Long-term oncologic outcomes of positive surgical margins following robot-assisted partial nephrectomy
Introduction
Positive surgical margin (PSM) status is almost universally considered an adverse pathologic factor that leads to higher rates of tumor recurrence, and in some cases, disease progression and death. Research in prostate and urothelial cancer, for example, has consistently shown adverse oncologic outcomes in the subset of patients with positive margins (1,2). However, in renal cell carcinoma (RCC), the literature has proven controversial, even for radical nephrectomy with adequate sample size and long-term follow up. While some reports have found an increase in recurrence after radical nephrectomy, well-established nomograms have not found this to be a significant predictor of cancer specific (CSS) or overall survival (OS) (3-5). Now, with increasing utilization of cross-sectional imaging and the resultant identification of small renal cortical neoplasms amenable to the partial nephrectomy (PN), this debate continues (6,7). Central to this debate is whether preventing loss of healthy renal parenchyma with PN outweighs the increased risk of incomplete tumor excision and adverse oncologic outcomes.
Studies have consistently demonstrated equivalent oncologic control with decreased perioperative morbidity for minimally invasive approaches when compared to open partial nephrectomy (8,9). Additionally, rapid adoption of the robotic approach by urologic surgeons has increased the number of patients undergoing robot-assisted partial nephrectomy (10). Local recurrence in many of these studies has been reported in 0.1% to 10% of patients (11). However, long-term follow-up is often lacking in these studies. And despite recurrences often occurring within the first five years, up to 10% of recurrences will occur greater than five years out from surgery (12-14). Further, confounding this data is the fact that most of these local recurrences are distant from the tumor bed and likely unrecognized multifocal tumor or de novo occurrence rather than true recurrence from a positive margin. As such, the effect of PSM on CSS and OS has been largely inconsistent and thus requires further elucidation.
In this study, we sought to elucidate the relationship between PSM after robot-assisted partial nephrectomy (RAPN) and the resulting long-term oncologic outcomes. Furthermore, we sought to identify clinicopathological variables that increase the likelihood of adverse oncologic outcomes following RAPN.
Methods
Our prospectively maintained, Institutional Review Board approved renal oncology database (IRB# 00009163) was queried to identify all patients having undergone RAPN from January 2008 to June 2017. All surgeries involved use of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, California, USA) and were performed by a single experienced surgeon with assistance from residents and fellows. Patients were stratified based on final pathologic margin status of either a PSM or a negative surgical margin (NSM). These groupings served as the basis for statistical analysis of outcomes.
Prior to surgery all patients underwent cross-sectional imaging to better characterize their renal tumor. Standard demographics and medical history were collected, in addition to determining clinical staging and calculating RENAL nephrometry score. Perioperative outcomes, including operative time, estimated blood loss (EBL), warm ischemia time (WIT), and hospital lengths of stay (LOS) were recorded. We also documented final tumor pathology data, including tumor size, histology, stage, and Fuhrman grade. Presence of a PSM included specimens found to be grossly positive, focally positive, or those abutting the surgical margin as defined by our institution’s pathology protocols.
Our technique for RAPN has been described previously in the literature (15,16). Once the tumor has been adequately exposed an ultrasound probe is advanced through the 12mm assistant port to allow for the operating surgeon to intra-operatively determine tumor boundaries and proximity of the tumor to vasculature and the pelvicalyceal system. The edge of the tumor is then scored under ultrasound guidance and the trajectory for excision is planned. We then control the hilar vasculature, which typically involves only clamping the renal artery. Tumor excision is then performed using cold scissors to avoid cautery artifact at the margin. We frequently utilize near-infrared fluorescence imaging with indocyanine green to assess ischemia to the kidney, to allow for super-selective clamping, to guide tumor excision via differential uptake between neoplastic and non-neoplastic parenchyma, and to assess vascularity at tissue edges after renorrhaphy.
Once discharged, patients are scheduled for follow-up in our clinic to monitor serum chemistries and review final pathology. Cross sectional imaging is performed at regularly scheduled intervals and monitored closely for evidence of tumor recurrence or presence of metastatic disease.
