Robotic microsurgery in male infertility and urology—taking robotics to the next level
Introduction
The contribution of male factor infertility is approximately 50% in infertile couples (1). Although about one third of male factor infertility is idiopathic, a significant number of infertile male patients have surgically correctable disorders such as a varicocele or vasal obstruction, either congenital or iatrogenic (2). Microsurgical reconstructive approaches are the standard of care in these anomalies of the male reproductive system (3). Microsurgery also plays a role as a diagnostic and therapeutic modality for men with non-obstructive azoospermia (NOA) (4).
Human evolution and our ability to develop and utilize new tools is an ongoing process that has progressed over billions of years. This innate ability or desire in our minds to progress is being described as a new framework called evolutionary psychology (5). Evolutionary psychology supports the hypothesis that knowledge acquisition and the adaptive regulation of behavior is a dynamic and progressive process. Thus our development and use of new technology in surgical procedures is likely to progress in a similar fashion.
Similar to the acceptance of robotic assisted laparoscopic surgery for a number of urological conditions, the use of robotic assisted microsurgery is in its infancy and may progress as cheaper, cost effective robotic microsurgical platforms become more accessible.
This article covers the current state of the art in robotic assisted microsurgical procedures in male infertility and urology: microsurgical vasectomy reversal, intra-abdominal vasovasostomy (for patient with prior inguinal hernia related inguinal vasal obstruction), microsurgical subinguinal varicocelectomy, microsurgical testicular sperm extraction (MicroTESE) and targeted microsurgical denervation of the spermatic cord for chronic orchialgia (6). Some novel adjunctive tools that assist in these procedures will also be presented.
The evolution of microsurgery in urology
Since the 1970s, there have been significant developments in urologic microsurgery. Silber introduced the operative microscope for urological procedures (7). Belker reported improved outcomes for vasovasostomy due to increased optical magnification with the microscope (8). The use of the microscope did bring with it the need for a stable platform for the surgeon to either stand or sit at the patient bedside while operating under the microscope. This led to the development of unique chairs, supportive armrests and other devices to help support and stabilize the surgeon’s arms at the bedside. This was a new skill set compared to operating with simple optical loupes.
Abbou et al. first reported the use of robotic assisted laparoscopic radical prostatectomy in 2000 to help alleviate some of the surgeon fatigue and technical limitation issues of laparoscopy (9). As robotic assisted laparoscopic procedures became more widespread, the potential for using this platform for robotic assisted microsurgery was also explored in animal studies (10,11). These studies were then followed by early human trials (12-14). Further exploration of the use of this platform in larger studies are ongoing (6).
The da Vinci surgical system (Intuitive Surgical Inc., Sunnyvale, CA, USA) is currently the only commercially available FDA approved system for urological procedures. As of October 2013, more than 2,500 systems have been installed in over 2,000 hospitals worldwide. The latest version of the system features a high-resolution 3-D view (with up-to 10-15× magnification) and three robotic instrument arms. These instruments are capable of six degrees of freedom, thus mimicking the surgeon’s hand, wrist and finger movements with 180° articulation and 540° rotation. It allows the surgeon to rotate an instrument to a greater degree than the human hand and provides some new maneuvering capability in microsurgery. The robotic instrument arms also eliminate physiologic tremors and provide motion scaling. The surgeon console provides a comfortable, ergonomic interphase to minimize surgeon fatigue. Having an extra third robotic instrument arm also allows the surgeon to control one additional instrument and be less reliant on the surgical bedside assistant. This extra arm can also hold adjunctive imaging or sensing tools such as a Doppler ultrasound probe and provide additional real-time inputs to aid the surgeon (15).
The surgeon console is also supported by specialized imaging software called TilePro (Intuitive Surgical Inc., Sunnyvale, CA, USA). This allows the surgeon to have up to three simultaneous real-time visual inputs in the console. These additional simultaneous image inputs could be a real-time Doppler ultrasound image to identify vascular structures and/or a real-time view from an optical phase-contrast microscope to evaluate seminal fluid or testicular tissue for any sperm (6). This multi-view ability provides the surgeon with an aircraft cockpit like experience with multi-simultaneous imaging/sensing data. Well experienced micro-surgeons (Goldstein M: an expert microsurgeon and flight surgeon) have previously commented on the similarity of the hand-eye coordination among performing microsurgery and flying high-performance aircraft between 50 and 500 feet at 500 knots (16). It would only seem intuitive, that robotic assisted microsurgery would only further bridge these similarities.
Robotics in the management of obstructive azoospermia (OA)
OA is defined as the absence of any spermatozoa (or sperm precursors) either in the semen or post-ejaculate urine due to a blockage anywhere along the male reproductive tract (2,17). OA can account for up to 40% of patients who have azoospermia (18). Obstruction could be due to vasectomy (most common cause), congenital (congenital absence of vas deferens), infection (epididymitis) or iatrogenic (due to scrotal, inguinal or transurethral surgery, for example, inguinal hernia repair) (19). Male infertility patients with OA have two options when considering treatment: (I) surgical correction or reconstruction of the obstruction, or (II) testicular or epididymal sperm retrieval with the use of assisted reproductive techniques (ART) to achieve a pregnancy (17). This paper will further describe the surgical reconstructive options for these patients and some novel robotic assisted options that have evolved.
