Dapoxetine and the treatment of premature ejaculation
Introduction
Rapid or premature ejaculation (PE) was first described in the medical literature in 1887 (1) and is widely accepted to be the most common sexual complaint in males (2). Among the multiple definitions and criteria for the diagnosis of PE, the most frequently cited are short time to ejaculation, inability to delay or control ejaculation and negative personal consequences (3-6).
PE can be subdivided into lifelong and acquired PE (7). Lifelong PE is defined by the International Society of Sexual Medicine (ISSM) as a male sexual dysfunction characterized by ejaculation that always or nearly always occurs before or within approximately one minute of vaginal penetration, the inability to delay ejaculation on all or nearly all vaginal penetrations, and negative personal consequences such as distress, bother, frustration and/or the avoidance of sexual intimacy (6). This definition is based on evidence that 80-90% of men with lifelong PE ejaculate within 60 seconds (8). Acquired PE characteristically develops later in life after a history of normal sexual function and ejaculatory control (9). Acquired PE is usually associated with urologic or psychological problems (10). This form of PE can often be remedied by treating the underlying etiology (10).
In 2006, two more PE subtypes, natural variable PE and premature-like ejaculatory dysfunction, were proposed (11). Natural variable PE is regarded as a normal variant of sexual performance, whereas premature-like ejaculatory dysfunction is defined as a complaint of PE superimposed on ejaculation time in the normal range (10). These new subtypes help physicians more precisely stratify patients and set treatment algorithms. Pharmacotherapy remains the basis of management of lifelong and acquired PE, whereas psychotherapy should be considered for patients with natural variable PE and premature like ejaculatory dysfunction (12).
Pathophysiology of PE
Ejaculation is comprised of two phases: emission and expulsion (13). The ejaculatory reflex requires the coordination of sympathetic, parasympathetic and somatic pathways, interlaced with central serotonergic and dopaminergic neuronal pathways (5,13). Emission is the deposition of sperm and seminal fluid into the posterior urethra by contraction of the seminal vesicles and the prostate gland and is mediated by the sympathetic nervous system (T10-L2) (13,14). The epididymis, vas deferens, seminal vesicles, prostate gland, prostatic urethra as well as the bladder neck are involved in the emission phase (13). Expulsion is the forceful antegrade ejection of sperm from the urethra and is controlled by somatic nerves (S2-4) (14). The external urethral sphincter relaxes and the ischiocavernosus, bulbocavernosus and other pelvic floor muscle undergo rhythmic synchronous contractions to allow antegrade flow of sperm out of the urethra. Concurrently, the smooth muscle of the bladder neck contracts to prevent retrograde flow (13).
A large body of research indicates that serotonin acting on the brain’s post-synaptic receptors exerts an overall inhibitory control on the ejaculatory process. As far back as 1976, administration of the serotonin (5-Hydroxytryptamine, 5-HT) precursor 5-Hydroxytryptophan was shown to inhibit male rat sexual behavior (15). 5-HT1A receptors have been demonstrated to exert a pro-ejaculatory effect on male sexual behavior. These receptors act on serotonergic neuronal cell bodies as a means of down regulating the release of 5-HT into the synaptic cleft. Hence, microinjections and a systemic delivery of 8-hydroxy-2-(di-n-propyl-amino) tetralin hydrobromide (8-OH-DPAT), a selective agonist of 5-HT1A receptors, elicits a diminished ejaculatory latency time in rats. There is limited evidence on the function of 5-HT1B and 5-HT2C receptors on ejaculation; however, the studies conducted implicate inhibitory activity for 5-HT1B and 5-HT2C (16,17). Both 5-HT2C and 5-HT1B receptors are distributed within the hypothalamus and in the lumbosacral areas of the spinal cord, along with 5-HT1A receptors (18).
The etiology of PE is multi-factorial in nature. Clinical evidence is limited and contradictory for many purported mechanisms. PE has been associated with both inherited and non-inherited neurobiological etiologies, pharmacological factors, urological pathology, endocrine disorders, and psychological/psychosocial mechanisms. Inherited defects in serotonergic control have been proposed to underlie a genetic basis of PE, possibly due to hyposensitive 5-HT2C and/or hypersensitive 5-HT1A receptors or increased expression of the serotonin transporter (1,18,19).
Acquired neurological diseases such as multiple sclerosis, peripheral neuropathies, spinal cord tumors, and a hypothetical hypersensitivity of the glans penis have been associated with PE; however, much of this evidence is limited and conflicting (20). Possible pharmacological causes of PE include bupropion intake and withdrawal of opioid/SSRI drug use (21-23). Urological factors include a short frenula, with one study reporting 43% of its lifetime PE patients having short frenula and improvement with frenulectomy (24). Researchers have linked hyperthyroidism to PE (25-28). As many as 72% of untreated hyperthyroid men were found to have PE according to one study and the mean IELTs increased dramatically after treatment (28). Some studies have noted a strong association between chronic prostatitis and PE. Improvements in PE and IELTs following antimicrobial therapy were reported (29-32). PE is strongly associated with psychosocial factors such as immature techniques for controlling ejaculation, conditioning from early hurried sexual experiences, alexithymia, anxiety, social phobias, and distressed emotions (33-36). Conversely, men with psychosocial burden have often leads to PE, leading to the question of which came first and making it difficult to scientifically establish causality (20).