Statistical comparisons were generated between patients with pathologically confirmed PSM and those with NSM. Student’s t-tests were performed for normally distributed data where means are presented, Kruskal-Wallis tests were used for skewed data where medians are presented, and Fischer’s exact tests were used for categorical measures. Kaplan-Meier estimates were generated for tumor recurrence and overall patient survival; comparisons were made between patients with PSM and NSM using log-rank tests. SAS Software (version 9.4, Cary, North Carolina, USA) was used to perform all statistical analyses.
Results
We identified a total of 432 patients who underwent RAPN for surgical management of a renal cortical neoplasm. There were 29 (6.7%) instances of pathologically confirmed PSM. Based on preoperative cross-sectional imaging, the most prominent nature of tumors in the PSM group was mesophytic (69%), while most tumors were exophytic (51%) in the NSM group. No completely endophytic tumors were seen in the PSM group, but they made up 9% of tumors in the NSM group. There were no statistical differences identified between the two groups for any of the remaining clinicopathologic variables, including patient comorbidities, tumor size, staging, tumor location within the kidney, and total contact surface area (Table 1).
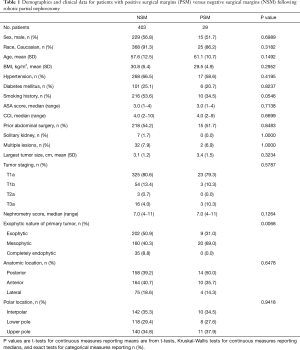
Full table
Perioperative, pathologic, and oncologic outcomes between patients with NSM and PSM were similar across nearly all variables we examined; however, warm ischemia time (WIT) was significantly longer in the PSM group despite no significant differences in pathologic tumor size, RENAL nephrometry score, operative time, or EBL between groups (Table 2). Distribution of tumor histology was also noted to be different between groups, with the rate of clear cell histology significantly higher in the NSM group and chromophobe histology significantly higher in the PSM group. Other variables, including LOS and Fuhrman grade, were all similar. Of the 29 identified PSM, seven abutted the surgical margin, ten were focally positive, and the remaining twelve had gross involvement of the surgical margin (Table 3).
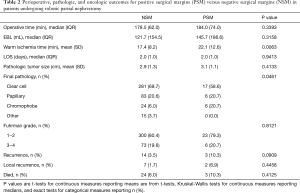
Full table

Full table
Median follow-up for the overall cohort was 45.1 months. We documented fourteen instances of disease recurrence, of which seven events were local recurrences, for patients with NSM. Of these patients with local recurrence, there were three instances of recurrence at or adjacent to the resection bed and four instances of new tumor development on the ipsilateral kidney at a site separate from the resection bed. The other seven events were distant recurrences, of which two involved the contralateral kidney and five involved distant sites (retroperitoneal lymph nodes, within the peritoneum, liver, adrenal gland, lungs, or skeletal metastases). Likewise, three patients with PSM experienced disease recurrence. Two of these patients had local recurrences, specifically one patient developed a recurrence adjacent to the site of tumor resection and the other patient developed a new tumor within the ipsilateral kidney. One patient with PSM was upstaged to pT3a disease on final pathology, developed pulmonary metastases within months of surgery, and died from their disease seven months post-operatively.
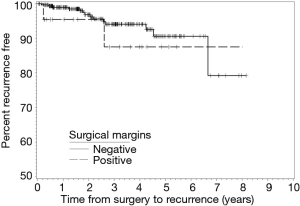
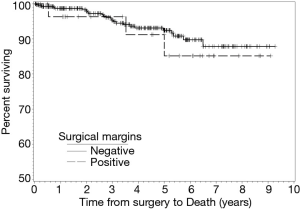
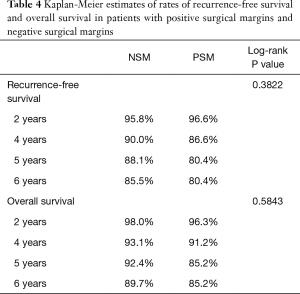
During follow-up, 24 patients with NSM died and three patients with PSM died. There were no statistical differences in the frequencies of tumor recurrence, local recurrence, or death between the NSM and PSM groups. Recurrence-free (RFS) and OS rates at two-, four-, five-, and six-year following RAPN are provided in Table 4. We identified no statistical differences in survival estimates, with log-rank P values of 0.3822 for RFS and 0.5843 for OS. Kaplan-Meier curves for RFS and OS are presented in Figure 1 and Figure 2, respectively.