Microsurgical vasectomy reversal is an option if the obstruction is in the vas deferens or epididymis (19). Success rates for microsurgical vasectomy reversal have been reported as high as 98% if bilateral vasovasostomy is performed (20). Recently, Chan et al. reported 92% patency rates with microsurgical vasoepididymostomy (21). These procedures are technically challenging and achieving great outcomes requires extensive clinical microsurgical experience and rigorous microsurgical training (16,22). Microsurgical procedures demand refined surgical skills including precise hand-eye coordination, fine dexterity and minimal hand tremor (23).
The skill demands on the microsurgeon and the pursuit of further exploring adjunctive tools in microsurgery initially led to the concept of robotic assisted microsurgery in 2003-2004. Robotic assistance in microsurgery was initially attempted in microsurgical vasectomy reversal procedures in animal models. The initial ex vivo and animal trials demonstrated advantages with robotic assistance such as: elimination of tremor, less operative duration and less sperm granuloma formation at the anastomosis site, with comparable patency rates (10,11,24). The first human case series in 2004 by Fleming et al. suggested greater ease and precision of suture placement and a shorter learning curve (12). The feasibility of robotic assisted vasectomy reversal was then further supported by another case report by De Naeyer et al. in 2007 (25).
Santomauro et al. recently showed the feasibility and effectiveness of different robotic assisted vasectomy reversal techniques (the one layer and two layer techniques) (26). They also compared the mean console time for experienced staff surgeons versus urology residents. Although the mean console time was 38 minutes for experienced staff and 54 minutes for residents, this was not statistically significant. This group reported a 93% patency rate (sperm in the ejaculate in twelve out of thirteen patients). This is a very interesting study in that it illustrates a fairly rapid learning curve for residents performing this technique and excellent outcomes comparable to some very experienced microsurgical series.
Our group has also compared robotic assisted microsurgical vasectomy reversal and standard microsurgical vasectomy reversal (27). Patency rates were higher for the robotic assisted microsurgical vasovasostomy (96%) versus the pure microsurgical vasovasostomy (80%), with a P value of 0.002. These included all reversals done by the same microsurgeon in his practice after completing fellowship training during the study period. Our group also documented significantly reduced operative duration with the robot versus pure microsurgery for both vasovasostomy (97 vs. 120 minutes, P=0.0003) and vasoepididymostomy (120 vs. 150 minutes, P=0.0008).
Technique
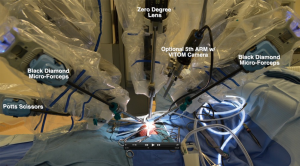
In robotic assisted microsurgery, the preparation of the anastomosis site and the anastomosis technique for vasectomy reversal is identical to the pure microsurgical technique. The difference compared to pure microsurgery is that the microsurgical anastomosis is performed using robotic micro EndoWrist instruments (Intuitive Surgical Inc., Sunnyvale, CA, USA). We utilize Black Diamond micro forceps in the left and right arms, and a Potts scissor in the fourth robotic instrument arm (Figure 1). These instrument arms are all controlled via finger manipulators in the surgeon console. The robot is docked from the right side of the patient and the patient is placed in the supine position. Our technique consists of a double layer anastomosis with five to seven double arm 10-0 nylon sutures for the inner mucosal lumen anastomosis and seven to nine 9-0 nylon sutures for the vasal muscularis anastomosis. We also place a 3-0 Prolene suture to re-approximate the adventitia and create a tension free anastomosis. Robotic vasoepididymostomy (RAVE) is similarly performed with the longitudinal intussusception technique (6,21). Two double arm 10-0 nylon sutures are utilized to involute the epididymal tubule lumen into the vasal mucosal lumen. The epididymal tunica is then circumferentially re-approximated to the vasal muscularis layer with five to six 9-0 Nylon sutures.
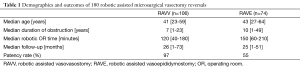
As of October 2013, we have performed 180 robotic assisted vasectomy reversals. Patients’ demographics, surgical and post-surgical outcomes are summarized in Table 1. Patients who have more than one million sperm per ejaculate any time after surgery is defined as patent.
