Contemporary and future insights into fertility preservation in male cancer patients
Introduction
Advances in anti-cancer therapies and supportive care have led to increased survival rates in cancer patients (1,2), with 5-year survival rates surpassing 70% in children and adolescents (3). This has resulted in a shift of focus from merely lengthening the patient’s lifespan to improving his quality of life (4,5). Fertility preservation is deemed an important aspect of post-treatment quality of life (6,7), especially since anti-cancer therapies are known to have gonadotoxic side effects (1,3). Approximately 15% to 30% of male cancer survivors lose their reproductive potential after treatment (2,6), causing much distress and unhappiness (8-10). Moreover, among those who recover, sperm parameters are likely to be reduced, thus having a negative impact on future fertility (3,11).
The World Health Organization (WHO) defines infertility as “the inability of a sexually active couple (at least three times per month), not using contraception, to achieve pregnancy within one year” (12). Since there is hitherto no cure for infertility, the only way to preserve male fertility is by sperm banking before treatment (4,5). Sperm banking involves sperm retrieval, usually by masturbation, and cryopreservation of the semen sample. Subsequently, the sample can be thawed and used in various assisted reproductive technologies (ART) to achieve pregnancy. Not only is sperm banking non-invasive and safe, but it has also been reported to be very effective (13). This option should therefore be offered to all patients before treatment commences because it is very difficult, if not impossible, to predict who will be rendered permanently sterile post-treatment (14,15). In fact, only 20% to 50% of patients regain their fertility within three years after treatment (16). At present, indications for sperm banking include, but are not limited to, couples who are physically separated (10,17), men with high-risk occupations (18,19), men about to undergo vasectomy (18,20) or potentially gonadotoxic therapies (17,20), sperm donation (21,22), and men with reproductive problems such as anejaculation, severe oligozoospermia and obstructive azoospermia (17,18).
In this review, we will discuss the effect of cancer and its treatment on male fertility, explain how and when sperm banking should take place, and explore current and future alternative strategies that can be employed should sperm be unobtainable due to the inability to masturbate or in cases of azoospermic and pre-pubertal patients. In addition, we will also elaborate on the benefits of sperm banking and possible barriers that may exist, resulting in the low utilization of sperm banking despite its effectiveness (23,24).
Effect of cancer and its treatment on male fertility
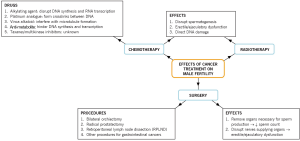
Cancer treatment involves cytotoxic chemotherapy, radiotherapy or radical surgical procedures (19,25), and these have the potential to affect one’s reproductive capacity by impairing spermatogenesis, damaging sperm DNA, and/or causing erectile or ejaculatory dysfunction (3,26). An outline of these effects can be seen in Figure 1. The presence of cancer itself can also impair fertility, and this will be elaborated upon in the following section. Iatrogenic infertility caused by anti-cancer treatment can be temporary or permanent and differs in severity between patients (4). A myriad of factors—pre-existing defects, endocrine disturbances, type of cancer, and dosage and duration of treatment—contribute to the patient’s likelihood of regaining fertility (27,28), making it practically impossible to predict who will be severely affected (11,29). Some patients may regain fertility in a few months’ time while others may take several years, but usually with suboptimal sperm quality (16,30). To date, the most gonadotoxic regimen is the combination of intensive chemotherapy and total body irradiation in bone marrow transplantation procedures (25).
Cancer
The presence of cancer may affect a patient’s fertility potential via different possible mechanisms even before any gonadotoxic treatment is given, and this is summarized in Table 1 (16,36). Men with testicular cancer and Hodgkin’s lymphoma are known to have impaired spermatogenesis and are likely to be oligozoospermic or azoospermic at the time of cancer diagnosis (29). It is also interesting to note that testicular cancer seems to affect the quantity, rather than the quality, of sperm produced (4). A study conducted by O’Flaherty et al. showed that sperm DNA integrity and compaction were compromised in patients with testicular cancer and Hodgkin’s lymphoma before chemotherapy (31). Although the exact mechanism by which cancer affects semen quality is not known (19), it is likely that pre-existing defects due to flawed development of the testes could contribute to testicular cancer (32,33), while abnormal cytokine secretion in the presence of cancer could result in Hodgkin’s lymphoma (34).

Full table
Cancer can also affect spermatogenesis via autoimmune, endocrine or systemic effects (5,17). For instance, testicular germ cell tumours (TGCTs) secrete β-human chorionic gonadotrophins, which depress spermatogenesis, while other tumours spur the production of antisperm antibodies, which could bind to sperm and prevent proper sperm function (35). Moreover, it has been established that the emotional stress experienced by patients who receive a diagnosis of cancer impairs spermatogenesis (5,30). It is therefore evident that cancer itself, prior to any treatment, can affect male fertility.
Chemotherapy
Chemotherapy regimens target proliferating cancer cells and thus, exert their effects on rapidly dividing spermatogonia as well (10,37). These drugs penetrate the blood-testes barrier and interrupt spermatogonial differentiation, hence hindering spermatogenesis (5) and causing oligozoospermia or azoospermia (38,39). Spermatogonial stem cells (SSCs) in the germinal epithelium, though comparatively less active, are also susceptible to permanent damage at higher doses (35,40). More mature germ cells such as spermatocytes and spermatids are less sensitive to chemotherapy because they have stopped dividing, and hence, the effects are only temporary. This may be the reason why some sperm can be found immediately after chemotherapy but gradually decrease in numbers over time (4). Due to their low proliferation rates, Leydig cells are relatively resistant to chemotherapy (35,36). However, there has been some evidence of damage to Leydig cells—increased luteinizing hormone (LH) levels with normal to low testosterone levels (41). In addition to disrupting spermatogenesis, cytotoxic chemotherapy may also contribute to erectile or ejaculatory dysfunction (42) or directly damage sperm DNA (10), resulting in the transmission of defective DNA and abnormal chromosomes to offspring (43).