Full table
Discussion
Partial nephrectomy requires the urologic surgeon achieve a balance between adequate excision of the renal neoplasm, while maintaining surrounding normal parenchyma to preserve post-operative renal function. It is rare, however, not entirely infrequent, that extirpation is unsuccessful and a PSM is identified on final pathology. Such findings have been inconsistently associated with clinical significance with respect to risk for recurrence of cancer, metastatic spread, or worse OS. With this controversy in mind, the primary objective of our investigation was to clarify the relationship between PSM and resultant oncologic outcomes in a cohort of patients having undergone RAPN with long-term follow-up available. Additionally, we sought to identify clinicopathologic variables that place patients at increased risk for PSM or adverse oncologic outcomes.
We report outcomes for 432 patients who underwent RAPN by a single experienced robotic surgeon with assistance from residents and fellows at a large, tertiary, academic medical center. Of the 29 (6.7%) patients with PSM identified on final pathology, three patients had a recurrence and three patients died over a median follow-up of forty-five months. Specifically, two patients developed a local recurrence, one patient developed distant metastasis, and, of the three patients who died, one died as a manifestation of their cancer. Moreover, the six-year RFS and OS estimates for patients with PSM were 80.4% and 85.2%, compared to 85.5% and 89.7% for patients with NSM, respectively. While we did not observe a difference in overall RENAL Nephrometry scores, there were significantly larger percentages of mesophytic tumors in the PSM group and exophytic tumors in the NSM group. While this finding may follow intuition in suggesting tumors located deeper within the parenchyma increase the risk of a PSM, the absence of purely endophytic tumors in our PSM group speaks to the contrary. Overall, we did not find PSM to be associated with worse oncologic outcomes compared to patients with NSM.
Several previous investigations have attempted to define the association between PSM following PN and long-term oncologic outcomes; however, these studies have reached varying conclusions. Kang et al. report oncologic outcomes over a median 32.5 months of follow-up for 1,813 patients with stage T1 lesions managed with PN (17). They identified 31 (1.7%) instances of PSM and determined surgical margin status was not significantly associated with recurrence (recurrence rates of 3.2% for PSM, 2.1% for NSM); moreover, there was no association between PSM and worse RFS. With a median follow-up of 3.4 years, Yossepowitch et al. report PSM in 77 (5.5%) out of 1,390 patients who underwent PN and conclude the five-year RFS and five-year metastatic progression-free survival was no different based on surgical margin status (18). Likewise, in a population-based analysis of the Ontario Cancer Registry, Ani et al. describe no differences in five-year CSS and OS for patients with PSM versus NSM over a median follow-up of 7.9 years (19). Overall, the above results and conclusions are consistent with those reported in the present study, which demonstrates no statistical association between PSM and worse oncologic outcomes, as well as no significant difference in six-year RFS and OS estimates.
To our knowledge, few reports exist in the literature documenting the implication of surgical margin status on long-term oncologic outcomes for cohorts of patients having exclusively undergone RAPN. A multi-center study provided by Lista et al. presents data on 339 patients who underwent RAPN for cT1 lesions and reports 22 (6.5%) documented PSM and zero local recurrences over 49 months of median follow-up (20). However, oncologic survival data is not reported. Several contemporary studies have reported excellent five-year oncologic outcomes for RAPN; however, these studies document outcomes for smaller patient cohorts compared to the present study and do not report oncologic outcomes with respect to surgical margin status (21-24). In the current study we present long-term oncologic outcomes for a larger cohort of patients with renal cortical neoplasms exclusively managed by RAPN. Our investigation indicates no association between PSM and worse RFS or OS and demonstrates no differences in six-year survival estimates.
