Comparison of prostate cancer detection rates between magnetic resonance imaging-targeted biopsy and transrectal ultrasound-guided biopsy according to Prostate Imaging Reporting and Data System in patients with PSA ≥4 ng/mL: a systematic review and meta-analysis
Introduction
Prostate cancer (PCa) is the second most common malignancy among men worldwide (1). Therefore, screening is very important for integrated management of PCa. The discovery of PSA not only increased the detection rate of PCa, but also decreased the mortality rate of PCa. However, PSA might increase the risk of overdiagnosis and overtreatment, which leads to the necessity of random and systematic sampling of the whole prostate under ultrasound guidance (2). Currently, the standard technique for PCa diagnosis is transrectal ultrasound-guided biopsy (TRUS-Bx) (3). Nevertheless, the limitation of TRUS-Bx random sampling for PCa is that gray-scale ultrasound cannot distinguish PCa tissue from benign prostatic tissue (4,5). Therefore, TRUS-Bx is less sensitive and specific to PCa.
According to the European Society of Urogenital Radiology (ESUR), multiparameter magnetic resonance imaging (mpMRI) has improved the imaging sensitivity of PCa, because of its increasing availability, advances in anatomical and functional data, as well as more and more studies have confirmed it (6,7). Therefore, the clinical guidelines recommend that although the results of initial TRUS biopsy are negative, the suspicions of PCa remain and mpMRI should be performed (8). Several radiologists used different mpMRI scores to indicate their suspicion of PCa, which contributed to great interference for clinicians (9). Due to the lack of a standardized diagnostic criteria for reporting results, it hinders the widespread acceptance of prostate MRI.
To improve the readers reliability and meaningful communication with clinicians, ESUR issued a consensus-based guide in 2012 called the Prostate Imaging Reporting and Data System (PI-RADS), and it was updated to version 2.0 in 2015 (7,10). It evaluates three imaging modalities of intraprostatic lesions with mpMRI using a five-point scale to predict the possibility of clinically significant prostate cancer (csPCa). Since the application of PI-RADS, numerous studies based on PI-RADS and mpMRI have been published (11-13). However, no one has conducted a systematic review and meta-analysis to assess the value of the selection of prostate biopsy methods by PI-RADS. Therefore, this study aimed to evaluate whether PI-RADS could be used as a MRI reporting system to compare the PCa detection rates between magnetic resonance imaging-targeted biopsy (MRI-TBx) and TRUS-Bx by collecting the data from all correlated articles.
Methods
We primarily aimed to systematically evaluate the diagnostic ability of PI-RADS to compare between MRI-TBx and TRUS-Bx in the detection of PCa, including csPCa and non-csPCa.
Search strategy
The online databases PubMed, Embase and Web of Science were searched from inception to find all relevant articles until October 1st, 2019. Systematic literature retrieval was carried out with the help of information experts in medical libraries. For each database, the search terms used were (“PI-RADS”) AND (“prostate cancer OR prostatic Cancer OR prostate Neoplasm OR prostate Neoplasms OR Prostatic Neoplasm OR Prostatic Neoplasms”) AND (“biopsy OR prostate biopsy”) AND (“nuclear magnetic resonance imaging” OR “nuclear magnetic resonance” OR “MRI”).
Inclusion and exclusion criteria
Inclusive studies focused on suspected PCa patients, who elevated PSA (≥4 ng/mL) and/or positive rectal digital examination (RDE). In addition, among these patients who underwent positive MRI (defined as finding suspicious PCa lesions in prostate MRI scans), the mpMRI results were scored independently by PI-RADS, and the patients with equivocal (PI-RADS 3) and intermediate/high-risk (PI-RADS 4/5) lesions were only selected, and of all, these patients were biopsy naïve. Meanwhile, the included studies examined the same person underwent MRI-TBx and followed by TRUS-Bx performed by a urologist, and compared the detection rate of PCa between these two methods in the most objective measure (14). Accordingly, we only included the articles comparing MRI-TBx with TRUS-Bx on the basis of PI-RADS in biopsy naïve patient.
Some studies were excluded according to the following criteria: (I) unable to extract the required information of PCa and determine the quality of research; (II) some articles such as non-case-control studies, case reports, letters, reviewed editorial articles; (III) when repeated or identical patients were used in multiple publications, only the most recent or complete studies were selected in this meta-analysis.