Treatment of PE
There are multiple psychological/behavioral treatments for PE, which may be used as a single therapy for natural variable PE or premature-like ejaculatory dysfunction or in combination with pharmacologic therapy for other subtypes of PE (10,37). Psychotherapy and sexual education can reduce patient anxiety, increase communication between a man and his partner, give patients more confidence, and modify many maladaptive sexual scripts (10,14,38). Behavioral therapy is primarily comprised of the “stop and start” technique, established by Semans (39) and a variation/modification of this technique, the ‘squeeze’ technique, proposed by Masters and Johnson (40). The aim of these methodologies is to help a patient maintain his sexual excitement just below the threshold for triggering ejaculation, by either stopping sexual activity or squeezing the head of the penis until the urge to ejaculate subsides (41). Desensitization of the penis via masturbation before sexual intercourse is a practice used by younger men and has proved effective in prolonging the ejaculatory period (42). These psychological/behavioral practices can lead to short-term improvement with overall success rates of 50-60% (43,44). However, as these methods require patient/partner commitment and practice to maintain viability, their efficacy decreases over time (45).
Topical local anesthetics such as lidocaine and/or prilocaine are the oldest drugs used for PE treatment. These are available in cream, gel and aerosol formulations (46,47). These agents delay ejaculation in theory by reducing the sensitivity of the glans penis. The use of topical anesthetics is a relatively efficacious, user friendly, and inexpensive modality for PE treatment (48). However, they can cause penile glans numbness and condom use or prior washing off before sexual activity is required to prevent transference of the drug to the vaginal mucosa (14).
Another potential medical treatment option for PE is the phosphodiesterase type 5 (PDE-5) inhibitors. PDE-5 inhibitors have traditionally been used to treat ED. Theoretically, a man trying to decrease his level of excitation to prevent PE may lead to ED, and conversely a man trying to excite himself to remedy his ED may experience PE. In theory, these are two sides of the same coin and may be superimposed upon each other (49). PE is observed in about 1/3 of patients complaining of ED (50). The efficacy of PE treatment with PDE-5 inhibitors reveals conflicting results. Several authors report better IELT with PDE-5 inhibitors administration for PE (51-53). However, in one well designed, randomized, double blind, placebo-controlled study, IELT was not significantly improved in the sildenafil group compared to placebo (54). A systematic review of PDE-5 inhibitors used in this context failed to provide strong evidence to support a role for PDE-5 inhibitors in the treatment of men with lifelong PE who maintain normal erectile function (55,56). Despite these results, sildenafil reduces anxiety, increase confidence, and gives a perception of ejaculatory control (54). There is some evidence to support the efficacy and safety of off-label on-demand or daily dosing of PDE-5 inhibitors in the treatment of lifelong PE in men with normal erectile function (51-53). However, treatment of lifelong PE with PDE5-inhibitors in such situations is not recommended (level of evidence 4) and further evidence-based research is encouraged to understand these conflicting data (14).
Tramadol hydrochloride is a synthetic opioid analgesic developed in the late 1970s (57). It is a centrally acting analgesic, which binds to both µ-opioid and gamma-aminobutyric acid (GABA) receptors. Secondarily, it inhibits the reuptake of norepinephrine and serotonin (14,57). Systematic reviews and recent published data support the efficacy and safety of on-demand use of tramadol as an alternative treatment for PE (58-61). Meta-analysis reveals that tramadol increases IELT by three minutes (58). However, long-term clinical efficacy, safety issues, and the potential for addiction need to be clarified before tramadol can be routinely used in clinical practice for the treatment of PE (14,59).
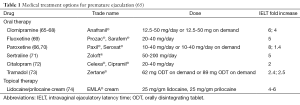
Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed medications for the treatment of a variety of mood disorders such as depression (62). The use of SSRIs to treat PE is based on the observation that delayed ejaculation and anorgasmia are common side effects of this class of drugs (41,63). SSRIs, either alone at low doses or in combination with psychosexual counseling are widely accepted as first line treatments for lifelong PE (14). Men with acquired PE generally receive targeted therapy aimed at resolving the underlying etiology of their PE, either with or without the addition of SSRIs (10). SSRIs act to block the axonal reuptake of serotonin from the synaptic cleft of central serotonergic neurons by 5-HT transporters, which desensitize the 5-HT1A and 5-HT1B receptors (64). The delay in ejaculation can occur within a few days; however, chronic administration for at least 2-3 weeks is necessary to maximize the drugs therapeutic effects (10). With the exception of fluvoxamine, most SSRIs have been shown to clinically delay ejaculatory time (Table 1) (75). Daily use of SSRIs increases geometric mean IELT by 2.6 to 13.2 fold (75).