Full table
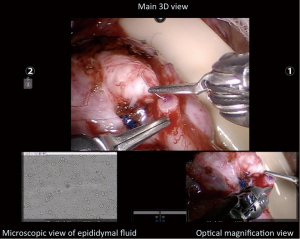
The three-dimensional, high-definition magnified view in the surgeon console with six degrees of rotational and articulation ability of the robotic micro instruments provides excellent hand-eye coordination and dexterity for the microsurgeon. Furthermore, elimination of tremor and a stable, ergonomic platform allows the microsurgeon to perform complex maneuvers in a very comfortable setting. The additional fourth instrument arm also eliminates the need for a skilled microsurgical assistant. Finally, the TilePro software in the surgeon console allows for simultaneous viewing of up to three image inputs (Figure 2). Figure 2 illustrates the typical surgeon’s view in a RAVE: there are two additional simultaneous image views: one view from the optical phase contrast microscope as the OR technician is assessing the fluid from the epididymal tubule for sperm and another image from the video telescope operating monitor (VITOM) optical magnification camera system (Karl Storz Inc., Tuttlingen, Germany). Having the capability of simultaneously evaluating the epididymal fluid while operating, improves the operative efficiency of the microsurgeon—the surgeon (15). The additional optical magnification of the VITOM camera system allows for additional optical magnification for the surgeon (this provides up to 15-20× magnification). This five arm robotic approach with the VITOM camera system also obviates the need for the surgeon to zoom in and zoom out during the procedure since each camera is set to different focal lengths—the 3D digital camera providing a more global view at 10-15× and the VITOM providing a high magnification optical view at 15-20× (15).
The use of robotic assistance for vasectomy reversal also creates some novel opportunities to take microsurgery to places that were once considered difficult to access, such as the intra-abdominal lower pelvis. Najari et al. recently described robotic assisted laparoscopic mobilization of the intra-abdominal vas deferens to allow for an external inguinal tension free anastomosis with standard microsurgical vasovasostomy in a patient who had vasal obstruction from a hernia repair (28). Trost et al. have taken this one step further, and have described the first bilateral intra-corporeal robotic microsurgical vasovasostomy in a patient who had bilateral vasal obstruction due to prior bilateral inguinal hernia repairs (29). This is truly a benefit for these patients, since the vasal reconstruction can now be performed intra-abdominally with very small inguinal incisions to mobilize the external vas deferens and then tunnel these proximal segments into the pelvis for the microsurgical reconstruction intra-corporeal. This technique also allows for a tension free anastomosis since it is usually difficult to mobilize very long intra-abdominal vas segments out to the scrotum, and it is technically easier to bring the testicular vas from the inguinal area into the pelvis.
Robotic assisted vasectomy reversal also provides for a convenient training tool for residents and fellows (30). Some of the newer robotic platforms allow for dual surgeon consoles to allow an experienced surgeon to operate with a trainee simultaneously.
Robotics in the management of non-obstructive azoospermia (NOA)
The diagnosis of testicular spermatogenesis failure or NOA is considered in the presence of azoospermia with no discrete blockage in the male reproductive tract. Failure of spermatogenesis can be due directly from intrinsic testicular deficiency (primary) or secondary to endocrine disorders (31). Although NOA can be differentiated from OA with clinical findings such as a hormone profile and physical exam, the definitive diagnosis can only be establish on histologic examination of testicular tissue. The current standard is that testicular biopsy not be used only to establish the diagnosis, but it should also be performed to retrieve sperm for use in ART (2).
There are various sperm retrieval techniques including needle aspiration from the epididymis or testis, percutaneous or open testicular biopsy and microsurgical testicular sperm extraction (micro-TESE). Micro-TESE provides the highest success in sperm retrieval (4).
The safety and effectiveness of robotic assisted micro-TESE (ROTESE) is being assessed and preliminary outcomes seem comparable to the pure microsurgical approach (6,15). The use of adjunctive simultaneous imaging technology in the future to better detect sperm during micro-TESE may create a role for the robotic micro-TESE platform.
Technique
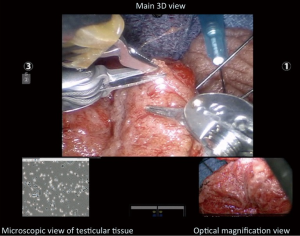
The surgical technique for ROTESE is similar to the standard microsurgical approach except the robot is utilized for the evaluation of the seminiferous tubules. Once the tunica is incised, the robotic platform is docked from the patient’s right side (patient in supine position). The Black Diamond micro forceps are used in the right arm, the micro-bipolar forceps in the left arm and the Potts scissors in the fourth arm (Figure 3). Figure 3 illustrates the surgeon view in the surgeon console during the ROTESE procedure. The primary 3D view (middle top) provides digital magnification at 10-15×, the left lower view provides simultaneous real-time imaging from the andrologist/embryologist’s phase contrast microscope as they are assessing the testicular tissue and the right lower image provides a 15-20× optical magnification view of the tubules.
Recently, utilization of a probe-based confocal laser endomicroscope for in situ localization of viable spermatozoa has been shown (32). Confocal laser endomicroscopy was able to identify fluorescent-labeled spermatozoa, spermatocytes and spermatogonia in the seminiferous tubules. Another group has also shown the utility of multi-photon microscopy in detecting spermatozoa in testicular tissue (33). Once these adjunctive imaging tools are more readily available for clinical use, they would be a natural fit for the robotic platform and could be incorporated rather easily and manipulated using the additional fourth arm.
