unstable elements which cause oxidative stress (13). Additionally, the rat model has been used to further investigate the effect of nitric oxide (NO) in testes with varicocele (29). NO can act as a free radical and result in oxidative stress, but also plays a role in various reproductive functions. NO acts as a vasodilator and helps regulate testicular vasculature. The rat model will continue to play a role in understanding how NO is involved in varicocele pathophysiology (30,31). There has also been study into the role of the anti-oxidant system in fighting the oxidant stress in varicocele patients. The rat model has been used to study the effect of various anti-oxidants as well as measure the expression of antioxidant molecules in response to varicocele (20,32).
Heat stress is one of the oldest theories on the pathophysiology of varicocele. The rat model has been utilized to demonstrate that varicocele results in increased testicular temperature (7). The benefit of the animal model is that testicular temperature is measured directly in the testes as opposed to the scrotal temperature, which is measured in human studies. The heat stress results in impairment of spermatogenesis, as development of sperm is dependent on optimal temperatures (33,34). The rat model has been influential in helping understand how heat stress affects patients with varicocele.
Conclusions
The rodent model is extremely important to researchers investigating the pathophysiology of varicocele. Partial occlusion of the left renal vein has been used with varied success to investigate many aspects of varicocele. Microsurgical ligation of the left gonadal vein draining into the common iliac results in a more effective varicocele model. Further research into varicocele pathophysiology may benefit through using this technique.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mehta A, Goldstein M. Microsurgical varicocelectomy: a review. Asian J Androl 2013;15:56-60. [PubMed]
- Oster J. Varicocele in children and adolescents. An investigation of the incidence among Danish school children. Scand J Urol Nephrol 1971;5:27-32. [PubMed]
- Najari BB, Li PS, Ramasamy R, et al. Microsurgical rat varicocele model. J Urol 2014;191:548-53. [PubMed]
- Russell JK. Varicocele in groups of fertile and subfertile males. Br Med J 1954;1:1231-3. [PubMed]
- Tanrikut C, Goldstein M, Rosoff JS, et al. Varicocele as a risk factor for androgen deficiency and effect of repair. BJU Int 2011;108:1480-4. [PubMed]
- Goldstein M. Surgical management of male infertility. In: Wein A, Novick AC, Partin AW, et al. eds. Campbell-Walsh Urology. Philadelphia, PA: Elsevier Saunders, 2012:648-87.
- Shiraishi K, Matsuyama H, Takihara H. Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. Int J Urol 2012;19:538-50. [PubMed]
- Sigman M. There is more than meets the eye with varicoceles: current and emerging concepts in pathophysiology, management, and study design. Fertil Steril 2011;96:1281-2. [PubMed]
- Saypol DC, Howards SS, Turner TT, et al. Influence of surgically induced varicocele on testicular blood flow, temperature, and histology in adult rats and dogs. J Clin Invest 1981;68:39-45. [PubMed]
- Hurt GS, Howards SS, Turner TT. Repair of experimental varicoceles in the rat. Long-term effects on testicular blood flow and temperature and cauda epididymidal sperm concentration and motility. J Androl 1986;7:271-6. [PubMed]
- Rajfer J, Turner TT, Rivera F, et al. Inhibition of testicular testosterone biosynthesis following experimental varicocele in rats. Biol Reprod 1987;36:933-7. [PubMed]
- Li H, Dubocq F, Jiang Y, et al. Effect of surgically induced varicocele on testicular blood flow and Sertoli cell function. Urology 1999;53:1258-62. [PubMed]
- Hsu HS, Chang LS, Chen MT, et al. Decreased blood flow and defective energy metabolism in the varicocele-bearing testicles of rats. Eur Urol 1994;25:71-5. [PubMed]