Data extraction
Data collection was independently carried out by two reviewers (Z.K and Q.ZQ). According to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (15), a flow chart to show the number of studies identified and included or excluded at each literature screening stage was established. If there were inconsistencies, we reviewed and discussed it with the third reviewers (X.JX). In the process of searching studies, we first browsed the titles or abstracts related to our chosen topic. If it was not clear, we would move on to the full text. The references cited in the included studies were also considered as potential related studies. The collection of data included the publication information (author and year); the method of recruitment; the publication information (race, mean age, mean PSA and mean volume of prostate); the collection and evaluation of MRI performance; PI-RADS scores; the number of cores collected by systems biopsy; the definition of csPCa; the detection rate of overall PCa, csPCa and non-csPCa based on different PI-RADS score.
Statistical analysis
The odds ratios (ORs) of 95% confidence intervals (95% CIs) were used to evaluate the correlations between MRI-TBx and TRUS-Bx in the diagnosis of PCa. The goodness-of-fit chi-square test to assess the sources of heterogeneity about such association was adopted, and P<0.05 regarded as significant imbalance (16). According to the P value of heterogeneity, the fixed effect model (Mantel-Haenszel method) or random effect model (DerSimonian-Laird method) were used to calculate the pooled ORs (17). Then, subgroup analysis was performed by Gleason score based on the results of prostate biopsy to explore its impact on heterogeneity. Sensitivity analysis was performed by excluding one single study and recalculating their ORs to test the stability and reliability of the overall meta-analysis. In addition, Begg’s funnel plot and Egger’s linear regression test were used to find the publication bias. When the P value was less than 0.05, it was regarded as significant selection bias. We utilized STATA software (version 12.0; StataCorp LP, College Station, TX) to deal with all above statistical analyses.
Results
Studies characteristics
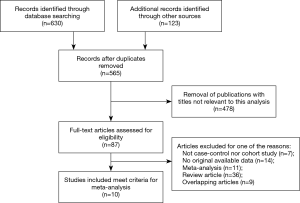
As shown in Figure 1, a total of 753 records were obtained by a systematic literature search from the above databases. According to the inclusion and exclusion criteria, 188 duplicates were removed. Subsequently, 478 records were excluded by screening titles and abstracts and 87 full-text articles were eligible. Besides, 77 articles were eliminated due to insufficient data after carefully reading the full texts. Finally, 10 articles were included in this meta-analysis (18-27); and all included articles were to assess the diagnostic value of MRI-TBx and TRUS-Bx for the detection of PCa. Among these, seven studies were about the influence of Gleason score for PCa diagnosis.
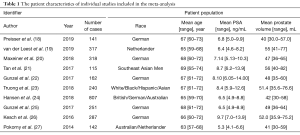
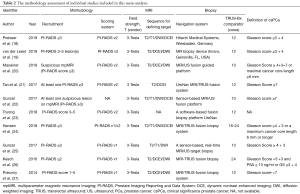
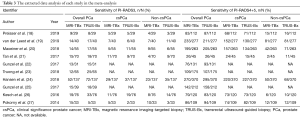
The patient characteristics about number of cases, race, age, prostate volume, and PSA levels of the selected studies were shown in Table 1. The race of most population was German, the rest also included Asian, British, Australian, Hispanic and so on. In addition, the methodology assessment of these studies included was also collected in this meta-analysis (Table 2). The different parameters, consisted of T2 or T1-weighted imaging, diffusion weighted imaging, and dynamic contrast enhancement (DCE) imaging on based on 3.0T MRI, were considered by radiologists to divide the suspicious lesions into different PI-RADS. The suspicion of PCa in all included studies on the basis of PI-RADS was reported on a scale of 3–5. In addition, the suspicious lesions of PI-RADS <3 were not included; because it was impossibly considered PCa. The detection rate of PCa, csPCa and non-csPCa was detected to compare the value of MRI-TBx and TRUS-Bx based on PI-RADS (Table 3).