The severity of damage depends most importantly on the type and total dosage of drug used, as well as the patient’s age (5,42,44). As expected, a higher cumulative dose of drugs given over a longer time period will result in more extensive damage (8). Alkylating agents, such as cyclophosphamide, procarbazine and chlorambucil (45), are the most gonadotoxic drugs because they interfere with DNA synthesis and RNA transcription, thus causing new mutations that may lead to apoptosis (46). Cisplatin, a platinum analogue, is also equally harmful as it causes crosslinks to form between DNA (23,46). Whereas vinca alkaloids interfere with microtubule formation thereby preventing mitosis from occurring, anti-metabolites hinder DNA synthesis and transcription (46). Furthermore, different combinations of drugs are usually given simultaneously in chemotherapeutic regimens, thus making it more challenging to predict their additive effects on reproductive function (27,47). Unfortunately, the effect of newer drugs like the taxanes and multikinase inhibitors are still unknown (19,46), although there have been indications that when used as an adjuvant, taxanes may enable cyclophosphamide to become more toxic (25).
To combat this problem, less gonadotoxic alternatives or lower doses of drugs are used whenever possible (5,48). Also, since chemotherapy targets rapidly proliferating cells, it has been proposed that hormonal manipulation such that the hypothalamus-pituitary-gonadal (HPG) axis is suppressed may cause spermatogenesis to slow down or even stop, hence protecting spermatogonia from the cytotoxic effects of chemotherapeutic drugs (16,49). In studies conducted on rats by Cespedes et al. and Kangasniemi et al., the administration of flutamide and a luteinizing hormone releasing hormone (LHRH) agonist successfully prevented chemotherapy from damaging the germinal epithelium (50,51). However, both Johnson et al. and Fosså et al. had earlier found that the results could not be produced in humans (49,52). As such, hormonal manipulation is not clinically recommended for patients (53).
Radiotherapy
As in chemotherapy, the rapidly dividing cells in the germinal epithelium of the testes are most susceptible to damage and can be permanently destroyed by irradiation (17,48). Radiation doses as low as 0.1-1.2 Gray (Gy) can damage spermatogonia morphologically, hence preventing spermatogenesis from occurring (19,25,37). This can be caused by direct DNA damage or by disturbing the HPG axis (19,35). Exposure to 2 to 3 Gy permanently damages spermatocytes (46), giving rise to azoospermia (19), while doses exceeding 4 Gy generally affect the spermatids and cause an even longer period of azoospermia (5,46). Again, the Leydig cells are more resistant to radiotherapy (48,54) and are only affected by doses above 15 Gy (19,25). In addition, radiation may also play a role in causing erectile dysfunction (39).
The extent of damage depends on various factors such as the total dose, radiation source, field of treatment, and whether it is fractionated (37,54). A higher dose of radiation not only causes more damage, but also increases the time needed for recovery, if at all (5). Radiation damage occurs when radiotherapy is used directly on the testes in testicular cancer (35,36) but is more commonly caused by scatter radiation from radiotherapy directed at the lower abdominal and pelvic regions (38,55). Although lead shields are always used to protect the testes, some scatter radiation is inevitable and can often be extremely gonadotoxic (16,37). Hormonal manipulation via administration of gonadotrophin releasing hormone (GnRH) agonists was used to decrease the rate of spermatogenesis and to reduce the gonadotoxic effects of radiotherapy without success (56).
Surgery
Cancer surgery may decrease the patient’s fertility potential if the organs necessary for reproduction need to be removed or the nerves supplying these organs are disrupted (42). In both cases, sperm counts decrease and erectile or ejaculatory dysfunction occurs (10,29). Bilateral orchiectomy in patients with testicular cancer will result in permanent azoospermia (38,55), whereas radical prostatectomy in patients with prostate cancer can lead to erectile dysfunction (38,39). Retroperitoneal lymph node dissection (RPLND) in testicular cancer patients may damage the autonomic pelvic plexus (4,46), causing retrograde ejaculation or anejaculation (55,57). However, nerve-sparing RPLND can be successfully carried out with the maintenance of normal ejaculatory function post-surgery (23). Other surgical procedures for gastrointestinal cancers in the lower abdomen and perineal regions may also damage nerves and affect ejaculation, resulting in infertility (4).
Process of sperm banking
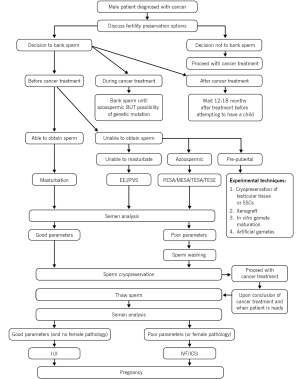
The entire process of sperm banking is complex and involves many steps from the initial cancer diagnosis to semen collection, sperm cryopreservation and eventually, the use of ART to hopefully result in a pregnancy. This is illustrated in Figure 2 and will be further elaborated on in the following sections.
Sperm retrieval
The first step of sperm banking involves collecting semen samples from patients by self-stimulation and masturbation (35,54). Not only is ejaculated sperm of the best quality, but masturbation is also inexpensive and safe (46). However, men must understand that masturbation cannot be carried out with lubrication, and that the entire ejaculate has to be collected in the sterile specimen cups provided (19). This is because the first part of the ejaculate usually contains the most sperm (30,35). Should the patient wish to masturbate in the privacy of his home, he must be instructed to keep the specimen at body temperature and bring it to the laboratory within the next hour (35). After collection, the samples will be left to liquefy at room temperature (22,30,58).
Men are usually encouraged to bank three samples with at least 48 hours of sexual abstinence between samples for maximal concentration of healthy sperm (14,19). However, patients with low sperm concentrations or poorer sperm parameters may be asked to provide more samples in order to pool a sufficient number of sperm for cryopreservation (5,35). In some cases, men who are unable to produce more than one sample due to urgency of treatment or health reasons should still bank their sperm as more advanced techniques are now available that enable a single motile sperm to fertilize an egg (14,55). Finally, for patients who are unable to masturbate or produce viable sperm, other alternative options of sperm retrieval are available, and this will be expanded upon later.
Cryopreservation
After liquefaction and before cryopreservation, the semen samples are analysed and the colour, viscosity, and semen parameters such as sperm count, motility and morphology are recorded (4,22,55). In the event that a sample has poor sperm characteristics, the sample can be enhanced via sperm washing procedures like swim-up or density gradient centrifugation (35,46). Swim-up involves centrifuging the sample and adding culture medium on the top—only motile sperm will be able to swim up into the media. On the other hand, density gradient centrifugation involves centrifuging the semen sample on top of a density gradient, allowing only the motile sperm to move in the direction of the sedimentation gradient and thus forming a pellet at the bottom (10). Both these techniques will allow only healthy, motile sperm to be selected from the seminal plasma and other cellular debris, hence improving the sample’s quality and concentration (10,35,46).