Although daily administration of these drugs improves ejaculatory latency, chronic use of SSRIs also increases the likelihood of unwanted adverse events. Common adverse effects include fatigue, yawning, nausea, diarrhea and perspiration, which are usually mild and gradually improve within a few weeks (48). This class of drugs is also associated with unwanted sexual adverse events. Decreased libido (41-64%), anorgasmia (31-53%), and impotence/erectile dysfunction (10-41%) have been observed following treatment with fluoxetine, paroxetine, fluvoxamine, sertraline, and citalopram (76,77). The sudden discontinuation of these medications or rapid dose reduction may lead to SSRI-withdrawal syndrome; a cluster of psychological and vegetative clinical symptoms occurring 3-4 days after drug withdrawal and lasting for longer than one week and sometimes accompanied by suicidal thoughts and actions (48,78). The ideal SSRI for treatment of PE should have rapid onset and clearance, good tolerability, fewer adverse effects, and be formulated for use as on-demand treatment (63). Dapoxetine is a short acting SSRI that fits the treatment requirements of PE by exhibiting these ideal parameters.
In this review, we further examine the pharmacokinetics, animal and clinical studies and the adverse events associated with the use of dapoxetine for the treatment of PE.
Dapoxetine
Structure and pharmacokinetics
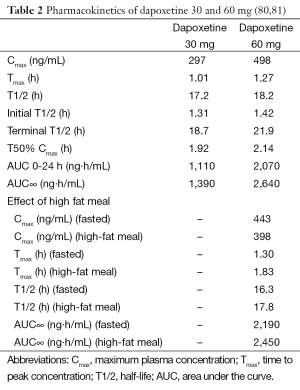
Dapoxetine (Priligy, Menarini, Italy) shares a similar mode of action with other SSRIs. Dapoxetine inhibits the serotonin reuptake transporter, with minimal inhibitory effects at the norepinephrine and dopamine reuptake transporters (41). The chemical name is (+)-(S)-(N), N-dimethyl-(a)-[2-(1-napthalenyloxy)ethyl]-benzenemethanamine hydrochloride. Its structure is similar to fluoxetine (79). The molecular weight of dapoxetine is 341.88 and is a water-soluble compound (38). The pKa is 8.6 and it is charged at a physiological pH of 5.87 (38). After administration, dapoxetine is rapidly absorbed (80). Rate of absorption of dapoxetine is slightly decreased by food as shown in Table 2. Elimination of dapoxetine is biphasic. The initial half-life for 30 and 60 mg doses of dapoxetine is approximately 1.31 and 1.42 hours respectively and 18.7 and 21.9 hours for the terminal half-life, respectively (80). Longer-acting SSRIs such as fluoxetine and paroxetine are absorbed much slower than dapoxetine (80). The half-lives of fluoxetine, paroxetine and sertraline range from 16 to 96 hours (82). On account of its short half-life, the steady state plasma concentrations of dapoxetine are reached within four days compared to 1-22 months for fluoxetine (83,84). Dapoxetine is extensively metabolized in the liver by cytochrome P450 isoenzymes CYP3A4 and CYP2D6 and is excreted primarily in the urine (38,83). Dapoxetine has no inhibitory or inductive effects on cytochrome P450 enzymes (38).
The metabolites of dapoxetine include dapoxetine-N-oxide, desmethyldapoxetine and didesmethyldapoxetine. Dapoxetine-N-oxide does not have any clinical efficacy, while desmethyldapoxetine and didesmethyldapoxetine have similar efficacy to dapoxetine, but as they comprise far smaller percentages of circulating dapoxetine species (less than 3%) their clinical effects are limited (80). At 24 hours, plasma dapoxetine concentrations drop to 3.5% and 3.9% of peak concentrations for 30 and 60 mg doses, respectively (80). The pharmacokinetics of dapoxetine are unaffected by multiple dosing with minimal apparent accumulation (80). In contrast, chronic use of paroxetine and sertraline has a 8- and 2-fold increases in plasma concentrations, respectively (85,86). Elimination of multiple doses is rapid. At 24 hours following the last dose on day 9, there is 5.5% and 6.6% of peak plasma dapoxetine concentrations left in the blood circulation for the 30 and 60 mg doses, respectively (80). Moreover, co-administration of PDE-5 inhibitors with dapoxetine has no effect on the pharmacokinetics of dapoxetine (81). This favorable pharmacokinetic profile makes dapoxetine the drug of choice for on-demand treatment of PE.