Full table
Full table
Full table
Quantitative synthesis results
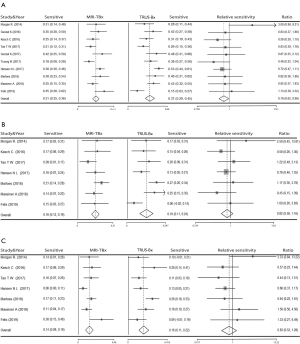
In the comparison of the diagnostic value of MRI-TBx and TRUS-Bx in PCa patients, there was a significant difference advantage of comparing MRI-TBx with TRUS-Bx for overall PCa detection (OR =0.78, 95% CI: 0.62–0.98) (MRI-TBx: sensitivity =0.31, 95% CI: 0.25–0.36; TRUS-Bx: sensitivity =0.37, 95% CI: 0.29–0.45) in PI-RADS 3 (Figure 2A). Basing subgroup analysis of Gleason score (csPCa: Gleason score ≥7; non-csPCa: Gleason score <7), a summary analysis of the detection rate of csPCa showed that no significant difference was found (OR =0.82, 95% CI: 0.58–1.16) (MRI-TBx: sensitivity =0.16, 95% CI: 0.12–0.19, TRUS-Bx: sensitivity =0.18, 95% CI: 0.11–0.24) (Figure 2B); meanwhile, no significant differences was also detected in non-csPCa patients (OR =0.83, 95% CI: 0.53–1.28) (MRI-TBx: sensitivity =0.14, 95% CI: 0.09–0.19, TRUS-Bx: sensitivity =0.16, 95% CI: 0.11–0.22) (Figure 2C).
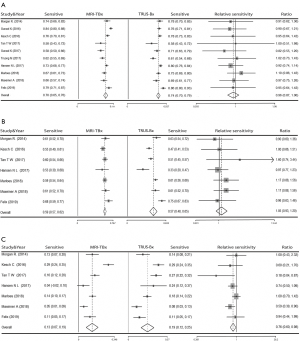
In PI-RADS 4 or 5, no significant results were detected between MRI-TBx and TRUS-Bx (OR =0.96, 95% CI: 0.87–1.06) (MRI-TBx: sensitivity =0.70, 95% CI: 0.65–0.76, TRUS-Bx: sensitivity =0.74, 95% CI: 0.70–0.79) for overall PCa detection (Figure 3A). The stratification analyses by Gleason score found that there was no significant difference in the detection rate of csPCa (OR =1.05, 95% CI: 0.93–1.20) (MRI-TBx: sensitivity =0.59, 95% CI: 0.57–0.62, TRUS-Bx: sensitivity =0.57, 95% CI: 0.48–0.65) (Figure 3B); However, TRUS-Bx had an advantage over MRI-TBx in non-csPCa patients (OR =0.76, 95% CI: 0.60–0.98) (MRI-TBx: sensitivity =0.13, 95% CI: 0.07–0.19, TRUS-Bx: sensitivity =0.19, 95% CI: 0.12–0.25) (Figure 3C).
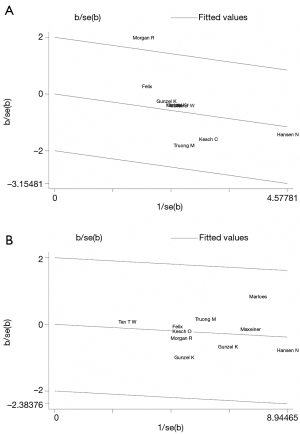
Test of heterogeneity
Subgroup analyses could decrease the heterogeneity, and Gleason score might contribute to one aspect of substantial heterogeneity. Figure 4 showed the results of a Galbraith radial plot, suggesting no obvious heterogeneity between these studies.
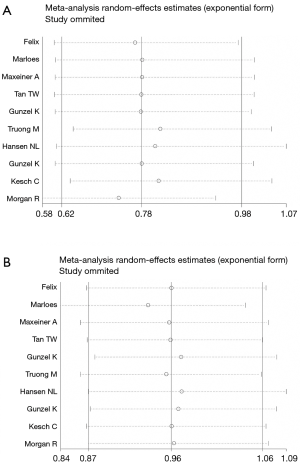
Sensitivity analysis
Sensitivity analysis was carried out by omitting one single study to check the influence of each individual study on the recalculated ORs by repeating the meta-analysis. The results demonstrated that the pooled ORs were not significantly influenced, thus to suggest that the results were robust and stability in this meta-analysis (Figure 5).
Publication bias
The Begg’s funnel plot and Egger’s test were applied to assess the publication bias for all data. The shapes of the funnel plots seemed symmetrically distributed in the funnel plots (Figure 6), indicating little evidence of significant publication bias across studies, which were confirmed by Egger’s test (PI-RADS 3: P=0.104; PI-RADS 4 or 5: P=0.651).