Cryoprotectant is then added to the sample to prevent the formation of ice crystals—inside or outside the cell—during cryopreservation (46). This is because cryoprotectants contain glycerol (and egg yolk), which helps reduce salt levels, decreases osmotic stress, and ultimately maintains the integrity of the sperm cell membrane (22). After equilibration, small aliquots of the mixture are frozen in separate vials for ease of thawing (16,25). Usually, an aliquot is frozen separately, then thawed and analysed again the following day. This ‘test-thaw’ will give a good indication of the quality of that particular semen sample after cryopreservation (4,22,35).
There are two methods for conducting cryopreservation—slow or controlled freezing and vitrification. With slow freezing, the most conventional and commonly used method, freezing medium is slowly added to the sample, which allows dehydration to occur during cooling (10). The vials are immersed in –20 °C for 15 to 30 minutes, then in –79 °C for another 15 to 30 minutes, and finally dipped into liquid nitrogen and stored at –196 °C until they are needed (10,30). These steps can be done manually or in a programmable freezer (10,35). Despite the effectiveness of this method, slow freezing takes up to 1.5 hours and exact protocols differ between labs (22,35).
In contrast, vials are quickly plunged into liquid nitrogen with vitrification (21), and this decreases the protocol time to five minutes (22). Vitrification completely avoids freezing, and consequently the formation of ice crystals, by causing the sample to form an amorphous solid state. However, vitrification is still a novel procedure and is not part of standard clinical practice (10,35).
In the process of cryopreservation, it is inevitable that sperm parameters will be drastically affected, especially that of motility (21,22). It is not uncommon to see a decrease in motility of 25% to 75% after thawing, and the acrosome structure and sperm nuclei may also be damaged (40,44). Furthermore, sperm concentration will be reduced due to dilution with the cryoprotectant (20). As such, in order to attempt a pregnancy, the vials may have to be pooled together to obtain enough viable sperm (5). Nevertheless, semen samples can be stored for up to 50 years in liquid nitrogen with no further damage incurred (59).
Use of sperm in assisted reproductive technologies (ART)
There are three main techniques used to achieve a pregnancy with thawed sperm—intrauterine insemination (IUI), in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). The decision to use a particular technique depends on the number and quality of thawed sperm available, female factors and individual preferences (19,35,40).
IUI is only used when the number of viable sperm post-thaw exceeds five million and the woman has at least one normal fallopian tube (35,60). In this procedure, a thin catheter is used to introduce the semen sample into the woman’s uterus (55). Two inseminations—one given two days prior to ovulation and the other on the day of ovulation itself—are necessary to increase chances of fertilization because sperm can only survive for 48 hours in the female (30). In some cases where ovarian stimulation is also employed, a few IUI cycles are sufficient to achieve pregnancy in 15% to 30% of women (60).
On the other hand, IVF and ICSI are more rigorous techniques that are used when sperm count and/or quality is too low, both of which are commonly seen in cancer patients (3,25), or when the woman has some abnormality in her reproductive tract (38). In both IVF and ICSI, oocytes are removed from the woman and fertilized ex vivo in the laboratory (47). The fertilized egg is allowed to develop into an embryo, which is then returned to the uterus to be implanted (55). In IVF, all motile sperm in the sample are added to the same petri dish as the oocyte in the hopes that fertilization will occur, but ICSI is more complicated (55). ICSI only requires a single viable sperm which will be directly injected into the oocyte (35,60). This circumvents the need for sperm to be of good quality and hence, patients are still encouraged to bank sperm even if there is a very small number of good quality sperm in the sample (9,20,26). In fact, this novel technique can also be employed to enable less mature sperm retrieved from the epididymis or testes to be of reproductive use (60). A study conducted by Chung et al. revealed that 75% of patients, including one with only a few motile spermatozoa, who attempted to father a child post-treatment were successful, thus lending credence to the feasibility of ART with cryopreserved sperm (61).
When sperm banking should occur
According to the American Society of Clinical Oncology (ASCO) [2006], “fertility preservation should be considered as early as possible during treatment planning” (6,62). Ideally, sperm banking should be completed before any potentially gonadotoxic treatment—chemotherapy, radiotherapy or surgery—commences (14,30,63). Patients who start on low-dose treatments should also be advised to bank sperm in case stronger treatment is indicated before their testes are able to fully recover from the initial milder gonadotoxic therapy (7,8). A study conducted by Ginsberg et al. reported that 60% of patients who banked sperm after treatment began were azoospermic (64). This highlights how susceptible the testes are to gonadotoxic treatments—even a single low dose of therapy can severely affect spermatogenesis (19,42). Moreover, providing semen samples before treatment also ensures that sperm DNA already affected by the cancer is not further damaged by therapy (5,20).
If treatment has already begun, patients can still bank their sperm until they become azoospermic (4,14). Although chemotherapy is capable of causing gene mutations, it is not known whether gonadotoxic treatments have any detrimental effect on existing sperm (11). However, animal studies have shown that young produced when the male is undergoing gonadotoxic therapy tend to have many genetic mutations (14). As a precautionary measure, preimplantation genetic diagnosis (PGD) is recommended when reproduction is attempted with sperm obtained during treatment (11). Therefore, it is safer to bank sperm before initiation of gonadotoxic therapy.
In cases where treatment has ended, patients are advised to wait 12 to 18 months before banking sperm or attempting to father a child (19,35,65). This is due to the fact that increased genetic and chromosomal abnormalities have been reported to last up to 18 months post-treatment (14,19,35).
What to do when sperm cannot be obtained
Unable to masturbate
Patients may find it difficult to masturbate due to physical, psychological, cultural or religious reasons (5). Some may be on medications or may feel too ill, stressed or anxious to perform the act (30,42,46) while others may have been brought up in a conservative environment in which masturbation is frowned upon by their culture and/or religion (19,55). Men with ejaculatory dysfunction due to spinal cord injuries, anejaculation or retrograde ejaculation are also unable to produce a semen sample (10,46).