Animal studies of dapoxetine
The long acting SSRIs, clomipramine, serotonin, fluoxetine and sertraline, inhibit increases in seminal vesicle pressure and the contractile responses induced by hypogastric nerve stimulation in the animal model of PE (87). However, dapoxetine appears to inhibit the ejaculatory reflex at a supraspinal level. Giuliano et al. studied the effect of dapoxetine on pudendal motoneuron reflex discharges (PMRD) elicited by bilateral electrical stimulation of the dorsal nerve of the penis in the rat model (88). The results revealed that dapoxetine significantly increased PMRD latency and was more efficient than paroxetine in inhibiting PMRD (88). At the supraspinal level, there are 5-HT neurons in the lateral paragigantocellular nucleus (LPGi), which is located in the ventral portion of the rostral medulla in the rat brain (89). Microstimulation of the medullary reticular formation decreases the amplitude and increases the latency of PMRD (90). Intrathecal and intravenous injection of dapoxetine in rats with LPGi lesions did not alter either PMRD latency or amplitude, whereas rats with intact LPGi experienced significant increases in latency and decreases in amplitude of PMRD. Hence, dapoxetine was shown to inhibit the ejaculatory expulsion reflex by modulating activity at a supraspinal level and it is now established that LPGi is a requisite brain structure for this effect (91). Clément’s behavioral study using Fos protein expression in the male rat as a marker of neuronal activity led to the identification of brain areas specifically involved in ejaculation (92). In rapidly ejaculating rats, the density of Fos expressing cells in the hypothalamus, amygdala, and LPGi were significantly higher than in the normal and sluggish categories (92,93). These results demonstrate that acute oral dapoxetine significantly prolongs latency and decreases the number of ejaculations in the rapid ejaculation rat model of PE when compared to controls (vehicle) (92). Fos expression levels in the hypothalamus, thalamus and amygdala were significantly lower in dapoxetine-treated rapid rats compared to vehicle-treated rapid rats (92). The rat model of PE clearly shows that dapoxetine significantly delays ejaculation by reducing neuronal activity in the excitatory thalamic and hypothalamic areas of the ejaculatory circuit.
Clinical studies of dapoxetine
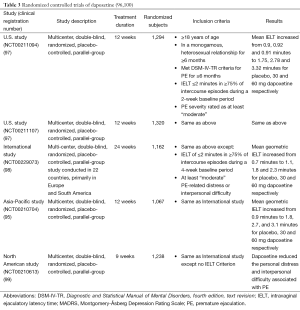
Because of its rapid action and short half-life, the on-demand use of dapoxetine makes it a popular alternative for treating PE (94-97). Currently, dapoxetine is approved for the treatment of PE in over 50 countries. Several randomized controlled trials (RCTs) demonstrated the efficacy and safety of dapoxetine on more than 6,000 men with PE in over 25 countries (95,97-99) (Table 3). Integrated analysis of these phase III trials of dapoxetine demonstrate a significant increase in geometric mean IELT, from baseline (0.8 min) with 30 mg (2.0 min) and 60 mg (2.3 min) vs. placebo (1.3 min) at 12 weeks (96). In addition to IELT, both doses of dapoxetine improved patient reported outcome measures compared to placebo (96). Dapoxetine was comparably effective both in men with lifelong and acquired PE (96,101,102).
Despite these favorable outcomes, the results of the integrated analysis of the clinical dapoxetine trials revealed that 30.4% of the subjects included into the study discontinued, mostly due to lack of efficacy and personal reasons (96). These findings were in accordance with those of a recent report that demonstrated 20% of lifelong PE patients decided not to start dapoxetine treatment and almost 90% of the ones who initiated this therapy discontinued within one year because the beneficial effect were below expectations (24.4%), cost (22.1%), side effects (19.8%), loss of interest in sex 19.8%, and lack of efficacy 13.9% (103).
Adverse events related to dapoxetine therapy were more common than placebo (56.1% vs. 35.1%) (96). Although these events were usually mild to moderate in severity, they still resulted in discontinuation from treatment, especially among patients who were treated with dapoxetine 60 mg (1.0%, 3.5%, 8.8%, and 10.0% of subjects with placebo, dapoxetine 30 mg prn, dapoxetine 60 mg prn, and dapoxetine 60 mg qd, respectively) (96). The adverse events included nausea (17.3%), dizziness (9.4%), headache (7.9%), diarrhoea (5.9%), somnolence (3.9%), fatigue (3.9%), insomnia (3.8%) and nasopharyngitis (3.1%). A recent dapoxetine postmarketing observational study confirmed its safety profile and low prevalence of adverse events, which were noted to be more common in patients aged >65 yr (21.4%) (104).
No drug-drug interactions associated with dapoxetine have been reported (105). In men with PE and comorbid ED, who were on a stable regimen of a PDE5 inhibitor, dapoxetine provided meaningful treatment benefit and was generally well tolerated (106).
Conclusions
There are a number of treatment options available for men who suffer from PE. These include psychological/behavioral therapy, topical anesthetic agents, PDE-5 inhibitors and tramadol hydrochloride. Off-label oral SSRIs are commonly prescribed for PE treatment; however, despite their efficacy, daily use of SSRIs comes with unwanted adverse events. Dapoxetine is a short-acting SSRI, designed specifically for the treatment of PE. Dapoxetine has demonstrated clinical efficacy and safety in five large, randomized, placebo-controlled phase III clinical trials. The postmarketing observational studies confirm its reliable safety profile and low prevalence of adverse events associated with its use. Dapoxetine is currently the oral drug of choice for on demand therapy of PE.
Acknowledgements
None.
Footnote
Conflicts of Interest: Ege Can Serefoglu is consultant for Allergan Inc. Irvwine, CA, USA. Wayne J.G. Hellstrom: American Medical Systems—Consultant or Advisor; Andromedical—Consultant or Advisor; Auxilium—Meeting Participant or Lecturer, Consultant or Advisor, Investigator; Allergan—Consultant or Advisor, Scientific Study or Trial; Coloplast—Consultant or Advisor, Investigator; Cook—Consultant or Advisor, Lecturer; Endo—Consultant or Advisor, Investigator, Lecturer; Johnson & Johnson—Consultant or Advisor, Meeting Participant or Lecturer, Investigator; Lilly, USA—Consultant or Advisor, Lecturer; NIH—Board Member, Officer, Trustee; Slate Pharmaceutical—Lecturer, Advisor, and Investigator; Theralogix—Board Member, Officer, Trustee; VIVUS—Advisor/Consultant, Investigator, Lecturer. And Premsant Sangkum and Rhamee Badr have no conflicts of interest to declare.