Discussion
Among a series of diagnostic criteria for PCa, the most important one is the result of biopsy, which is considered as the gold diagnostic criterion for PCa nowadays (28). Therefore, the sensitivity and specificity of prostate biopsy has become the focus of more and more researchers. At present, MRI-TBx and TRUS-Bx are coexisting in clinic; However, the selection of two puncture methods is still controversial (29,30). Schoots et al. suggested that the selection of puncture method was associated with pathological grading of PCa (29). Besdies, Schouten et al. reported that the missing detection of biopsy was related to the location of PCa (30). Thus, all these characteristics of PCa are included in PI-RADS (7). Following the publication of PI-RADS, standardized MRI reports have been used by more and more radiologists (31,32). The definitions of the PI-RADS can be expressed in terms of volume (measured on T2 or contrast-enhanced images) or radiological characteristics, so we used some prominent features of MRI changes, such as the difference between the lesion and its surrounding background prostate, the visibility of ADC values or any parameters used (10). Therefore, the PI-RADS of targeted biopsy samples and standard biopsy samples was extracted from each literature, to judge whether the use of PI-RADS reporting criteria could be helpful to distinguish the practicability between systematic biopsy and MRI-targeted biopsy.
Choosing MRI examination before prostate biopsy not only has advantages of the highly sensitivity for detecting PCa, but may also show the location of the lesion (33,34). However, as a new monitoring tool, it might require PCa-suspected patients to bear more financial burdens. Meanwhile, the result of MRI might influence the selection of the way of prostate biopsy, and help clinicians to determine the patients with PSA ≥4 ng/mL to choose the most appropriate biopsy. In this meta-analysis, the results indicated that in the patients with biopsy naïve in PI-RADS 3, the detection rate of TRUS-Bx was better than that of MRI-TBx, while in PI-RADS 4 or 5, no significant difference in detection rate of PCa was found. Then, it is important to recognize that clinically significant disease’s detection might differ between MRI-TBx and TRUS-Bx. Gleason score as the subgroup were further investigated, and these patients were divided into two groups (csPCa: Gleason score ≥7; non-csPCa: Gleason score <7). The results showed that the patients with PI-RADS 4 or 5 in Gleason score <7, TRUS-Bx was better than with MRI-TBx in the detection rate of non-csPCa; Yet no significant difference between MRI-TBx and TRUS-Bx was found in Gleason score ≥7.
The main reason for a negative MRI-TBx when patients with PI-RADS 3 or Gleason score <7 was the possibility of invisible PCa on MRI, and numerous studies about the relationship between MRI findings and pathological specimens of prostatectomy showed that 30–48% of PCa patients were underestimated on MRI (35,36). In addition, the failure of MRI-TBx might also be caused by varying agreement of inter-reader on MRI imaging, patient movement, prostate movement/deformation, incorrect match of image planes, especially at the base of the prostate (37). Furthermore, the slightly lower detection rate of PCa by MRI-TBx in our cohort might also be related to differences in the biopsy learning curve (18). In the screened literature, both TRUS-Bx and MRI-TBx are performed on the same patient, and targeted biopsy was first performed. If MRI-TBx is first performed, needle tracks after biopsy may make TRUS-Bx more likely to puncture sample tissues of the same position as MRI-TBx, even if they are blinding. Meanwhile, some researchers suggested that finding suspicious lesions on MRI might have a positive impact on TRUS-Bx results (29,38). This deviation results in the thinking whether targeted biopsy prior to systematic biopsy affect the results of this meta-analysis. Although these patients are interested in MRI in active monitoring, it is not only an early re-classification strategy, but also a monitoring tool. The results of MRI-TBx depend on the identification of suspicious MRI lesions, which may lead to potential bias.
Ultimately, several limitations still existed in our meta-analysis as follows: (I) the included studies were mainly conducted on Germans, only five studies had other population. Thus, more researches should pay attention to the influence of ethnicity factors to avoid selection bias in the subsequent years; (II) the threshold effect and obvious heterogeneity existed in this study, which might be due to large differences in reagent resources, lifestyle, ethnicity, gender, age, cores of biopsy, type of determination, and critical values; (III) the present meta-analysis included only 10 studies, which might undermine the reliability of our results; (IV) evaluating negative results is necessary, but we cloud not get the original data of included studies. Therefore, more well-designed studies should be conducted on large sample sizes to verify the guidance value of selecting the better prostate biopsy for PCa-suspected patients.