Several investigations with contrasting conclusions to the current study have been published, adding to the controversy surrounding the implication of a PSM in the setting of PN. Recently, Petros et al. matched thirty-four patients with PSM following PN for RCC to one hundred patients with NSM, concluding PSM resulted in increased risk for worse oncologic survival outcomes (25). Moreover, in a follow-up analysis of this data, PSM was also found to be associated with recurrence of disease in the surgical tumor bed (26). In an analysis of 770 patients, Kwon et al. reported an increased risk of local recurrence in patients with PSM and “high malignant potential histology,” specifically the presence of clear cell, collecting duct, or papillary type II variants of RCC, as well as presence of sarcomatoid differentiation (27). A sub-group analysis performed by Shah et al. stratified pathologic specimens into either low-risk or high-risk groupings based on tumor stage and grade. The presence of PSM and high-risk pathology (stage pT2-pT3 and/or Fuhrman grade III−IV) was found to significantly increase a patient’s risk for disease recurrence at five years (28). Importantly, this association was not present for patients with low-risk pathology (stage pT1 or Fuhrman grade I−II), indicating that all PSM may not necessarily be equal and that other factors related to tumor aggressiveness in the presence of a PSM may place patients at an increased risk for recurrence.
The present study is limited by its retrospective nature of data collection from a prospectively maintained database. Several factors intrinsic to our patient cohort may allow for better interpretation of results. With the majority of patients in our cohort having stage pT1 tumors and Fuhrman grade I-II on final pathology, it is more difficult to confidently define the relationship between a PSM in tumors with high risk features than in tumors with low risk features, for which a significant association with worse long-term oncologic outcomes does not appear to exist. Certainly, additional studies examining both standard pathologic variables, as well as genomic markers of tumor aggressiveness, are warranted to better identify risk factors for worse oncologic outcomes in the setting of PSM.
PSM have been inconsistently associated with clinical significance with respect to risk for recurrence of cancer, metastatic spread, or worse overall survival. While PSM are relatively uncommon in the setting of RAPN, their presence still serves as a potential risk factor for disease recurrence or worse oncologic outcomes. In instances of PSM, immediate secondary intervention is most likely unnecessary and more attentive long-term clinical follow up, with especially vigilant monitoring in patients with high risk features, may be more advisable.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ashok K. Hemal) for the series “Robotic-assisted Urologic Surgery” published in Translational Andrology and Urology. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The series “Robotic-assisted Urologic Surgery” was commissioned by the editorial office without any funding or sponsorship. AKH served as the unpaid Guest Editor of the series and serves as the unpaid editorial board member of Translational Andrology and Urology from May 2019 to Apr 2021. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our prospectively maintained, Institutional Review Board approved renal oncology database (IRB# 00009163) was queried to identify all patients having undergone RAPN from January 2008 to June 2017.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dotan ZA, Kavanagh K, Yossepowitch O, et al. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol 2007;178:2308-12. [Crossref] [PubMed]
- Zhang L, Wu B, Zha Z, et al. Surgical margin status and its impact on prostate cancer prognosis after radical prostatectomy: a meta-analysis. World J Urol 2018;36:1803-15. [Crossref] [PubMed]
- Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 2003;97:1663-71. [Crossref] [PubMed]
- Frank I, Blute M, Cheville J, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade, and necrosis: the SSIGN score. J Urol 2002;168:2395-400. [Crossref] [PubMed]
- Abu-Ghanem Y, Ramon J, Berger R, et al. Positive surgical margin following radical nephrectomy is an independent predictor of local recurrence and disease-specific survival. World J Surg Oncol 2017;15:193. [Crossref] [PubMed]
- Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer 2008;113:78-83. [Crossref] [PubMed]