References
- Waldinger MD. The neurobiological approach to premature ejaculation. J Urol 2002;168:2359-67. [PubMed]
- Jannini EA, Lenzi A. Epidemiology of premature ejaculation. Curr Opin Urol 2005;15:399-403. [PubMed]
- International Classification of Diseases and Related Health Problems. 10 ed. Geniva: World Health Organization, 1994.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th Ed. Text Revision. Washington, D.C.: American Psychiatric Publishing Inc, 2000.
- Rowland D, Mcmahon CG, Abdo C, et al. Disorders of orgasm and ejaculation in men. J Sex Med 2010;7:1668-86. [PubMed]
- McMahon CG, Althof SE, Waldinger MD, et al. An evidence-based definition of lifelong premature ejaculation: report of the International Society for Sexual Medicine (ISSM) ad hoc committee for the definition of premature ejaculation. J Sex Med 2008;5:1590-606. [PubMed]
- Schapiro B. Premature ejaculation, a review of 1130 cases. J Urol 1943;50:374-9.
- Waldinger M, Hengeveld M, Zwinderman A, et al. An empirical operationalization of DSM-IV diagnostic criteria for premature ejaculation. Int J Psychiatry Clin Pract 1988;2:287-93.
- Godpodinoff ML. Premature ejaculation: clinical subgroups and etiology. J Sex Marital Ther 1989;15:130-4. [PubMed]
- Waldinger MD. Premature ejaculation: state of the art. Urol Clin North Am 2007;34:591-9. vii-viii. [PubMed]
- Waldinger MD, Schweitzer DH. Changing paradigms from a historical DSM-III and DSM-IV view toward an evidence-based definition of premature ejaculation. Part I--validity of DSM-IV-TR. J Sex Med 2006;3:682-92. [PubMed]
- Waldinger MD. Recent advances in the classification, neurobiology and treatment of premature ejaculation. Adv Psychosom Med 2008;29:50-69. [PubMed]
- Giuliano F, Clément P. Physiology of ejaculation: emphasis on serotonergic control. Eur Urol 2005;48:408-17. [PubMed]
- McMahon CG, Jannini E, Waldinger M, et al. Standard operating procedures in the disorders of orgasm and ejaculation. J Sex Med 2013;10:204-29. [PubMed]
- Ginton A. Copulation in noncopulators: effect of PCPA in male rats. Pharmacol Biochem Behav 1976;4:357-9. [PubMed]
- Hillegaart V, Ahlenius S. Facilitation and inhibition of male rat ejaculatory behaviour by the respective 5-HT1A and 5-HT1B receptor agonists 8-OH-DPAT and anpirtoline, as evidenced by use of the corresponding new and selective receptor antagonists NAD-299 and NAS-181. Br J Pharmacol 1998;125:1733-43. [PubMed]
- Pomerantz SM, Hepner BC, Wertz JM. 5-HT1A and 5-HT1C/1D receptor agonists produce reciprocal effects on male sexual behavior of rhesus monkeys. Eur J Pharmacol 1993;243:227-34. [PubMed]
- Giuliano F, Clement P. Serotonin and premature ejaculation: from physiology to patient management. Eur Urol 2006;50:454-66. [PubMed]
- Waldinger MD, Berendsen HH, Blok BF, et al. Premature ejaculation and serotonergic antidepressants-induced delayed ejaculation: the involvement of the serotonergic system. Behav Brain Res 1998;92:111-8. [PubMed]
- Buvat J. Pathophysiology of premature ejaculation. J Sex Med 2011;8:316-27. [PubMed]
- Abdollahian E, Javanbakht A, Javidi K, et al. Study of the efficacy of fluoxetine and clomipramine in the treatment of premature ejaculation after opioid detoxification. Am J Addict 2006;15:100-4. [PubMed]
- Adson DE, Kotlyar M. Premature ejaculation associated with citalopram withdrawal. Ann Pharmacother 2003;37:1804-6. [PubMed]
- Kravos M. Bupropion-associated premature ejaculation. Pharmacopsychiatry 2010;43:156-7. [PubMed]
- Gallo L, Perdonà S, Gallo A. The role of short frenulum and the effects of frenulectomy on premature ejaculation. J Sex Med 2010;7:1269-76. [PubMed]
- Carosa E, Di Sante S, Rossi S, et al. Ontogenetic profile of the expression of thyroid hormone receptors in rat and human corpora cavernosa of the penis. J Sex Med 2010;7:1381-90. [PubMed]
- Kulikov AV, Zubkov EA. Chronic thyroxine treatment activates the 5-HT2A serotonin receptor in the mouse brain. Neurosci Lett 2007;416:307-9. [PubMed]
- Cahangirov A, Cihan A, Murat N, et al. Investigation of the neural target level of hyperthyroidism in premature ejaculation in a rat model of pharmacologically induced ejaculation. J Sex Med 2011;8:90-6. [PubMed]