Robotics in the management of varicocele
The presence of a varicocele leads to a two-fold increase in the likelihood of having abnormal semen analysis parameters in men seeking infertility treatment (34). Varicocelectomy can lead to significant improvements in semen analysis parameters and a recent meta-analysis showed significant improvements in sperm count and motility regardless of the varicocelectomy technique (35). The sub-inguinal microsurgical varicocelectomy approach has higher spontaneous pregnancy rates, lower postoperative recurrence and lower complication rates when compared to other techniques (36).
The initial report showing the safety, feasibility and comparable outcomes of robotic assistance in sub-inguinal microsurgical varicocelectomy was published by Shu et al. (13) in 2008. This group described elimination of tremor and the stable, ergonomic platform as benefits of the robotic approach. Our group further explored this technique in a canine spermatic cord model (prospective randomized control trial) and showed a significantly faster operative time with robotic assisted microsurgical varicocelectomy (RAVx) when compared to the standard microsurgical approach (15). Recently, Mechlin and McCullough reported preliminary results of their initial experience with RAVx (37). They found no difference in terms of operative time when comparing RAVx to standard microsurgical varicocelectomy. However, they noted that there was a learning curve to RAVx and that their robotic operative time was progressively diminishing in their more recent cases.
Technique
A sub-inguinal approach is utilized to access the spermatic cord beyond the external inguinal ring. The cord is then brought up to the skin and held in place using a tongue blade platform. The cremasteric muscle layer is then separated and dilated veins are found and ligated with 3-0 silk ties using robotic microsurgical instruments (6). The Black diamond micro-forceps are used in the right arm, the micro-bipolar forceps in the left arm and the curved monopolar scissors in the fourth arm. Previous studies have shown that 75% of patients have multiple testicular arteries in the spermatic cord at the subinguinal level (38). 95% of these arteries are surrounded by adherent veins (38). Thus, to avoid any inadvertent injury to the testicular arteries during the varicocelectomy, we routinely utilize a micro Doppler ultrasound probes to assess the location of the arteries and veins. The use of the robotic platform allows us to use this probe real-time with the additional arm to sense flow in the artery while performing vein ligation simultaneously with the other two arms. There are two micro Doppler ultrasound probes available currently. VTI (Vascular Technology Inc., Nashua, NH, USA) provides an easy to use, audible, disposable micro Doppler probe (Figure 4). Aloka (Hitachi-Aloka, Tokyo, Japan) has a micro-ultrasound Doppler probe (Figure 4) that provides full depth ultrasound imaging of the spermatic cord with Doppler flow sensing as well. The output from this probe can be sent directly to the surgeon console to provide real-time simultaneous imaging while the surgeon is operating.
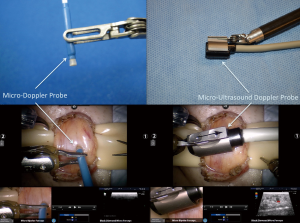
Our group has performed 238 RAVx procedures from June 2008 to October 2013. The median robotic OR duration was 20 minutes. Seventy-six percent of patients with oligospermia had a significant improvement in sperm count and/or motility and 28% of patients with azoospermia were converted to oligospermia. Ninety-two percent of patients with testicular pain had a significant reduction in their pain scores. Eighty-four percent of the patients, who had testicular pain, also had robotic assisted targeted microsurgical denervation of the spermatic cord procedure at the same time as the varicocelectomy.
Robotics in the management of chronic groin or scrotal content pain
Chronic groin or scrotal content pain (CGSCP) is defined as a discomfort or pain lasting more than three months in the groin, scrotum, testis or epididymis (39,40). Although some patients have a previous history of vasectomy, inguinal hernia repair, infection, and trauma, the exact mechanism of CGSCP is still unknown. There is no demonstrable etiological factor (idiopathic pain) in approximately 40 percent of the patients (39-43).
The management of CGSCP begins with medical treatment including analgesics, anti-inflammatories and antibiotics. Neurotransmitter inhibitors (such as gabapentin) may also provide benefit especially in patients who have neuropathic pain (40). Surgical treatment modalities such as microsurgical denervation of the spermatic cord (MDSC), epididymectomy and orchiectomy are employed only when all non-invasive medical options fail. Among available surgical options, MDSC appears to offer high success rates with the least amount of extirpative surgery (43,44). Our group recently explored the anatomic basis of the MDSC procedure in men who had CGSCP. The study illustrated a unique distribution of Wallerian Degeneration in nerve bundles located at three specific areas in the spermatic cord: the cremasteric muscle area, the peri-vasal tissues and the posterior peri-arterial/lipomatous tissues (45). Wallerian degeneration has been shown to cause chronic pain in peripheral nerves in other regions such as the arm or leg. This has helped us in developing a targeted approach to MDSC to further minimize or refine the amount of ligation performed during the procedure. The goal is just to ligate tissues in the spermatic cord that are likely to harbor the nerves with Wallerian degeneration, while preserving the bulk of the cord.