- Bhargavan M, Sunshine J. Utilization of radiology services in the United States: levels and trends in modalities, regions, and populations. Radiology 2005;234:824-32. [Crossref] [PubMed]
- Xia L, Wang X, Xu T, et al. Systematic Review and Meta-Analysis of Comparative Studies Reporting Perioperative Outcomes of Robot-Assisted Partial Nephrectomy Versus Open Partial Nephrectomy. J Endourol 2017;31:893-909. [Crossref] [PubMed]
- Garisto J, Bertolo R, Dagenais J, et al. Robotic versus open partial nephrectomy for highly complex renal masses: Comparison of perioperative, functional, and oncological outcomes. Urol Oncol 2018;36:471.e1-471.e9. [Crossref] [PubMed]
- Patel HD, Mullins J, Pierorazio P, et al. Trends in Renal Surgery: Robotic Technology is Associated with Increased Use of Partial Nephrectomy. J Urol 2013;189:1229-35. [Crossref] [PubMed]
- Laganosky DD, Filson CP, Master VA. Surgical Margins in Nephron-Sparing Surgery for Renal Cell Carcinoma. Curr Urol Rep 2017;18. [Crossref] [PubMed]
- Breda A, Konijeti R, Lam J. Patterns of recurrence and surveillance strategies for renal cell carcinoma following surgical resection. Expert Rev Anticancer Ther 2007;7:847-62. [Crossref] [PubMed]
- Ljungberg B, Alamdari F, Rasmuson T, et al. Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int 1999;84:405-11. [Crossref] [PubMed]
- Brookman-May S, May M, Shariat SF, et al. Features associated with recurrence beyond 5 years after nephrectomy and nephron-sparing surgery for renal cell carcinoma: Development and internal validation of a risk model (PRELANE score) to predict late recurrence based on a large multicenter database. Eur Urol 2013;64:472-7. [Crossref] [PubMed]
- Mufarrij PW, Krane LS, Rajamahanty S, et al. Does Nephrometry Scoring of Renal Tumors Predict Outcomes in Patients Selected for Robot-Assisted Partial Nephrectomy? J Endourol 2011;25:1649-53. [Crossref] [PubMed]
- Krane LS, Manny TB, Hemal AK. Is Near Infrared Fluorescence Imaging Using Indocyanine Green Dye Useful in Robotic Partial Nephrectomy: A Prospective Comparative Study of 94 Patients. Urology 2012;80:110-6. [Crossref] [PubMed]
- Kang HW, Lee SK, Kim WT, et al. Surgical margin does not influence recurrence rate in pT1 clear cell renal cell carcinoma after partial nephrectomy: A multicenter study. J Surg Oncol 2016;114:70-4. [Crossref] [PubMed]
- Yossepowitch O, Thompson RH, Leibovich BC, et al. Positive Surgical Margins at Partial Nephrectomy: Predictors and Oncological Outcomes. J Urol 2008;179:2158-63. [Crossref] [PubMed]
- Ani I, Finelli A, Alibhai SM, et al. Prevalence and impact on survival of positive surgical margins in partial nephrectomy for renal cell carcinoma: A population-based study. BJU Int 2013;111:E300-5. [Crossref] [PubMed]
- Lista G, Buffi NM, Lughezzani G, et al. Margin, ischemia, and complications system to report perioperative outcomes of robotic partial nephrectomy: A European multicenter observational study (EMOS project). Urology 2015;85:589-95. [Crossref] [PubMed]
- Andrade HS, Zargar H, Caputo PA, et al. Five-year Oncologic Outcomes After Transperitoneal Robotic Partial Nephrectomy for Renal Cell Carcinoma. Eur Urol 2016;69:1149-54. [Crossref] [PubMed]
- Vartolomei MD, Matei DV, Renne G, et al. Robot-assisted Partial Nephrectomy: 5-yr Oncological Outcomes at a Single European Tertiary Cancer Center. Eur Urol Focus 2019;5:636-41. [Crossref] [PubMed]
- Beauval JB, Peyronnet B, Benoit T, et al. Long-term oncological outcomes after robotic partial nephrectomy for renal cell carcinoma: a prospective multicentre study. World J Urol 2018;36:897-904. [Crossref] [PubMed]
- Bertolo R, Garisto J, Dagenais J, et al. Transperitoneal Robot-assisted Partial Nephrectomy with Minimum Follow-up of 5 Years: Oncological and Functional Outcomes from a Single Institution. Eur Urol Oncol 2019;2:207-13. [Crossref] [PubMed]
- Petros FG, Metcalfe MJ, Yu KJ, et al. Oncologic outcomes of patients with positive surgical margin after partial nephrectomy: a 25-year single institution experience. World J Urol 2018;36:1093-101. [Crossref] [PubMed]
- Wood EL, Adibi M, Qiao W, et al. Local Tumor Bed Recurrence Following Partial Nephrectomy in Patients with Small Renal Masses. J Urol 2018;199:393-400. [Crossref] [PubMed]
- Kwon EO, Carver BS, Snyder ME, et al. Impact of positive surgical margins in patients undergoing partial nephrectomy for renal cortical tumours. BJU Int 2007;99:286-9. [Crossref] [PubMed]
- Shah PH, Moreira DM, Okhunov Z, et al. Positive Surgical Margins Increase Risk of Recurrence after Partial Nephrectomy for High Risk Renal Tumors. J Urol 2016;196:327-34. [Crossref] [PubMed]