- Cihan A, Demir O, Demir T, et al. The relationship between premature ejaculation and hyperthyroidism. J Urol 2009;181:1273-80. [PubMed]
- El-Nashaar A, Shamloul R. Antibiotic treatment can delay ejaculation in patients with premature ejaculation and chronic bacterial prostatitis. J Sex Med 2007;4:491-6. [PubMed]
- Sadeghi-Nejad H, Seftel A. Sexual dysfunction and prostatitis. Curr Urol Rep 2006;7:479-84. [PubMed]
- Screponi E, Carosa E, Di Stasi SM, et al. Prevalence of chronic prostatitis in men with premature ejaculation. Urology 2001;58:198-202. [PubMed]
- Trinchieri A, Magri V, Cariani L, et al. Prevalence of sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome. Arch Ital Urol Androl 2007;79:67-70. [PubMed]
- Corona G, Mannucci E, Petrone L, et al. Psycho-biological correlates of free-floating anxiety symptoms in male patients with sexual dysfunctions. J Androl 2006;27:86-93. [PubMed]
- Tignol J, Martin-Guehl C, Aouizerate B, et al. Social phobia and premature ejaculation: a case-control study. Depress Anxiety 2006;23:153-7. [PubMed]
- Michetti PM, Rossi R, Bonanno D, et al. Dysregulation of emotions and premature ejaculation (PE): alexithymia in 100 outpatients. J Sex Med 2007;4:1462-7. [PubMed]
- Rowland DL, Patrick DL, Rothman M, et al. The psychological burden of premature ejaculation. J Urol 2007;177:1065-70. [PubMed]
- Perelman MA. A new combination treatment for premature ejaculation: a sex therapist’s perspective. J Sex Med 2006;3:1004-12. [PubMed]
- Feige AM, Pinsky MR, Hellstrom W. Dapoxetine for premature ejaculation. Clin Pharmacol Ther 2011;89:125-8. [PubMed]
- Semans JH. Premature ejaculation: a new approach. South Med J 1956;49:353-8. [PubMed]
- Masters WH, Johnson VE. eds. Human sexual inadequacy. Boston: Little&Brown, 1970.
- Hellstrom WJ. Emerging treatments for premature ejaculation: focus on dapoxetine. Neuropsychiatr Dis Treat 2009;5:37-46. [PubMed]
- de Carufel F, Trudel G. Effects of a new functional-sexological treatment for premature ejaculation. J Sex Marital Ther 2006;32:97-114. [PubMed]
- Grenier G, Byers ES. Rapid ejaculation: a review of conceptual, etiological, and treatment issues. Arch Sex Behav 1995;24:447-72. [PubMed]
- Metz ME, Pryor JL, Nesvacil LJ, et al. Premature ejaculation: a psychophysiological review. J Sex Marital Ther 1997;23:3-23. [PubMed]
- De Amicis LA, Goldberg DC, Lopiccolo J, et al. Clinical follow-up of couples treated for sexual dysfunction. Arch Sex Behav 1985;14:467-89. [PubMed]
- Berkovitch M, Keresteci AG, Koren G. Efficacy of prilocaine-lidocaine cream in the treatment of premature ejaculation. J Urol 1995;154:1360-1. [PubMed]
- Henry R, Morales A, Wyllie MG. TEMPE: Topical Eutectic-Like Mixture for Premature Ejaculation. Expert opinion on drug delivery 2008;5:251-61. [PubMed]
- Porst H. An overview of pharmacotherapy in premature ejaculation. J Sex Med 2011;8:335-41. [PubMed]
- Jannini EA, Lombardo F, Lenzi A. Correlation between ejaculatory and erectile dysfunction. Int J Androl 2005;28 Suppl 2:40-5. [PubMed]
- Corona G, Petrone L, Mannucci E, et al. Psycho-Biological correlates of rapid ejaculation in patients attending an andrologic unit for sexual dysfunctions. Eur Urol 2004;46:615-22. [PubMed]
- Salonia A, Maga TO, Colombo RE, et al. A prospective study comparing paroxetine alone versus paroxetine plus sildenafil in patients with premature ejaculation. J Urol 2002;168:2486-9. [PubMed]
- Mattos RM, Marmo Lucon A, Srougi M. Tadalafil and fluoxetine in premature ejaculation: prospective, randomized, double-blind, placebo-controlled study. Urol Int 2008;80:162-5. [PubMed]
- Aversa A, Pili M, Francomano D, et al. Effects of vardenafil administration on intravaginal ejaculatory latency time in men with lifelong premature ejaculation. Int J Impot Res 2009;21:221-7. [PubMed]
- McMahon CG, Stuckey BG, Andersen M, et al. Efficacy of sildenafil citrate (Viagra) in men with premature ejaculation. J Sex Med 2005;2:368-75. [PubMed]
- McMahon CG, McMahon CN, Leow LJ, et al. Efficacy of type-5 phosphodiesterase inhibitors in the drug treatment of premature ejaculation: a systematic review. BJU Int 2006;98:259-72. [PubMed]
