How to perform a robotic pyeloplasty utilizing the da Vinci SP platform: tips and tricks
Introduction
Minimally invasive pyeloplasty was first described by Kavoussi and Peters, as well as Schuessler et al. in 1993 who presented the technique and early experience with laparoscopic pyeloplasty (1,2). As the technique matured and gained wider adoption, the da Vinci robotic platform was introduced into the arsenal of minimally invasive surgery. The first report and series of robotic-assisted pyeloplasty was in 2003 (3,4). The adoption of minimally invasive pyeloplasty, especially robotic pyeloplasty, steadily increased throughout the 2000s (5).
The multiport platform of the da Vinci S, Si, and Xi systems® have allowed for complex procedures to be performed that were previously only done in an open fashion. As the technique was refined and platform more widely disseminated, increased interest developed to moving the multiple ports to a common opening to aid in convalescence and cosmesis. Single site modification of multiport platforms has been reported for pyeloplasty (6,7). However, true single port surgery was not possible until the da Vinci SP platform® (Intuitive Surgical, Sunnyvale, CA) introduction in 2018. Multiple reports of urologic procedures with this platform in live patients have been described (8-10), including limited data in single-port robotic-assisted pyeloplasty (spRAP) (11,12). We present our approach, technique, and experience with spRAP.
Description and tips
Preoperative preparation
It is imperative to undergo a formal training course prior to utilizing the da Vinci SP® robotic platform. This should include classroom and wet lab (animal and/or cadaver) training to gain simulated experience with novel technical and logistical changes specific to the SP (8). Given the multiple novel aspects, the operating surgeon and staff should familiarize themselves with the platform, docking, arm movements, and instruments prior to operating in patients. Additionally, patient choice is important. A minimum of 10 cm of working space is needed (or 12 cm if using the roundtooth forceps) to be able to completely articulate the arms. In adults, this amount of space is typically present after abdominal insufflation for spRAP. Early in one’s learning curve we would caution against the use of the single port platform in patients with significant previous abdominal surgery, as intra-abdominal adhesions can hinder the ability to achieve safe and adequate working space.
Patient positioning and initial incision
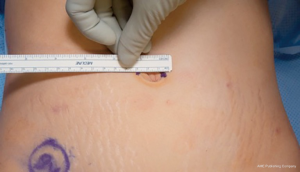
We position and secure the patient in a sloppy lateral flank position to allow for maximum bed rotation from the supine to flank position. We perform a tilt test before sterile preparation and draping of the patient to ensure no unintended movement during rotation of the bed intraoperatively. A 2.5 cm periumbilical incision hidden within the umbilicus is made and carried down to the level of the abdominal fascia (Figure 1). We prefer rolling the patient to the supine position to aid in safe trocar placement. Care should be taken, especially in patients with prior surgery, to carefully open the fascia and lyse any intraabdominal adhesions. In this setting, an additional 5mm laparoscopic trocar in the left mid-subcostal space can be placed to assess feasibility of SP trocar placement. Upon opening the fascia, the apices of the fascial incision should be tagged with heavy suture. Not only does this facilitate closure, but also assists with trocar placement.
Trocar placement and robotic docking
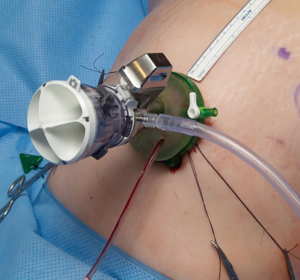
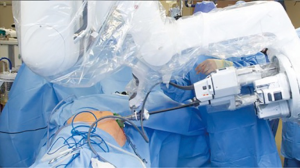
The SP trocar should be completely assembled with blunt trocar inside the port cannula. Using counter tension with the fascial sutures, the trocar should be gently placed in the abdominal cavity. No additional port placement has been needed in our experience with spRAP. If an assistant port is desired, a GelPOINT® or GelPOINT Mini® (Applied Medical, Rancho San Margarita, CA) access port can be used as previously described by Hebert et al. (11) (Figure 2). The regular GelPOINT® allows for optimal spacing between the SP and assistant trocar which enables for a greater range of movement than what is possible with the GelPOINT Mini®. It is important to note that the incision needed for such access ports is larger than the 2.5 cm incision required for the SP trocar alone, generally 2.7–3 cm if accounting for the addition of a 5 mm assistant port. There can be issues with pressure-induced ischemia of the surrounding skin with an incision that is too small. After port placement, the patient can then be rolled with the bed to the lateral position. The robot is then docked over the flank (Figure 3).