Laudano et al. recent performed an animal study (rat model) to illustrate the safety and efficacy of the MDSC procedure (46). They showed a significant decrease in median number of nerve fibers remaining around the vas deferens after the MDSC procedure compared to the sham control (MDSC =3.5 nerves vs. sham =15.5 nerves, P=0.003). The animals were survived and then reassessed after two months. No deleterious effects on spermatogenesis or vas patency were seen in the experimental groups when compared with the sham rats.
Technique
Robotic assisted targeted microsurgical denervation (RTMDSC) was first described by our group in 2010 (14). Our technique differs from standard MDSC described by Levine (47) in that in includes a more conservative targeted ligation of tissues in the cord and also involves the use of the robotic platform. The standard MDSC technique involves ligation of the bulk of the spermatic cord except for the testicular arteries, vas deferens (in cases of previous vasectomy—this is ligated as well) and lymphatics. RTMDSC focuses and involves ligation of only three specific areas in the spermatic cord where we illustrated nerves with Wallerian Degeneration in our previous studies (6,45,47). The bulk of the internal spermatic cord sheath and internal cord are preserved. A subinguinal approach is utilized. The spermatic cord is brought up to the skin. Medial, posterior and lateral cauterization of the peri-cord tissues is performed to ligate branches of the ilio-inguinal and genito-femoral nerves in the external inguinal ring area. The spermatic cord is now secured on a tongue-blade platform. The robot is now brought in from the right side of the patient (supine position). A black diamond micro-forceps is used in the right arm, micro-bipolar forceps in the left arm and a curved monopolar scissor is used in the fourth arm. The cremasteric muscle layer is carefully ligated using the curved monopolar scissors, the peri-vasal sheath is also carefully ligated while preserving the deferential artery and the vas deferens, and finally the posterior lipomatous/peri-arterial tissues are ligated. The internal spermatic sheath and inner cord are completely preserved.
After standard MDSC, there are some nerve fibers still left behind in the peri-vasal tissues as shown by Laudano et al. (46). Since this peri-vasal tissue area was shown in our previous study to be the one area with the highest density of nerves with Wallerian degeneration, we also perform hydrodissection of the vas deferens to further ligate any small diameter nerve fibers that may be in this tissue while preserving the vasa-vasorum (vascular plexus on the vas deferens). Previous animal studies have shown the efficacy of this technology for this application (48).
In order to decrease the risk of neuroma formation and scarring around the spermatic cord, we also wrap the cord with a bio-inert matrix (AxoGuard, Axogen, Gainesville, FL, USA) at the completion of the RTMDSC (49).
A recent modification of our technique has been the utilization of the flexible fiber-optic CO2 laser (OmniGuide, Cambridge, MA, USA) (Figure 5) to perform the ligation/ablation of the three key tissues mentioned above during RTMDSC. We recently performed a comparative study on a fresh human cadaver to assess the degree of peripheral thermal injury or damage to surrounding tissues when utilizing monopolar cautery versus CO2 laser ablation (50). The study illustrated a significantly decreased amount of peripheral thermal damage with CO2 energy compared to standard monopolar electrocautery (50). Thus, we have now instituted the use of this laser for the tissue ablation during RTMDSC for a more precise and controlled dissection.

We have performed 546 RTMDSC procedures from October 2008 to October 2013 (546 spermatic cords in 463 patients). The median robotic operative duration was 15 minutes (range, 10-150 minutes). Pre-operative and post-operative pain was assessed using an externally validated pain assessment tool: PIQ-6 (QualityMetric Inc., Lincoln, RI, USA). 84.8% (463/546 cases) had a significant reduction (>50%) in pain after the RTMDSC procedure by six months postoperative. Within this group, 70.5% (385 cases) had complete resolution of pain and 14.3% (78 cases) had a greater than fifty percent reduction in their pain score. RTMDSC did not relieve any pain in 15.2% [83] testicular units. The complications were limited to one testicular ischemia, two testicular artery injuries (repaired intra-op with no long term sequel), one vasal injury (repaired intra-op with no long term sequel), ten hematomas, three seromas, and five wound infections.
Use of the robot for RTMDSC procedures offers some advantages to microsurgeons in terms of providing an additional instrument arm obviating the need for a skilled microsurgical assistant, allows for the easy integration of various imaging and sensing modalities at the surgeon console to improve surgical efficiency, and most importantly provides an ergonomic platform that eliminates tremor and reduced surgeon fatigue. The use of robotics for microsurgery at our institution has allowed us to improve our surgical throughput (decreased operative duration and ability to perform more procedures in the same amount of time) and thus has reduced the out-of-pocket costs to the patients to levels comparable to standard microsurgery. The caveat to this is of course the need for high volume to create such a situation. However, as the prices of robotic platforms fall in the future and as more platforms are developed, the use of these robots is likely to become less cost-prohibitive.