- Asimakopoulos AD, Miano R, Finazzi Agrò E, et al. Does current scientific and clinical evidence support the use of phosphodiesterase type 5 inhibitors for the treatment of premature ejaculation? a systematic review and meta-analysis. J Sex Med 2012;9:2404-16. [PubMed]
- Giuliano FA. Tramadol for the treatment of premature ejaculation. Eur Urol 2012;61:744-5. [PubMed]
- Wu T, Yue X, Duan X, et al. Efficacy and safety of tramadol for premature ejaculation: a systematic review and meta-analysis. Urology 2012;80:618-24. [PubMed]
- Wong BL, Malde S. The use of tramadol “on-demand” for premature ejaculation: a systematic review. Urology 2013;81:98-103. [PubMed]
- Yang L, Qian S, Liu H, et al. Role of tramadol in premature ejaculation: a systematic review and meta-analysis. Urol Int 2013;91:197-205. [PubMed]
- Eassa BI, El-Shazly MA. Safety and efficacy of tramadol hydrochloride on treatment of premature ejaculation. Asian J Androl 2013;15:138-42. [PubMed]
- von Wolff A, Hölzel LP, Westphal A, et al. Selective serotonin reuptake inhibitors and tricyclic antidepressants in the acute treatment of chronic depression and dysthymia: A systematic review and meta-analysis. J Affect Disord 2013;144:7-15. [PubMed]
- Giuliano F, Hellstrom WJ. The pharmacological treatment of premature ejaculation. BJU Int 2008;102:668-75. [PubMed]
- Olivier B, van Oorschot R, Waldinger MD. Serotonin, serotonergic receptors, selective serotonin reuptake inhibitors and sexual behaviour. Int Clin Psychopharmacol 1998;13 Suppl 6:S9-14. [PubMed]
- Althof SE, Abdo CH, Dean J, et al. International society for sexual medicine’s guidelines for the diagnosis and treatment of premature ejaculation. J Sex Med 2010;7:2947-69. [PubMed]
- Waldinger MD, Zwinderman AH, Olivier B. On-Demand treatment of premature ejaculation with clomipramine and paroxetine: a randomized, Double-Blind Fixed-Dose study with stopwatch assessment. Eur Urol 2004;46:510-5. [PubMed]
- Althof SE, Levine SB, Corty EW, et al. A double-blind crossover trial of clomipramine for rapid ejaculation in 15 couples. J Clin Psychiatry 1995;56:402-7. [PubMed]
- Goodman RE. An assessment of clomipramine (Anafranil) in the treatment of premature ejaculation. J Int Med Res 1980;8:53-9. [PubMed]
- Kara H, Aydin S, Agargun MY, et al. The efficacy of fluoxetine in the treatment of premature ejaculation: a Double-Blind placebo controlled study. J Urol 1996;156:1631-2. [PubMed]
- Waldinger MD, Hengeveld MW, Zwinderman AH. Paroxetine treatment of premature ejaculation: a double-blind, randomized, placebo-controlled study. Am J Psychiatry 1994;151:1377-9. [PubMed]
- McMahon CG. Treatment of premature ejaculation with sertraline hydrochloride: a single-blind placebo controlled crossover study. J Urol 1998;159:1935-8. [PubMed]
- Atmaca M, Kuloglu M, Tezcan E, et al. The efficacy of citalopram in the treatment of premature ejaculation: a placebo-controlled study. Int J Impot Res 2002;14:502-5. [PubMed]
- Bar-Or D, Salottolo KM, Orlando A, et al. A randomized double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of two doses of the tramadol orally disintegrating tablet for the treatment of premature ejaculation within less than 2 minutes. Eur Urol 2012;61:736-43. [PubMed]
- Busato W, Galindo CC. Topical anaesthetic use for treating premature ejaculation: a double-blind, randomized, placebo-controlled study. BJU Int 2004;93:1018-21. [PubMed]
- Waldinger MD, Zwinderman AH, Schweitzer DH, et al. Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta-analysis. Int J Impot Res 2004;16:369-81. [PubMed]
- Montejo AL, Llorca G, Izquierdo JA, et al. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry 2001;62 Suppl 3:10-21. [PubMed]
- Montejo-González AL, Llorca G, Izquierdo JA, et al. SSRI-induced sexual dysfunction: fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. J Sex Marital Ther 1997;23:176-94. [PubMed]
- McCarty E, Dinsmore W. Dapoxetine: an evidence-based review of its effectiveness in treatment of premature ejaculation. Core Evid 2012;7:1-14. [PubMed]
- Sorbera LA, Castaner J, Castaner RM. Dapoxetine hydrochloride. Drugs Future 2004;29:1201-5.