Conclusions
In summary, this meta-analysis revealed that using TRUS-Bx was better than MRI-TBx for the diagnosis of PCa in PI-RADS 3; Besides, TRUS-Bx had an advantage over MRI-TBx in the detection for non-csPCa in PI-RADS 4 or 5. Therefore, PI-RADS could be used as an MRI evaluation system in the selection of prostate biopsy. More large-scale multicenter prospective randomized controlled trials are warranted to verify the effectiveness of PI-RADS in the guidance of PCa patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. [Crossref] [PubMed]
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol 2017;71:630-42. [Crossref] [PubMed]
- Heijmink SW, van Moerkerk H, Kiemeney LA, et al. A comparison of the diagnostic performance of systematic versus ultrasound-guided biopsies of prostate cancer. Eur Radiol 2006;16:927-38. [Crossref] [PubMed]
- Wildeboer RR, Postema AW, Demi L, et al. Multiparametric dynamic contrast-enhanced ultrasound imaging of prostate cancer. Eur Radiol 2017;27:3226-34. [Crossref] [PubMed]
- Wu LM, Xu JR, Gu HY, et al. Usefulness of diffusion-weighted magnetic resonance imaging in the diagnosis of prostate cancer. Acad Radiol 2012;19:1215-24. [Crossref] [PubMed]
- Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. [Crossref] [PubMed]
- Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 2013;63:125-40. [Crossref] [PubMed]
- Lin WC, Muglia VF, Silva GE, et al. Multiparametric MRI of the prostate: diagnostic performance and interreader agreement of two scoring systems. Br J Radiol 2016;89:20151056. [Crossref] [PubMed]
- Barentsz JO, Weinreb JC, Verma S, et al. Synopsis of the PI-RADS v2 Guidelines for Multiparametric Prostate Magnetic Resonance Imaging and Recommendations for Use. Eur Urol 2016;69:41-9. [Crossref] [PubMed]
- Wegelin O, Exterkate L, van der Leest M, et al. The FUTURE Trial: A Multicenter Randomised Controlled Trial on Target Biopsy Techniques Based on Magnetic Resonance Imaging in the Diagnosis of Prostate Cancer in Patients with Prior Negative Biopsies. Eur Urol 2019;75:582-90. [Crossref] [PubMed]
- Padhani AR, Weinreb J, Rosenkrantz AB, et al. Prostate Imaging-Reporting and Data System Steering Committee. PI-RADS v2 Status Update and Future Directions. Eur Urol 2019;75:385-96. [Crossref] [PubMed]
- Schoots IG, Nieboer D, Giganti F, et al. Is magnetic resonance imaging-targeted biopsy a useful addition to systematic confirmatory biopsy in men on active surveillance for low-risk prostate cancer? A systematic review and meta-analysis. BJU Int 2018;122:946-58. [Crossref] [PubMed]
- Arumainayagam N, Kumaar S, Ahmed HU, et al. Accuracy of multiparametric magnetic resonance imaging in detecting recurrent prostate cancer after radiotherapy. BJU Int 2010;106:991-7. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Magosi LE, Goel A, Hopewell JC, et al. Identifying systematic heterogeneity patterns in genetic association meta-analysis studies. PLoS Genet 2017;13:e1006755. [Crossref] [PubMed]
- Garcia-Alamino JM, Bankhead C, Heneghan C, et al. Impact of heterogeneity and effect size on the estimation of the optimal information size: analysis of recently published meta-analyses. BMJ Open 2017;7:e15888. [Crossref] [PubMed]
- Preisser F, Theissen L, Wenzel M, et al. Performance of Combined Magnetic Resonance Imaging/Ultrasound Fusion-guided and Systematic Biopsy of the Prostate in Biopsy-naive Patients and Patients with Prior Biopsies. Eur Urol Focus 2019. [Epub ahead of print].