Vasectomy reversal is also a viable surgical treatment option in patients who have post-vasectomy pain (51). The efficacy of robotic assisted vasectomy reversal for post-vasectomy pain has been explored by our group (52). A total of 24 robotic vasectomy reversals [22 robotic assisted vasovasostomy (RAVV) and 2 RAVE] were performed in patients who had post-vasectomy pain. Eighty-five percent of the patients had a significant reduction in pain (>50% reduction in pain score). However, larger studies with long-term follow-up are needed to assess its clinical utility for this indication.
Robotic microsurgery training
In standard microsurgery, it has been well established that training in a microsurgery laboratory improves the surgeon’s confidence, reduces stress and reduces the operating duration (23). Similarly, it is likely that training on a robotics platform will likely lead to similar benefits for robotic microsurgery.
A classification of robotic microsurgical training models and examples for each model are summarized in Table 2 (53). Initially simulators and practicing with non-living non-biologic materials are recommended to become familiar with the robotic system (53).

Full table
Our group performed a study recently to assess how skills may be acquired for robotic microsurgery using non-traditional approaches (54). In this study, one group of robot naïve participants trained for robotic microsurgery by building Lego® structures with the robot using all three arms. This group was compared to another similar group that trained by practicing repetitive robotic microsurgical anastomosis on a synthetic vas deferens (Syndaver®) model. When these two groups were tested before and after the training sessions (by performing an anastomosis on the synthetic vas), both groups demonstrated a significant similar improvement in their robot skills. This opens up a whole avenue of potential exercises and tasks that may help improve a microsurgeon’s robotic skills indirectly.
A progression on to non-living biological models is then recommended such as vas deferens segments from major radical cystectomy specimens (53). Ruggiero has also shown the feasibility of using earthworms for robotic microsurgery training (55). Finally, performing robotic microsurgical vascular anastomosis on living models such as rat and rabbit are recommended (53).
Whether one can transfer previous microsurgery skills to robotic microsurgery is another concern in training. Karamanoukian et al. conducted a study (56) where they compared microsurgical vascular anastomosis of fully trained vascular surgeons and mid-level surgical residents on the robotic system. They found no significant difference between the groups and so previous microsurgical experience did not seem to affect the learning curves on the robot. Ramdhian et al. compared learning curves of robotic anastomosis and standard microsurgical anastomosis on a robot and microsurgical naïve surgeon (57). Although the learning curve for standard microsurgical anastomosis was faster than for robotic anastomosis, the difference was not statistically significant (57).
One additional area of importance for robotic assisted procedures is the training and experience of the operating room staff. Since robotic procedures entail a team effort, training of all team members including the anesthesiologist, circulator, scrub nurse and surgical assistant are crucial for the effective performance of the team (58).
Robotics in the future
Cost and the lack of tactile feedback are some challenges of the current commercially available da Vinci robotic system. However, as technology evolves, it is likely that these limitations will be overcome with newer and competing platforms.
Recently, a video illustrating the working principles of novel robotic system called SPORT-Single Port Orifice Robotic Technology (Titan Medical Inc., Toronto, Ontario, Canada) was released (59). This video shows the deployment of a 3D high definition camera and two flexible robotic instrument arms through a single port platform. This same group is also working on the development of a multi-port surgical system called Amadeus, which features tactile feedback. Raven is another surgical robot from the University of Washington funded by the Department of Defense, which focuses on telesurgery for military applications (60,61). The Sofie (Surgeons Operating Force-feedback Interface Eindhoven) is another robot from Eindhoven University of Technology in the Netherland that is being developed with tactile feedback (61).
All these technological advances may provide more tools and options to allow surgeons to shift from standard microsurgery to robotic assisted microsurgery assistance in the future.