- Modi NB, Dresser MJ, Simon M, et al. Single- and multiple-dose pharmacokinetics of dapoxetine hydrochloride, a novel agent for the treatment of premature ejaculation. J Clin Pharmacol 2006;46:301-9. [PubMed]
- Dresser MJ, Kang D, Staehr P, et al. Pharmacokinetics of dapoxetine, a new treatment for premature ejaculation: Impact of age and effects of a high-fat meal. J Clin Pharmacol 2006;46:1023-9. [PubMed]
- Hiemke C, Hartter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther 2000;85:11-28. [PubMed]
- Feige AM, Pinsky MR, Hellstrom W. Dapoxetine for premature ejaculation. Clin Pharmacol Ther 2011;89:125-8. [PubMed]
- Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol 1996;78:203-8. [PubMed]
- PAXIL® (paroxetine hydrochloride) Tablets and Oral Suspension [package insert]. Research Triangle Park, NC: GlaxoSmithKline, 2007.
- ZOLOFT® (sertraline hydrochloride) Tablets and Oral Concentrate [package insert]. New York, NY: Pfizer Inc, 2005.
- Kim SW, Lee SH, Paick JS. In vivo rat model to measure hypogastric nerve stimulation-induced seminal vesicle and vasal pressure responses simultaneously. Int J Impot Res 2004;16:427-32. [PubMed]
- Giuliano F, Bernabé J, Gengo P, et al. Effect of acute dapoxetine administration on the pudendal motoneuron reflex in anesthetized rats: comparison with paroxetine. J Urol 2007;177:386-9. [PubMed]
- Andrezik JA, Chan-Palay V, Palay SL. The nucleus paragigantocellularis lateralis in the rat. Anat Embryol (Berl) 1981;161:355-71. [PubMed]
- Johnson RD, Hubscher CH. Brainstem microstimulation differentially inhibits pudendal motoneuron reflex inputs. Neuroreport 1998;9:341-5. [PubMed]
- Clément P, Bernabé J, Gengo P, et al. Supraspinal site of action for the inhibition of ejaculatory reflex by dapoxetine. Eur Urol 2007;51:825-32. [PubMed]
- Clément P, Laurin M, Compagnie S, et al. Effect of dapoxetine on ejaculatory performance and related brain neuronal activity in rapid ejaculator rats. J Sex Med 2012;9:2562-73. [PubMed]
- Pattij T, de Jong TR, Uitterdijk A, et al. Individual differences in male rat ejaculatory behaviour: searching for models to study ejaculation disorders. Eur J Neurosci 2005;22:724-34. [PubMed]
- Hellstrom WJ, Althof S, Gittelman M, et al. Dapoxetine for the treatment of men with premature ejaculation (PE): dose-finding analysis. J Urol 2005;173:238-abstract 877.
- McMahon C, Kim SW, Park NC, et al. Treatment of premature ejaculation in the Asia-Pacific region: results from a phase III double-blind, parallel-group study of dapoxetine. J Sex Med 2010;7:256-68. [PubMed]
- McMahon CG, Althof SE, Kaufman JM, et al. Efficacy and safety of dapoxetine for the treatment of premature ejaculation: integrated analysis of results from five phase 3 trials. J Sex Med 2011;8:524-39. [PubMed]
- Pryor JL, Althof SE, Steidle C, et al. Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: an integrated analysis of two double-blind, randomised controlled trials. Lancet 2006;368:929-37. [PubMed]
- Buvat J, Tesfaye F, Rothman M, et al. Dapoxetine for the treatment of premature ejaculation: results from a randomized, Double-Blind, Placebo-Controlled phase 3 trial in 22 countries. Eur Urol 2009;55:957-67. [PubMed]
- Kaufman JM, Rosen RC, Mudumbi RV, et al. Treatment benefit of dapoxetine for premature ejaculation: results from a placebo-controlled phase III trial. BJU Int 2009;103:651-8. [PubMed]
- Hellstrom WJ. Update on treatments for premature ejaculation. Int J Clin Pract 2011;65:16-26. [PubMed]
- Jannini EA. Editorial comment on: Dapoxetine for the treatment of premature ejaculation: results from a randomized, double-blind, placebo-controlled phase 3 trial in 22 countries. Eur Urol 2009;55:967-8. [PubMed]
- Porst H, Mcmahon CG, Althof SE, et al. Baseline characteristics and treatment outcomes for men with acquired or lifelong premature ejaculation with mild or no erectile dysfunction: integrated analyses of two phase 3 dapoxetine trials. J Sex Med 2010;7:2231-42. [PubMed]
- Mondaini N, Fusco F, Cai T, et al. Dapoxetine treatment in patients with lifelong premature ejaculation: the reasons of a “Waterloo”. Urology 2013;82:620-4. [PubMed]
- Mirone V, Arcaniolo D, Rivas D, et al. Results from a Prospective Observational Study of Men with Premature Ejaculation Treated with Dapoxetine or Alternative Care: The PAUSE Study. Eur Urol 2013. [Epub ahead of print].
- Dresser MJ, Desai D, Gidwani S, et al. Dapoxetine, a novel treatment for premature ejaculation, does not have pharmacokinetic interactions with phosphodiesterase-5 inhibitors. International Journal of Impotence Research 2006;18:104-10. [PubMed]
- McMahon CG, Giuliano F, Dean J, et al. Efficacy and safety of dapoxetine in men with premature ejaculation and concomitant erectile dysfunction treated with a phosphodiesterase type 5 inhibitor: randomized, placebo-controlled, phase III study. J Sex Med 2013;10:2312-25. [PubMed]