In cases where oral sympathomimetics fail to result in ejaculation (14,19), electro-ejaculation (EEJ), penile vibrostimulation (PVS) or retrieval of sperm from post-coital urine can be carried out to obtain sperm for cryopreservation (27). EEJ is a painful procedure which is performed under general anaesthesia (1,35). A probe is inserted via the anus and placed against the anterior rectal wall. The application of electricity stimulates the prostate gland and seminal vesicles, causing ejaculation (35,42,55). However, EEJ should not be performed when patients are thrombocytopenic or leukopenic as the procedure may give rise to excessive bleeding or infection (4,55). Samples obtained by EEJ usually have a normal concentration but individual sperm are likely to have poor motility, morphology and viability (16,22). These samples are therefore more effectively used in IVF or ICSI rather than IUI (19,35). PVS is simpler and does not require anaesthesia (35). A vibrator is placed against the frenulum of the penis to stimulate the dorsal penile and pudendal nerves and cause ejaculation (35,46). However, this should not be used on boys who have not masturbated previously as it might have psychological side effects. As for patients suffering from retrograde ejaculation, sperm can be obtained from the urine after orgasm (5).
Azoospermia
As mentioned earlier, some patients are azoospermic even before therapy commences because of the effects of cancer (42). Hence, novel techniques have been developed to extract sperm directly from the testes or the epididymis (5,25). For obstructive azoospermia, percutaneous or microsurgical epididymal sperm aspiration (PESA/MESA) can be carried out to obtain sperm from the epididymis. In cases of non-obstructive azoospermia, testicular sperm aspiration or extraction (TESA/TESE) must be carried out under anaesthesia to extract sperm from the testes (3,5,10).
PESA is the easiest technique because no microsurgical equipment or skill is needed. Under local anaesthesia, a 21-gauge butterfly needle is inserted into the caput epididymis and fluid is drawn up into the attached tube. This procedure is repeated until sufficient fluid is collected. However, due to the lack of visual guidance with a microscope, it is easy to inadvertently puncture blood vessels and cause bleeding (10,46). Alternatively, sperm can be extracted from the epididymis via MESA, and this is the preferred technique for patients with obstructive azoospermia (42,46). In MESA, patients are anaesthetized and the procedure is performed with the aid of an operating microscope. This allows for easy identification and directed insertion of the needle into individual epididymis tubules for aspiration of the fluid into a syringe. Again, this is repeated until sufficient fluid is collected (10,46). The fluid collected via PESA or MESA is then analysed and processed in the lab (10). Sufficiently motile and viable sperm can usually be obtained for ART via MESA (14,46).
In TESA, a needle is inserted into three different locations of the testes (upper, centre and lower segments) and the samples are extracted via negative pressure. The extracted fluid is then analysed for sperm in the lab (10). TESE is the more commonly used option for patients with non-obstructive azoospermia (46). After being cut transversely at its centre and at its upper and lower poles, each testis is then lightly squeezed so that some of the tissue bulges outward. The protruding tissue is excised, transferred into culture media, and sent to the lab to extract sperm cells (10,46). This technique can also be used in cases of testicular cancer where the testes have been removed from the body by orchiectomy (5,66). Sperm obtained from TESE can only be used for ART as only certain sections of the testes will contain sufficiently mature sperm (4,10).
A more recent improvement to TESE is microdissection TESE (mTESE). This technique uses microsurgical equipment to identify larger seminiferous tubules that are more likely to be active in spermatogenesis (10,46). Not only does mTESE minimize the loss of testicular tissue (especially in patients with atrophied testes), but it also prevents the accidental puncture of neighbouring blood vessels (10). Moreover, mTESE has been shown to be more effective than regular TESE, obtaining approximately 18% more healthy, viable sperm from the testes (5).
Pre-pubertal
Another subset of patients from whom sperm cannot be extracted from is pre-pubertal boys, whose reproductive systems have not begun spermatogenesis. There is currently no known method of preserving fertility in such patients, but research into various techniques is being carried out (25,54). These include the cryopreservation of testicular tissue or SSCs, xenografting gonadal tissues, in vitro gamete maturation, and the use of artificial gametes (20,25,47). Although results have generally been encouraging, there are still safety, ethical and legal issues that must be addressed before they can be implemented clinically (54).
Cryopreservation of testicular tissue or spermatogonial stem cells (SSCs)
Cryopreservation of testicular tissue or SSCs is the most promising method (5,67). This involves the extraction of testicular tissue, prior to gonadotoxic therapy, to be cryopreserved. When the patient desires to have children, the tissue can be thawed and re-implanted into the patient. Theoretically, SSCs will be recognized by the Sertoli cells and due to their innate ability to self-renew and differentiate, spermatogenesis will resume, restoring gonadal function to the patient (11,37,63). Otherwise, it is expected that by then, advances in technology will find a way to stimulate spermatogenesis from cryopreserved tissue or SSCs (5,35,54).
Furthermore, it was found that cryopreservation of testicular tissue instead of SSCs alone is more likely to preserve the natural function of SSCs. This is because freezing the tissue allows for the SSCs’ surroundings to be preserved as well, hence maintaining the support system they need for proper functioning (5,46). At present, this method has only been successfully carried out in rodents and its use in humans is still experimental (11,19). In these animal models, spermatogenesis was successfully re-initiated when the SSCs were returned to the animal post-treatment (16).
Despite the advantages of this method, there are certain safety and ethical issues that need further consideration. Firstly, there is a possibility that in the process of returning thawed testicular tissue to the cured patient, malignant cells may be transplanted as well (14,47). To circumvent this problem, it may be safer to isolate SSCs from the testicular tissue and only transplant those back into the patient (37,42,68). However, as explained above, this will compromise the ability of the SSCs to produce sperm. Alternatively, the SSCs can be allowed to mature in vitro and only the mature sperm will be used in ART (14,68). Another ethical issue is that the procedure may be too invasive for young patients who may not be of age to give consent for themselves (5,37).
Xenograft
Testicular tissue extracted from a patient may also be transplanted into a host animal to provide a suitable environment for sperm maturation, after which sperm can be extracted for use in IVF or ICSI (35,47). Nagano et al. showed that human SSCs were able to persist and proliferate in mouse testes (69), thus lending support for this method. However, in the use of sperm derived from xenotransplanted SSCs, there is a risk of interspecies transmission of animal DNA, viruses or infections to humans (11,47,54). Therefore, more measures have to be implemented to address these issues before xenotransplantation of gonadal tissue can be considered for clinical use.