- Laven JS, Wensing CJ. Induction of varicocele in the dog: I. Partial ligation of the left renal vein does not induce a varicocele in the dog. J Androl 1989;10:9-16. [PubMed]
- Pascual JA, Lemmi C, Rajfer J. Variability of venous anatomy of rat testis: application to experimental testicular surgery. Microsurgery 1992;13:335-7. [PubMed]
- Turner TT. The study of varicocele through the use of animal models. Hum Reprod Update 2001;7:78-84. [PubMed]
- Turner TT, Howards SS. The venous anatomy of experimental left varicocele: comparison with naturally occurring left varicocele in the human. Fertil Steril 1994;62:869-75. [PubMed]
- Lewis MH, Moffat DB. The venous drainage of the accessory reproductive organs of the rat with special reference to prostatic metabolism. J Reprod Fertil 1975;42:497-502. [PubMed]
- Ohtsuka A. Microvascular architecture of the pampiniform plexus-testicular artery system in the rat: a scanning electron microscope study of corrosion casts. Am J Anat 1984;169:285-93. [PubMed]
- Cam K, Simsek F, Yuksel M, et al. The role of reactive oxygen species and apoptosis in the pathogenesis of varicocele in a rat model and efficiency of vitamin E treatment. Int J Androl 2004;27:228-33. [PubMed]
- Kilinc F, Guvel S, Kayaselcuk F, et al. p53 expression and apoptosis in varicocele in the rat testis. J Urol 2004;172:2475-8. [PubMed]
- Barqawi A, Caruso A, Meacham RB. Experimental varicocele induces testicular germ cell apoptosis in the rat. J Urol 2004;171:501-3. [PubMed]
- Tek M, Cayan S, Yilmaz N, et al. The effect of vascular endothelial growth factor on spermatogenesis and apoptosis in experimentally varicocele-induced adolescent rats. Fertil Steril 2009;91:2247-52. [PubMed]
- Fazlioglu A, Yilmaz I, Mete O, et al. The effect of varicocele repair on experimental varicocele-induced testicular germ cell apoptosis. J Androl 2008;29:29-34. [PubMed]
- Onur R, Semerciöz A, Orhan I, et al. The effects of melatonin and the antioxidant defence system on apoptosis regulator proteins (Bax and Bcl-2) in experimentally induced varicocele. Urol Res 2004;32:204-8. [PubMed]
- Shiratsuchi A, Kawasaki Y, Ikemoto M, et al. Role of class B scavenger receptor type I in phagocytosis of apoptotic rat spermatogenic cells by Sertoli cells. J Biol Chem 1999;274:5901-8. [PubMed]
- Kilinç F, Kayaselcuk F, Aygun C, et al. Experimental varicocele induces hypoxia inducible factor-1alpha, vascular endothelial growth factor expression and angiogenesis in the rat testis. J Urol 2004;172:1188-91. [PubMed]
- Shiraishi K, Naito K. Involvement of vascular endothelial growth factor on spermatogenesis in testis with varicocele. Fertil Steril 2008;90:1313-6. [PubMed]
- De Stefani S, Silingardi V, Micali S, et al. Experimental varicocele in the rat: early evaluation of the nitric oxide levels and histological alterations in the testicular tissue. Andrologia 2005;37:115-8. [PubMed]
- Türker Köksal I, Erdoğru T, Gülkesen H, et al. The potential role of inducible nitric oxide synthase (iNOS) activity in the testicular dysfunction associated with varicocele: an experimental study. Int Urol Nephrol 2004;36:67-72. [PubMed]
- Abbasi M, Alizadeh R, Abolhassani F, et al. Effect of aminoguanidine in sperm DNA fragmentation in varicocelized rats: role of nitric oxide. Reprod Sci 2011;18:545-50. [PubMed]
- Semercioz A, Onur R, Ogras S, et al. Effects of melatonin on testicular tissue nitric oxide level and antioxidant enzyme activities in experimentally induced left varicocele. Neuro Endocrinol Lett 2003;24:86-90. [PubMed]
- Goldstein M, Eid JF. Elevation of intratesticular and scrotal skin surface temperature in men with varicocele. J Urol 1989;142:743-5. [PubMed]
- Yamaguchi M, Sakatoku J, Takihara H. The application of intrascrotal deep body temperature measurement for the noninvasive diagnosis of varicoceles. Fertil Steril 1989;52:295-301. [PubMed]