Initial dissection
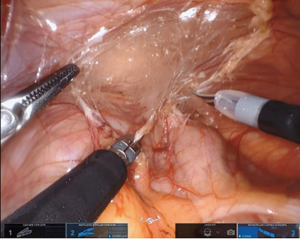
We favor the camera at 12 o’clock position with scissors at 3 o’clock (arm 3), working forceps (Maryland or atraumatic) at 6 o’clock (arm 2), and atraumatic forceps at 9 o’clock (arm 1) for the initial dissection. We find the Cadiere forceps superior to the roundtooth for retraction (8). In standard fashion, the colon overlying the UPJ of interest is reflected medially (Figure 4). On the right side, the duodenum is carefully Kocherized. These steps can be performed without difficulty, however, it is important to take into account the different movement capabilities of the robot. When working toward the left of the surgical field, retraction is done with arm 1 and arms 2 and 3 are used for dissection. Conversely, when working toward the right, arm 3 is used for retraction and arms 1 and 2 are used for dissection. When changing field of view or if the arms need to be repositioned, the entire boom with arms and camera move as one unit opposed to the prior independent movement of these structures.
Sutures can be passed at any time during the case and no assistant port is required for this step. Suture passing can be accomplished by removing a working arm and having the bedside assistant pass the suture through the arm cannula with a laparoscopic instrument. A ½ circle 17 mm suture needle can be passed through the SP trocar. Alternatively, suture can be passed through an assistant port if present. It is important to attempt suture passage with the cannula outside the patient prior to the case to avoid issues intraoperatively. We prefer passing the anticipated sutures needed after reflection of the colon so that they can be stored in the paracolic gutter. The sutures can be kept out of the way of the rest of the dissection and then easily accessed when needed.
Dissection of ureteropelvic junction
The ureter can then be identified, with or without preoperative stent placement. The dissection is carried cephalad to the UPJ, with care to not skeletonize the ureter completely from its blood supply (Figure 5). If a crossing vessel is encountered, this should be preserved to prevent loss of perfusion to renal parenchyma (Figure 6). Careful dissection of the UPJ should be performed, especially if suction is not being utilized, as the lack of suction port can pose difficulty with even minimal bleeding over the area of dissection. The use of external suction can be employed with flexible nasotracheal suction tubing passed alongside the SP trocar, which does not require extending the standard 2.5 cm incision. The suction can then be moved to area of need with robotic arms as previously described by our group (9). Additional absorbable hemostat gauze can be used to control unwanted capillary bleeding during the surgical dissection.
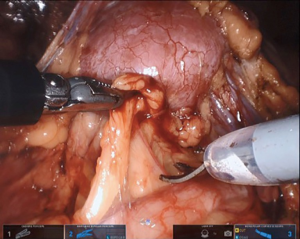
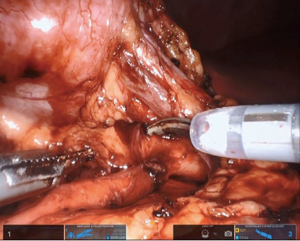
Maryland forceps available in the SP platform can be utilized to facilitate dissection of the crossing vessel from surrounding tissue, as it enables bipolar electrocautery for meticulous hemostasis. If there is significant redundant renal pelvis, this can be hitched transabdominally by pexing tissue to the abdominal wall or with an external hitch stitch utilizing a Keith needle passed extracorporeally by the bedside assistant. Alternatively, the camera can be moved to the 6 o’clock position and the 12 o’clock position arm can be used for retraction.
Management of obstruction
After the proximal ureter and renal pelvis have been identified and completely mobilized, the surgeon can perform any method of repair felt appropriate. We prefer a dismembered pyeloplasty due to the versatility in managing crossing vessels, intrinsic obstruction or iatrogenic injury. Any stenotic segment is excised and the renal pelvis and proximal ureter are spatulated. We utilize a 4-0 braided polyglactin 910 on a ½ circle 17 mm tapered needle, which easily passes through the SP instrument channel, for the ureteropelvic anastomosis. We begin on the posterior wall of the UPJ anastomosis (Figure 7). If an assistant port is placed, the suture can be passed through that port at this time (if not passed already as described above). Due to proximal displacement of the endowrist and a slight reduction in the degree of flexion relative to multiport platforms, efficient needle advancement through the targeted tissue requires additional movement at the elbow of the working SP needle driver.
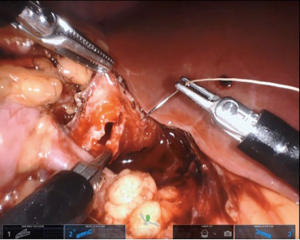
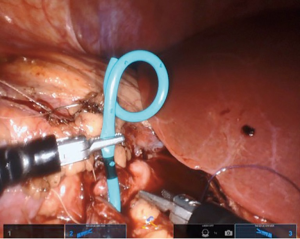
If a stent is not placed before the robotic portion of the case, we prefer to pass the stent after the back wall of the anastomosis has been completed. A ureteral stent can be placed over a wire transabdominally, as our group has described previously (9). In a direct downward line from the abdominal wall to the anastomosis, a 14-gauge angiocatheter is passed through the abdominal wall and the inner needle is removed. This allows one to extracorporeally pass a 0.035 inch hydrophilic-tipped, PTFE-coated nitinol core hybrid wire into the proximal ureter and antegrade into the bladder (Figure 8). The soft angiocatheter is then removed and a double J ureteral stent is advanced over the wire and into the bladder (Figure 9). The anterior wall of the pyeloplasty is then closed in a running fashion (Figure 10). For stents placed in this fashion, we recommend performing flexible cystoscopy with the patient in a frog-legged position to confirm the distal stent curl is seated within the bladder before awaking the patient from anesthesia.