In vitro gamete maturation
In vitro maturation (IVM) of SSCs is yet another method that may solve the problem of infertility in pre-pubertal cancer patients. As briefly mentioned above, SSCs extracted from the patient before treatment can be developed into mature sperm cells for IVF or ICSI (46,63). Although this removes the possibility of returning cancer cells to the cured patient, the full intricacies of the support network and environment of the cell culture required for proper maturation are hitherto not understood (37,47). As such, there are concerns about the possibility of improper sperm maturation and subsequent birth defects (5,37).
Artificial gametes
Finally, a newer technique that has been proposed is the creation of artificial gametes (47,70). Geijsen et al. showed that mouse embryonic stem cells can be manipulated in the laboratory to produce sperm cells (71). Nayernia et al. further demonstrated that these sperm cells could be used to produce live offspring. Unfortunately, the offspring in that study were unhealthy and died young (72). In addition to these safety issues, there are ethical concerns regarding the creation of artificial gametes that result in live births (47).
Benefits of sperm banking
Aside from the obvious benefit that sperm banking will preserve the reproductive potential of the patient after cancer treatment and enable him to have biological children (46,73), there are also many positive psychological and emotional effects that will aid the patient in coping with his cancer diagnosis (13,28).
Firstly, it is known that the loss of fertility is a significant cause of anxiety and distress in many patients, especially for those who have yet to complete their family (5,74). Knowing that they have cryopreserved sperm in case they are rendered infertile by treatment will assuage their worries and reduce their fears of being childless (16,20,38). Not only will this help them to cope better, but they will also have a better quality of life after treatment (15,75,76). Additionally, when a physician discusses sperm banking with a patient, this reinforces the belief in long-term survival and reassures the patient that his diagnosis is not fatal (54,73). With the mindset that they will eventually be cured of cancer, patients and their families will be more optimistic and cooperative in the treatment plan too (25). Moreover, in the midst of all the uncertainties and feelings of helplessness, sperm banking gives patients a sense of achievement and control over their lives (77). Therefore, it can be seen that sperm banking has many psychological and emotional benefits, and this is further supported by the fact that 80% of cancer patients who banked their sperm were happy with their decision (4).
Barriers that prevent sperm banking
Despite the relative ease and reliability of sperm banking as a method of preserving fertility potential and its accompanying benefits, it continues to be underutilized among cancer patients. For example, Babb et al. found that only 42 out of 79 patients’ banked sperm, and only half of those who banked sperm proceeded to use their samples in ART (78). Furthermore, in a separate study where questionnaires were given to patients undergoing radical prostatectomies, only 20% of them wanted to bank their sperm although 84% of them felt there was a need for sperm cryopreservation to be offered (24). As such, this section will discuss the barriers that exist from the physician’s and patient’s perspectives as well as more general barriers such as legal issues and the fate of unused sperm.
Physician
One of the reasons that physicians fail to offer the option of sperm banking to patients is lack of time—both during consultation and before treatment begins. During consultation, the oncologist must not only break the news of the cancer diagnosis to the patient, but he must also explain the effects of cancer and the treatment required. With the tight schedule of a busy clinic, physicians have insufficient time to explain and discuss the issue of sperm banking with their patients (79-81). Additionally, there is often a need to start life-saving treatment as soon as possible and hence, physicians are reluctant to advise sperm banking, which will postpone treatment (48,57).
Many physicians also lack knowledge regarding sperm banking and its benefits as well as the facilities that are available for patients. Oncologists may not be aware of the latest developments in fertility techniques and do not have relevant education materials for the patient (9,18). For example, not knowing that only a single motile sperm is required in ICSI may cause physicians to prematurely dismiss a patient’s suitability for sperm banking (38,62,82). Physicians also tend to underestimate the importance of fertility and subsequently leave it out of routine discussions with their patients (13,63). Moreover, oncologists are unaware of the nearest and most convenient sperm banking facilities that they can refer their patients to (57,59,83).
Another barrier faced by physicians is the sensitivity of the issue. Physicians may feel uncomfortable discussing fertility with their patients, especially with adolescents, and therefore choose to completely avoid the topic (13,45,82). Finally, the last barrier elucidated from interviews and surveys is the perceived high cost of sperm banking. Oncologists tend to overestimate the costs of sperm banking and therefore, knowing a patient’s financial situation, may refrain from suggesting the option at all (59,81,83).
Patient
Even if sperm banking is offered, patients may not choose to take the option. The main reason cited is the lack of information (25% of interviewees) that hindered patients from making an informed decision (57,58,79). Even if patients proactively searched the Internet for information, Merrick et al. found that resources had incomplete information and were not reader-friendly in terms of design and language (84). As such, patients had insufficient information regarding the effects of cancer on fertility (18) and were equally uninformed about the procedure (45,83).
The next most common reason is patient uncertainty over the desire for biological children (especially for adolescents) (8), or the need for additional children, especially if they have already completed their families (78,79). Moreover, patients may be anxious that offspring produced from cryopreserved sperm will be abnormal, unhealthy, have birth defects, or have a higher risk of cancer (31,47,85).
Additionally, some patients fear that sperm banking will postpone life-saving cancer treatment (8,84,86), while others may feel too ill to provide a sample (6,8) or too stressed to make such a decision (30,38,77). Sperm banking is also often considered too sensitive for discussion, especially with adolescents (8,27,87), and is deemed to be immoral in certain cultures and religions such as the Evangelicals (8,27,75,88). Finally, some patients are unable to afford the cost of sperm banking (18,30,55), which includes freezing, storage, as well as the type of ART and the number of cycles required to achieve pregnancy (66).
General
It has also been found that very few patients return after gonadotoxic treatment to use their cryopreserved sperm in ART procedures. In a study conducted by Girasole et al., only 3 of the 31 patients had used or were intending to use the sperm (23), while in another study by Menon et al., a mere 2.2% of patients used their sperm (81). Tournaye et al. established the possible reasons for low utilization—recovery of normal reproductive health (41%), death of patient (37%) and no desire for biological children (7%) (11). Other suggested reasons include the fear that offspring will inherit the disease, uncertainty of their prognoses and the cost of ART (16,25,40). Moreover, some patients refuse to dispose of their sperm even when fertility was regained because they wanted it as backup should there be a relapse (73). With such low utilization rates, sperm banking appears to be a waste of resources, hence physicians and patients may feel it is unnecessary (85).