Conclusions
Robotic assistance for microsurgical procedures in male infertility and urology appears to be a possible adjunct to standard microsurgery. It has several advantages including elimination of tremor, multi-view magnification, additional instrument arms, and enhanced dexterity with articulating instrument arms. The current literature supports that these procedures appear to be safe and feasible. However, larger, prospective studies are needed to demonstrate the clinical benefits over standard microsurgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod 1991;6:811-6. [PubMed]
- Jungwirth A, Giwercman A, Tournaye H, et al. European Association of Urology guidelines on Male Infertility: the 2012 update. Eur Urol 2012;62:324-32. [PubMed]
- Goldstein M, Tanrikut C. Microsurgical management of male infertility. Nat Clin Pract Urol 2006;3:381-91. [PubMed]
- Schlegel PN. Nonobstructive azoospermia: a revolutionary surgical approach and results. Semin Reprod Med 2009;27:165-70. [PubMed]
- Cosmides L, Tooby J. Evolutionary psychology: new perspectives on cognition and motivation. Annu Rev Psychol 2013;64:201-29. [PubMed]
- Parekattil SJ, Gudeloglu A. Robotic assisted andrological surgery. Asian J Androl 2013;15:67-74. [PubMed]
- Silber SJ. Microsurgery in clinical urology. Urology 1975;6:150-3. [PubMed]
- Belker AM. Urologic microsurgery--current perspectives: I. Vasovasostomy. Urology 1979;14:325-9. [PubMed]
- Abbou CC, Hoznek A, Salomon L, et al. Remote laparoscopic radical prostatectomy carried out with a robot. Report of a case. Prog Urol 2000;10:520-3. [PubMed]
- Kuang W, Shin PR, Matin S, et al. Initial evaluation of robotic technology for microsurgical vasovasostomy. J Urol 2004;171:300-3. [PubMed]
- Schiff J, Li PS, Goldstein M. Robotic microsurgical vasovasostomy and vasoepididymostomy: a prospective randomized study in a rat model. J Urol 2004;171:1720-5. [PubMed]
- Fleming C. Robot-assisted vasovasostomy. Urol Clin North Am 2004;31:769-72. [PubMed]
- Shu T, Taghechian S, Wang R. Initial experience with robot-assisted varicocelectomy. Asian J Androl 2008;10:146-8. [PubMed]
- Parekattil SJ, Cohen MS. Robotic surgery in male infertility and chronic orchialgia. Curr Opin Urol 2010;20:75-9. [PubMed]
- Parekattil SJ, Brahmbhatt JV. Robotic approaches for male infertility and chronic orchialgia microsurgery. Curr Opin Urol 2011;21:493-9. [PubMed]
- Goldstein M. The making of a microsurgeon. J Androl 2006;27:161-3. [PubMed]
- Tanrikut C, Goldstein M. Obstructive azoospermia: a microsurgical success story. Semin Reprod Med 2009;27:159-64. [PubMed]
- Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol 1989;142:62-5. [PubMed]
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Male Reproduction and Urology. The management of infertility due to obstructive azoospermia. Fertil Steril 2008;90:S121-4. [PubMed]
- Patel SR, Sigman M. Comparison of outcomes of vasovasostomy performed in the convoluted and straight vas deferens. J Urol 2008;179:256-9. [PubMed]
- Chan PT. The evolution and refinement of vasoepididymostomy techniques. Asian J Androl 2013;15:49-55. [PubMed]
- Li PS, Schlegel PN, Goldstein M. Use of silicone medical grade tubing for microsurgical vasovasostomy training. Urology 1992;39:556-7. [PubMed]
- Mehta A, Li PS. Male infertility microsurgical training. Asian J Androl 2013;15:61-6. [PubMed]
- Kuang W, Shin PR, Oder M, et al. Robotic-assisted vasovasostomy: a two-layer technique in an animal model. Urology 2005;65:811-4. [PubMed]
- de Naeyer G, van Migem P, Schatteman P, et al. Robotic assistance in urological microsurgery: initial report of a successful in-vivo robot-assisted vasovasostomy. J Robot Surg 2007;1:161-2. [PubMed]
- Santomauro MG, Choe CH, James O, et al. Robotic vasovasostomy: description of technique and review of initial results. J Robot Surg 2012;6:217-21.
- Parekattil SJ, Gudeloglu A, Brahmbhatt J, et al. Robotic assisted versus pure microsurgical vasectomy reversal: technique and prospective database control trial. J Reconstr Microsurg 2012;28:435-44. [PubMed]
- Najari BB, Li PS, Mehta A, et al. V1593 robotic-assisted laparoscopic mobilization of the vas deferens for correction of obstructive azoospermia induced by mesh herniorrhaphy. J Urol 2013;189:e654.
- Trost L, Parekattil S, Wang J, et al. Intracorporeal Robot-Assisted Microsurgical Vasovasostomy for the Treatment of Bilateral Vasal Obstruction Occurring Following Bilateral Inguinal Hernia Repairs with Mesh Placement. J Urol 2013. [Epub ahead of print]. [PubMed]
- Mechlin C, Mccullough A. V. 1591 robotic microsurgical vasectomy reversal: initial experience and surgical outcomes from a single academic center. J Urol 2013;189:e653-4.
- Schlegel PN. Causes of azoospermia and their management. Reprod Fertil Dev 2004;16:561-72. [PubMed]
- Trottmann M, Liedl B, Becker A, et al. 847 probe-based confocal laser endomicroscopy (pcle)–a new imaging technique for in situ localization of vital spermatozoa. Eur Urol 2013;12:e844.