- van der Leest M, Cornel E, Israel B, et al. Head-to-head Comparison of Transrectal Ultrasound-guided Prostate Biopsy Versus Multiparametric Prostate Resonance Imaging with Subsequent Magnetic Resonance-guided Biopsy in Biopsy-naive Men with Elevated Prostate-specific Antigen: A Large Prospective Multicenter Clinical Study. Eur Urol 2019;75:570-8. [Crossref] [PubMed]
- Maxeiner A, Kittner B, Blobel C, et al. Primary magnetic resonance imaging/ultrasonography fusion-guided biopsy of the prostate. BJU Int 2018;122:211-8. [Crossref] [PubMed]
- Tan TW, Png KS, Lee CH, et al. MRI Fusion-Targeted Transrectal Prostate Biopsy and the Role of Prostate-Specific Antigen Density and Prostate Health Index for the Detection of Clinically Significant Prostate Cancer in Southeast Asian Men. J Endourol 2017;31:1111-6. [Crossref] [PubMed]
- Gunzel K, Cash H, Buckendahl J, et al. The addition of a sagittal image fusion improves the prostate cancer detection in a sensor-based MRI /ultrasound fusion guided targeted biopsy. BMC Urol 2017;17:7. [Crossref] [PubMed]
- Truong M, Feng C, Hollenberg G, et al. A Comprehensive Analysis of Cribriform Morphology on Magnetic Resonance Imaging/Ultrasound Fusion Biopsy Correlated with Radical Prostatectomy Specimens. J Urol 2018;199:106-13. [Crossref] [PubMed]
- Hansen NL, Barrett T, Kesch C, et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy-naive men with suspicion of prostate cancer. BJU Int 2018;122:40-9. [Crossref] [PubMed]
- Gunzel K, Haas M, Maxeiner A, et al. Predictive Parameters Identifying Men Eligible for a Sole MRI/Ultrasound Fusion-Guided Targeted Biopsy without an Additional Systematic Biopsy. Urol Int 2017;98:15-21. [Crossref] [PubMed]
- Kesch C, Radtke JP, Distler F, et al. Multiparametric MRI and MRI-TRUS fusion biopsy in patients with prior negative prostate biopsy. Urologe A 2016;55:1071-7. [Crossref] [PubMed]
- Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol 2014;66:22-9. [Crossref] [PubMed]
- Tangen CM, Goodman PJ, Till C, et al. Biases in Recommendations for and Acceptance of Prostate Biopsy Significantly Affect Assessment of Prostate Cancer Risk Factors: Results From Two Large Randomized Clinical Trials. J Clin Oncol 2016;34:4338-44. [Crossref] [PubMed]
- Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68:438-50. [Crossref] [PubMed]
- Schouten MG, van der Leest M, Pokorny M, et al. Why and Where do We Miss Significant Prostate Cancer with Multi-parametric Magnetic Resonance Imaging followed by Magnetic Resonance-guided and Transrectal Ultrasound-guided Biopsy in Biopsy-naive Men? Eur Urol 2017;71:896-903. [Crossref] [PubMed]
- Kirkham AP, Haslam P, Keanie JY, et al. Prostate MRI: who, when, and how? Report from a UK consensus meeting. Clin Radiol 2013;68:1016-23. [Crossref] [PubMed]
- Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol 2013;64:544-52. [Crossref] [PubMed]
- Kaplan I, Oldenburg NE, Meskell P, et al. Real time MRI-ultrasound image guided stereotactic prostate biopsy. Magn Reson Imaging 2002;20:295-9. [Crossref] [PubMed]
- Tang Y, Liu Z, Tang L, et al. Significance of MRI/Transrectal Ultrasound Fusion Three-Dimensional Model-Guided, Targeted Biopsy Based on Transrectal Ultrasound-Guided Systematic Biopsy in Prostate Cancer Detection: A Systematic Review and Meta-Analysis. Urol Int 2018;100:57-65. [Crossref] [PubMed]
- Rud E, Klotz D, Rennesund K, et al. Detection of the index tumour and tumour volume in prostate cancer using T2-weighted and diffusion-weighted magnetic resonance imaging (MRI) alone. BJU Int 2014;114:E32-42. [Crossref] [PubMed]
- Bratan F, Niaf E, Melodelima C, et al. Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: a prospective study. Eur Radiol 2013;23:2019-29. [Crossref] [PubMed]
- Cash H, Maxeiner A, Stephan C, et al. The detection of significant prostate cancer is correlated with the Prostate Imaging Reporting and Data System (PI-RADS) in MRI/transrectal ultrasound fusion biopsy. World J Urol 2016;34:525-32. [Crossref] [PubMed]
- Fourcade A, Payrard C, Tissot V, et al. The combination of targeted and systematic prostate biopsies is the best protocol for the detection of clinically significant prostate cancer. Scand J Urol 2018;52:174-9. [Crossref] [PubMed]