In cases of patient death, it is also difficult to determine if it is legal and/or ethical for surviving relatives to use sperm posthumously to produce a child (69). For now, this is only allowed if unambiguous consent to do so was given by the patient when he was alive (16,27,55). Moreover, laws regarding sperm cryopreservation differ across countries. For example, the United Kingdom and Canada allow donation and cryopreservation of gametes and embryos for young cancer patients, but more conservative countries like Switzerland and Italy have outlawed procedures like gamete donation and embryo freezing (47). As such, complex legislations may hinder the process of sperm banking too.
How to overcome these barriers
Not all barriers are insurmountable. Other members of the oncology team, such as nurses can be trained to discuss fertility options with the patients and counsel and support them where needed (59,73). Additionally, appropriate and useful education materials using various platforms such as pamphlets, videos or interactive media can be designed to help patients make decisions about sperm banking (3,74,83). As previously highlighted, the introduction of ICSI eliminates the need for multiple samples of good quality to be collected (11). Hence, the collection of a single semen sample should not delay treatment significantly (57).
Physicians’ lack of knowledge regarding fertility issues can be improved by education and training (6,55,82). A simple Internet search will identify the locations of nearby sperm banking facilities (6,59). Alternatively, some sperm banks provide cryopreservation kits that can be returned via post after the semen sample is collected at home. This makes the entire process very convenient and comfortable for the patient (6,19). Furthermore, in order to standardize the level of care provided by all physicians, protocols can be implemented for the discussion of sperm banking with patients (2,82). In fact, ASCO’s recently updated guidelines state that all health care providers should be willing to discuss fertility preservation options and “present sperm cryopreservation as the only established fertility preservation method” as other methods are still experimental (53).
Although costs differ among banks, it is projected that the approximate annual cost of storing three ejaculate samples is between $300-$500 (6). A part of it may be covered by insurance, especially if the patient has cancer, and some banks also offer payment plans (6,59). To avoid awkward situations where adolescents are too embarrassed to talk about fertility in front of their parents, separate discussions should be conducted (59,74). Parents should also be advised on how to approach the topic with their children in an appropriate manner (1).
Conclusions and future directions
In conclusion, cancer and its treatment (chemotherapy, radiotherapy and/or surgery) can potentially impair fertility and therefore, it is important to cryopreserve sperm samples before any form of gonadotoxic treatment commences. In cases where sperm cannot be retrieved by the conventional method of masturbation, there are alternative techniques that can be employed such as EEJ, MESA and TESE. With the numerous benefits of sperm banking and its relative ease and convenience, more effort should be put into overcoming the barriers that prevent its utilization so that post-treatment cancer patients can enjoy a better quality of life. Most importantly, there is a need to increase awareness and knowledge of sperm banking among healthcare providers (physicians, nurses and counsellors alike) and the general public as the whole process requires extensive coordination between all parties (89). Also, more research is needed to develop techniques of preserving fertility in adolescent pre-pubertal patients.
Acknowledgements
Funding: This study was supported by funds from the Center for Reproductive Medicine, Cleveland Clinic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- de Vries MC, Bresters D, Engberts DP, et al. Attitudes of physicians and parents towards discussing infertility risks and semen cryopreservation with male adolescents diagnosed with cancer. Pediatr Blood Cancer 2009;53:386-91. [PubMed]
- Johnson MD, Cooper AR, Jungheim ES, et al. Sperm banking for fertility preservation: A 20-year experience. Eur J Obstet Gynecol Reprod Biol 2013;170:177-82. [PubMed]
- Neal MS, Nagel K, Duckworth J, et al. Effectiveness of sperm banking in adolescents and young adults with cancer: a regional experience. Cancer 2007;110:1125-9. [PubMed]
- Williams DH. Sperm banking and the cancer patient. Ther Adv Urol 2010;2:19-34. [PubMed]
- Katz DJ, Kolon TF, Feldman DR, et al. Fertility preservation strategies for male patients with cancer. Nat Rev Urol 2013;10:463-72. [PubMed]
- Reebals JF, Brown R, Buckner EB. Nurse practice issues regarding sperm banking in adolescent male cancer patients. J Pediatr Oncol Nurs 2006;23:182-88. [PubMed]
- Nahata L, Cohen LE, Lehmann LE, et al. Semen analysis in adolescent cancer patients prior to bone marrow transplantation: when is it too late for fertility preservation? Pediatr Blood Cancer 2013;60:129-32. [PubMed]
- Klosky JL, Randolph ME, Navid F, et al. Sperm cryopreservation practices among adolescent cancer patients at risk for infertility. Pediatr Hematol Oncol 2009;26:252-60. [PubMed]
- Rabah DM, Wahdan IH, Merdawy A, et al. Oncologists’ knowledge and practice towards sperm cryopreservation in arabic communities. J Cancer Surviv 2010;4:279-83. [PubMed]
- Gupta S, Agarwal A, Sharma R, et al. Recovery, preparation, storage and utilization of sperm for fertility preservation. J Reprod Stem Cell Biotechnol 2013;1:150-68.
- Tournaye H, Goossens E, Verheyen G, et al. Preserving the reproductive potential of men and boys with cancer: current concepts and future prospects. Hum Reprod Update 2004;10:525-32. [PubMed]
- Hamada AJ, Montgomery B, Agarwal A. Male infertility: a critical review of pharmacologic management. Expert Opin Pharmacother 2012;13:2511-31. [PubMed]
- Gilbert E, Adams A, Mehanna H, et al. Who should be offered sperm banking for fertility preservation? A survey of UK oncologists and haematologists. Ann Oncol 2011;22:1209-14. [PubMed]
- Shin D, Lo KC, Lipshultz LI. Treatment options for the infertile male with cancer. J Natl Cancer Inst Monogr 2005.48-50. [PubMed]
- Pacey A, Merrick H, Arden-Close E, et al. Implications of sperm banking for health-related quality of life up to 1 year after cancer diagnosis. Br J Cancer 2013;108:1004-11. [PubMed]
- Lass A, Akagbosu F, Brinsden P. Sperm banking and assisted reproduction treatment for couples following cancer treatment of the male partner. Hum Reprod Update 2001;7:370-7. [PubMed]
- Oehninger S. Strategies for fertility preservation in female and male cancer survivors. J Soc Gynecol Investig 2005;12:222-31. [PubMed]
- Ping P, Zhu WB, Zhang XZ, et al. Sperm banking for male reproductive preservation: a 6-year retrospective multi-centre study in china. Asian J Androl 2010;12:356-62. [PubMed]
- Williams DH 4th. Fertility preservation in the male with cancer. Curr Urol Rep 2013;14:315-26. [PubMed]
- Menkveld R. Bank your future: Insemination and semen cryopreservation. F, V & V IN OBGYN 2010.68-73.