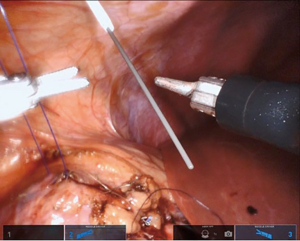
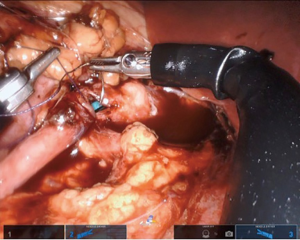
Completing the procedure
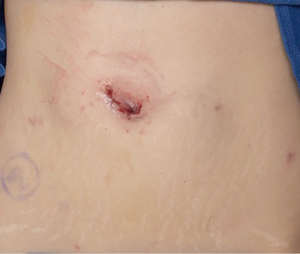
For standard cases, no drain is placed. If a drain is required, a 15 Fr closed suction round drain can be placed through a separate small stab incision concealed below the level of the waistline. Instruments should be removed under direct vision and the camera can be removed. The operating table can be moved to for the patient to be in the supine position and the SP trocar can be removed after allowing for pneumoperitoneum to be expelled. The facial tagging sutures can be used to facilitate abdominal wall fascia closure. Depending on patient body habitus, a deep subcuticular stitch is used to facilitate skin closure. Skin glue is also applied over the incision. The incision should be concealed within the umbilicus and ultimately be cosmetically acceptable (Figure 11). The postoperative course consists of catheter removal and hospital dismissal on postoperative day one. The ureteral stent is removed in the office 4–6 weeks postoperatively.
Conclusions
spRAP is safe and feasible. It can be performed in a truly single-port fashion, without an additional assistant port (11). If suction is desired, this can also be accomplished without an additional port or extension of the incision (9). Other groups have utilized a single-incision with the SP trocar and an assistant port through the same incision (12). In the three cases performed at our institution, all patients were discharged on postoperative day one, no patient experienced a perioperative complication, and all had a patent, non-obstructed repair on follow-up imaging.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ashok K. Hemal) for the series “Robotic-assisted Urologic Surgery” published in Translational Andrology and Urology. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2019.11.08). The series “Robotic-assisted Urologic Surgery” was commissioned by the editorial office without any funding or sponsorship. MTG: Consultant for Intuitive Surgical. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kavoussi LR, Peters CA. Laparoscopic pyeloplasty. J Urol 1993;150:1891-4. [Crossref] [PubMed]
- Schuessler WW, Grune MT, Tecuanhuey LV, et al. Laparoscopic dismembered pyeloplasty. J Urol 1993;150:1795-9. [Crossref] [PubMed]
- Gettman MT, Neururer R, Bartsch G, et al. Anderson-Hynes dismembered pyeloplasty performed using the da Vinci robotic system. Urology 2002;60:509-13. [Crossref] [PubMed]
- Gettman MT, Peschel R, Neururer R, et al. A Comparison of Laparoscopic Pyeloplasty Performed with the daVinci Robotic System versus Standard Laparoscopic Techniques: Initial Clinical Results. Eur Urol 2002;42:453-7; discussion 457-8. [Crossref] [PubMed]
- Jacobs BL, Lai JC, Seelam R, et al. Variation in the Use of Open Pyeloplasty, Minimally Invasive Pyeloplasty, and Endopyelotomy for the Treatment of Ureteropelvic Junction Obstruction in Adults. J Endourol 2017;31:210-5. [Crossref] [PubMed]
- Desai MM, Rao PP, Aron M, et al. Scarless single port transumbilical nephrectomy and pyeloplasty: first clinical report. BJU Int 2008;101:83-8. [Crossref] [PubMed]
- Kaouk JH, Goel RK, Haber GP, et al. Robotic single-port transumbilical surgery in humans: initial report. BJU Int 2009;103:366-9. [Crossref] [PubMed]
- Agarwal DK, Sharma V, Toussi A, et al. Initial Experience with da Vinci Single-port Robot-assisted Radical Prostatectomies. Eur Urol 2020;77:373-9. [Crossref] [PubMed]
- Hebert KJ, Joseph J, Gettman M, et al. Technical Considerations of Single Port Ureteroneocystostomy Utilizing da Vinci SP Platform. Urology 2019;129:236. [Crossref] [PubMed]
- Kaouk J, Garisto J, Bertolo R. Robotic Urologic Surgical Interventions Performed with the Single Port Dedicated Platform: First Clinical Investigation. Eur Urol 2019;75:684-91. [Crossref] [PubMed]
- Agarwal DK, Viers BR, Chow GK, et al. First Robot-Assisted Laparoscopic Pyeloplasty Utilizing the da Vinci SP System. Videourology 2019;33.
- Heo JE, Kang SK, Koh DH, et al. Pure single-site robot-assisted pyeloplasty with the da Vinci SP surgical system: Initial experience. Investig Clin Urol 2019;60:326-30. [Crossref] [PubMed]