- Najari BB, Ramasamy R, Sterling J, et al. Pilot study of the correlation of multiphoton tomography of ex vivo human testis with histology. J Urol 2012;188:538-43. [PubMed]
- The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. World Health Organization. Fertil Steril 1992;57:1289-93. [PubMed]
- Schauer I, Madersbacher S, Jost R, et al. The impact of varicocelectomy on sperm parameters: a meta-analysis. J Urol 2012;187:1540-7. [PubMed]
- Cayan S, Shavakhabov S, Kadioğlu A. Treatment of palpable varicocele in infertile men: a meta-analysis to define the best technique. J Androl 2009;30:33-40. [PubMed]
- Mechlin C, Mccullough A. V. 1590 robotic microsurgical varicocele repair: initial experience and surgical outcomes from a single academic center. J Urol 2013;189:e652-3.
- Hopps CV, Lemer ML, Schlegel PN, et al. Intraoperative varicocele anatomy: a microscopic study of the inguinal versus subinguinal approach. J Urol 2003;170:2366-70. [PubMed]
- Davis BE, Noble MJ, Weigel JW, et al. Analysis and management of chronic testicular pain. J Urol 1990;143:936-9. [PubMed]
- Levine L. Chronic orchialgia: evaluation and discussion of treatment options. Ther Adv Urol 2010;2:209-14. [PubMed]
- Christiansen CG, Sandlow JI. Testicular pain following vasectomy: a review of postvasectomy pain syndrome. J Androl 2003;24:293-8. [PubMed]
- McMahon AJ, Buckley J, Taylor A, et al. Chronic testicular pain following vasectomy. Br J Urol 1992;69:188-91. [PubMed]
- Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: long-term results from a single center. J Urol 2008;180:949-53. [PubMed]
- Oliveira RG, Camara C, Alves Jde M, et al. Microsurgical testicular denervation for the treatment of chronic testicular pain initial results. Clinics (Sao Paulo) 2009;64:393-6. [PubMed]
- Parekattil SJ, Gudeloglu A, Brahmbhatt JV, et al. Trifecta nerve complex: potential anatomical basis for microsurgical denervation of the spermatic cord for chronic orchialgia. J Urol 2013;190:265-70. [PubMed]
- Laudano MA, Osterberg EC, Sheth S, et al. Microsurgical Denervation of Rat Spermatic Cord: Safety and Efficacy Data. BJU Int 2013. [Epub ahead of print]. [PubMed]
- Levine LA. Microsurgical denervation of the spermatic cord. J Sex Med 2008;5:526-9. [PubMed]
- Gudeloglu A, Iqbal Z, Parekattil SJ, et al. Hydrodissection for improved microsurgical denervation of the spermatic cord: prospective blinded randomized control trial in a rat model. Fertil Steril 2011;96:S87-8.
- Parekattil SJ, Gudeloglu A, Brahmbhatt J, et al. Prospective randomized control trial of a neuroprotective wrap for the spermatic cord after denervation for chronic orchialgia. Fertil Steril 2011;96:S231.
- Gudeloglu A, Brahmbhatt J, Parekattil S. Prospective comparison of flexible fiberoptic CO2 laser and standard monopolar cautery for robotic microsurgical denervation of the spermatic cord procedure. Fertil Steril 2013;100:S123-4.
- Horovitz D, Tjong V, Domes T, et al. Vasectomy reversal provides long-term pain relief for men with the post-vasectomy pain syndrome. J Urol 2012;187:613-7. [PubMed]
- Brahmbhatt J, Gudeloglu A, Parekattil S. The efficacy of robotic-assisted vasectomy reversal for post-vasectomy pain. Fertil Steril 2013;100:S216.
- Liverneaux PA, Hendriks S, Selber JC, et al. Robotically assisted microsurgery: development of basic skills course. Arch Plast Surg 2013;40:320-6. [PubMed]
- Gudeloglu A, Brahmbhatt J, Priola K, et al. Robotic assisted lego® construction as a model for robotic microsurgery skills training. Fertil Steril 2012;98:S147.
- Ruggiero GM. Earthworms. Telemicrosurgery: Springer, 2013:53-7.
- Karamanoukian RL, Bui T, McConnell MP, et al. Transfer of training in robotic-assisted microvascular surgery. Ann Plast Surg 2006;57:662-5. [PubMed]
- Ramdhian RM, Bednar M, Mantovani GR, et al. Microsurgery and telemicrosurgery training: a comparative study. J Reconstr Microsurg 2011;27:537-42. [PubMed]
- Ben-Or S, Nifong LW, Chitwood WR Jr. Robotic surgical training. Cancer J 2013;19:120-3. [PubMed]
- Titan Medical Inc. Produces Video Illustrating the Dexterity of its SPORT(TM) Single Port Orifice Robotic Technology. Press release Oct. 9, 2013, 9:04 a.m. EDT.
- Rosen J, Lum M, Sinanan M, et al. Raven: developing a surgical robot from a concept to a transatlantic teleoperation experiment. Surgical robotics: Springer, 2011:159-97.
- Lendvay TS, Hannaford B, Satava RM. Future of robotic surgery. Cancer J 2013;19:109-19. [PubMed]