- Thachil JV, Jewett MA. Preservation techniques for human semen. Fertil Steril 1981;35:546-8. [PubMed]
- Anger JT, Gilbert BR, Goldstein M. Cryopreservation of sperm: indications, methods and results. J Urol 2003;170:1079-84. [PubMed]
- Girasole CR, Cookson MS, Smith JA Jr, et al. Sperm banking: use and outcomes in patients treated for testicular cancer. BJU Int 2007;99:33-6. [PubMed]
- Salonia A, Capogrosso P, Castiglione F, et al. Sperm banking is of key importance in patients with prostate cancer. Fertil Steril 2013;100:367-72.e1.
- Diedrich K, Fauser BC, Devroey P, et al. Cancer and fertility: strategies to preserve fertility. Reprod Biomed Online 2011;22:232-48. [PubMed]
- Hallak J, Kolettis PN, Sekhon VS, et al. Sperm cryopreservation in patients with testicular cancer. Urology 1999;54:894-9. [PubMed]
- Tomlinson MJ, Pacey AA. Practical aspects of sperm banking for cancer patients. Hum Fertil (Camb) 2003;6:100-5. [PubMed]
- Eiser C, Arden-Close E, Morris K, et al. The legacy of sperm banking: how fertility monitoring and disposal of sperm are linked with views of cancer treatment. Hum Reprod 2011;26:2791-8. [PubMed]
- Bizet P, Saias-Magnan J, Jouve E, et al. Sperm cryopreservation before cancer treatment: a 15-year monocentric experience. Reprod Biomed Online 2012;24:321-30. [PubMed]
- Kaempfer SH, Hoffman DJ, Wiley FM. Sperm banking: A reproductive option in cancer therapy. Cancer Nurs 1983;6:31-8. [PubMed]
- O’Flaherty C, Vaisheva F, Hales BF, et al. Characterization of sperm chromatin quality in testicular cancer and hodgkin’s lymphoma patients prior to chemotherapy. Hum Reprod 2008;23:1044-52. [PubMed]
- agarwal A, Allamaneni SS. Disruption of spermatogenesis by the cancer disease process. J Natl Cancer Inst Monogr 2005.9-12.
- Crha I, Ventruba P, Zakova J, et al. Survival and infertility treatment in male cancer patients after sperm banking. Fertil Steril 2009;91:2344-8. [PubMed]
- Rueffer U, Breuer K, Josting A, et al. Male gonadal dysfunction in patients with hodgkin’s disease prior to treatment. Ann Oncol 2001;12:1307-11. [PubMed]
- Wang JH, Muller CH, Lin K. Optimizing fertility preservation for pre- and postpubertal males with cancer. Semin Reprod Med 2013;31:274-85. [PubMed]
- Howell S, Shalet S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol Metab Clin North Am 1998;27:927-43. [PubMed]
- Wyns C, Curaba M, Vanabelle B, et al. Options for fertility preservation in prepubertal boys. Hum Reprod Update 2010;16:312-28. [PubMed]
- Bonetti TC, Pasqualotto FF, Queiroz P, et al. Sperm banking for male cancer patients: social and semen profiles. Int Braz J Urol 2009;35:190-7. [PubMed]
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med 2009;360:902-11. [PubMed]
- Apperley JF, Reddy N. Mechanism and management of treatment-related gonadal failure in recipients of high dose chemoradiotherapy. Blood Rev 1995;9:93-116. [PubMed]
- Howell SJ, Shalet SM. Testicular function following chemotherapy. Hum Reprod Update 2001;7:363-9. [PubMed]
- Levine J. Fertility preservation in children and adolescents with cancer. Minerva Pediatr 2011;63:49-59. [PubMed]
- Howell SJ, Shalet SM. Fertility preservation and management of gonadal failure associated with lymphoma therapy. Curr Oncol Rep 2002;4:443-52. [PubMed]
- Freour T, Mirallie S, Jean M, et al. Sperm banking and assisted reproductive outcome in men with cancer: a 10 years’ experience. Int J Clin Oncol 2012;17:598-603. [PubMed]
- Edge B, Holmes D, Makin G. Sperm banking in adolescent cancer patients. Arch Dis Child. 2006;91:149-52. [PubMed]
- Stahl PJ, Stember DS, Mulhall JP. Options for fertility preservation in men and boys with cancer. Adv Exp Med Biol 2012;732:29-39. [PubMed]
- Pacey AA. Fertility issues in survivors from adolescent cancers. Cancer Treat Rev 2007;33:646-55. [PubMed]
- Hobbie WL, Ogle SK, Ginsberg JP. Fertility concerns for young males undergoing cancer therapy. Semin Oncol Nurs 2009;25:245-50. [PubMed]
- Johnson DH, Linde R, Hainsworth JD, et al. Effect of a luteinizing hormone releasing hormone agonist given during combination chemotherapy on posttherapy fertility in male patients with lymphoma: preliminary observations. Blood 1985;65:832-6. [PubMed]
- Cespedes RD, Peretsman SJ, Thompson IM Jr, et al. Protection of the germinal epithelium in the rat from the cytotoxic effects of chemotherapy by a luteinizing hormone-releasing hormone agonist and antiandrogen therapy. Urology 1995;46:688-91. [PubMed]
- Kangasniemi M, Wilson G, Parchuri N, et al. Rapid protection of rat spermatogenic stem cells against procarbazine by treatment with a gonadotropin-releasing hormone antagonist (nal-glu) and an antiandrogen (flutamide). Endocrinology 1995;136:2881-8. [PubMed]
- Fosså SD, Klepp O, Norman N. Lack of gonadal protection by medroxyprogesterone acetate-induced transient medical castration during chemotherapy for testicular cancer. Br J Urol 1988;62:449-53. [PubMed]
- Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol 2013;31:2500-10. [PubMed]
- Wallace WH, Thomson AB. Preservation of fertility in children treated for cancer. Arch Dis Child 2003;88:493-6. [PubMed]
- Leonard M, Hammelef K, Smith GD. Fertility considerations, counseling, and semen cryopreservation for males prior to the initiation of cancer therapy. Clin J Oncol Nurs 2004;8:127-31, 145. [PubMed]
- Brennemann W, Brensing KA, Leipner N, et al. Attempted protection of spermatogenesis from irradiation in patients with seminoma by D-tryptophan-6 luteinizing hormone releasing hormone. Clin Investig 1994;72:838-42. [PubMed]
- Achille MA, Rosberger Z, Robitaille R, et al. Facilitators and obstacles to sperm banking in young men receiving gonadotoxic chemotherapy for cancer: The perspective of survivors and health care professionals. Hum Reprod 2006;21:3206-16. [PubMed]
- Justice T, Christensen G. Sperm cryopreservation methods. Methods Mol Biol 2013;927:209-15. [PubMed]
- Schover LR, Brey K, Lichtin A, et al. Oncologists’ attitudes and practices regarding banking sperm before cancer treatment. J Clin Oncol 2002;20:1890-7. [PubMed]
- Hirsh A. Male subfertility. BMJ 2003;327:669-72. [PubMed]
- Chung JP, Haines CJ, Kong GW. Sperm cryopreservation for Chinese male cancer patients: a 17-year retrospective analysis in an assisted reproductive unit in Hong Kong. Hong Kong Med J 2013;19:525-30.
- Salonia A, Gallina A, Matloob R, et al. Is sperm banking of interest to patients with nongerm cell urological cancer before potentially fertility damaging treatments? J Urol 2009;182:1101-7. [PubMed]
- Ginsberg JP, Carlson CA, Lin K, et al. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Hum Reprod 2010;25:37-41. [PubMed]
- Ginsberg JP, Ogle SK, Tuchman LK, et al. Sperm banking for adolescent and young adult cancer patients: sperm quality, patient, and parent perspectives. Pediatr Blood Cancer 2008;50:594-8. [PubMed]
- Bahadur G, Ozturk O, Muneer A, et al. Semen quality before and after gonadotoxic treatment. Hum Reprod 2005;20:774-81. [PubMed]
- Nangia AK, Krieg SA, Kim SS. Clinical guidelines for sperm cryopreservation in cancer patients. Fertil Steril 2013;100:1203-9. [PubMed]
- Silva CA, Bonfa E, Ostensen M. Maintenance of fertility in patients with rheumatic diseases needing antiinflammatory and immunosuppressive drugs. Arthritis Care Res (Hoboken) 2010;62:1682-90. [PubMed]
- Goossens E, Van Saen D, Tournaye H. Spermatogonial stem cell preservation and transplantation: From research to clinic. Hum Reprod 2013;28:897-907. [PubMed]
- Nagano M, Patrizio P, Brinster RL. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil Steril 2002;78:1225-33. [PubMed]
- Anderson RA. Fertility preservation techniques: Laboratory and clinical progress and current issues. Reproduction 2008;136:667-9. [PubMed]
- Geijsen N, Horoschak M, Kim K, et al. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 2004;427:148-54. [PubMed]
- Nayernia K, Nolte J, Michelmann HW, et al. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell 2006;11:125-32. [PubMed]
- Pacey AA, Eiser C. Banking sperm is only the first of many decisions for men: What healthcare professionals and men need to know. Hum Fertil (Camb) 2011;14:208-17. [PubMed]
- Huyghe E, Martinetti P, Sui D, et al. Banking on fatherhood: pilot studies of a computerized educational tool on sperm banking before cancer treatment. Psychooncology 2009;18:1011-4. [PubMed]
- Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer 2009;53:281-4. [PubMed]
- Crawshaw M. Male coping with cancer-fertility issues: Putting the ‘social’ into biopsychosocial approaches. Reprod Biomed Online 2013;27:261-70. [PubMed]
- Crawshaw MA, Glaser AW, Hale JP, et al. Young males’ experiences of sperm banking following a cancer diagnosis–a qualitative study. Hum Fertil (Camb) 2008;11:238-45. [PubMed]
- Babb A, Farah N, Lyons C, et al. Uptake and outcome of assisted reproductive techniques in long-term survivors of SCT. Bone Marrow Transplant 2012;47:568-73. [PubMed]
- Schover LR, Brey K, Lichtin A, et al. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol 2002;20:1880-9. [PubMed]
- Rofeim O, Gilbert BR. Normal semen parameters in cancer patients presenting for cryopreservation before gonadotoxic therapy. Fertil Steril 2004;82:505-6. [PubMed]
- Menon S, Rives N, Mousset-Simeon N, et al. Fertility preservation in adolescent males: experience over 22 years at rouen university hospital. Hum Reprod 2009;24:37-44. [PubMed]
- Shnorhavorian M, Kroon L, Jeffries H, et al. Creating a standardized process to offer the standard of care: Continuous process improvement methodology is associated with increased rates of sperm cryopreservation among adolescent and young adult males with cancer. J Pediatr Hematol Oncol 2012;34:e315-9. [PubMed]
- Chapple A, Salinas M, Ziebland S, et al. Fertility issues: The perceptions and experiences of young men recently diagnosed and treated for cancer. J Adolesc Health 2007;40:69-75. [PubMed]
- Merrick H, Wright E, Pacey AA, et al. Finding out about sperm banking: what information is available online for men diagnosed with cancer? Hum Fertil (Camb) 2012;15:121-8. [PubMed]
- Ragni G, Somigliana E, Restelli L, et al. Sperm banking and rate of assisted reproduction treatment: Insights from a 15-year cryopreservation program for male cancer patients. Cancer 2003;97:1624-9. [PubMed]
- Peddie VL, Porter MA, Barbour R, et al. Factors affecting decision making about fertility preservation after cancer diagnosis: a qualitative study. BJOG 2012;119:1049-57. [PubMed]
- Müller J, Sønksen J, Sommer P, et al. Cryopreservation of semen from pubertal boys with cancer. Med Pediatr Oncol 2000;34:191-4. [PubMed]
- Crawshaw MA, Glaser AW, Pacey AA. The use of pornographic materials by adolescent male cancer patients when banking sperm in the UK: legal and ethical dilemmas. Hum Fertil (Camb) 2007;10:159-63. [PubMed]
- Yee S, Buckett W, Campbell S, et al. A national study of the provision of oncology sperm banking services among canadian fertility clinics. Eur J Cancer Care (Engl) 2013;22:440-9. [PubMed]


